Abstract
Parasite drug resistance and difficulties in developing effective vaccines have precipitated the search for alternative therapies for malaria. The success of passive immunization suggests that immunoglobulin (Ig)-based therapies are effective. To further explore the mechanism(s) by which antibody mediates its protective effect, we generated human chimeric IgG1 and IgA1 and a single-chain diabody specific for the C-terminal 19-kDa region of Plasmodium yoelii merozoite surface protein 1 (MSP119), a major target of protective immune responses. These novel human reagents triggered in vitro phagocytosis of merozoites but, unlike their parental mouse IgG2b, failed to protect against parasite challenge in vivo. Therefore, the Fc region appears critical for mediating protection in vivo, at least for this MSP119 epitope. Such antibodies may serve as prototype therapeutic agents, and as useful tools in the development of in vitro neutralization assays with Plasmodium parasites. (Blood. 2003;102:4424-4430)
Introduction
Malaria continues to kill approximately 2 million people each year.1 The success of passive immunization in humans and animals suggests that immunoglobulin (Ig)-based therapies could be effective.2,3 Manipulating antibody (Ab) genes allows the design of Ig with defined class and specificity, targeting protective epitopes on the parasite surface. An appropriate target is the 19-kDa C-terminal region of merozoite surface protein 1 (MSP119). This polypeptide displays limited sequence polymorphism,4 is expressed by all vertebrate life-cycle stages,5 and acts as a major target of the erythrocyte invasion-inhibitory Ab response in individuals immune to Plasmodium falciparum malaria.6 The mechanisms whereby Ig mediates protective immunity in malaria remain unclear.7 A primary role of Ab appears to involve blocking of merozoite invasion of erythrocytes.8 However, studies using human Ab suggest that the protective effects are mediated through interaction with specific receptors for the Ig Fc region (FcR) expressed on various effector cells.9,10 Using Plasmodium yoelii in a murine model to address these possibilities, we generated human chimeric IgG1 and IgA1 Abs recognizing an epitope on P yoelii MSP119. We have investigated the potential of these intact Abs, and a novel diabody with specificity for both MSP119 and a human receptor for IgG Fc, FcγRI, as novel malaria therapeutics.
Materials and methods
Construction of expression vectors
Variable heavy (VH) and light (VL) complementary DNAs (cDNAs) were cloned from mouse hybridomas B10 (anti-MSP119) and M22 (anti-human FcγRI) by reverse transcriptase-polymerase chain reaction (RT-PCR) using degenerate primers (5′-SARGTNMARCTGCAGSAGTC-3′ and 5′-TGAGGAGACGGTGACCGTGGTYCCTTGGCCCC-3′ for VH and 5′-GAYATTGAGCTCACMCARWCTMCA-3′ and 5′-CCGTTTBAKCTCGAGCTTKGTSCC-3′ for VL).11,12 An alternative primer incorporating a BssHII restriction enzyme site was used to reamplify B10 VH to facilitate subcloning. The IgA1 heavy-chain constant region was subcloned as a BamHI-SalI fragment from an α chain gene-containing plasmid13 into pAD-Gal4-2.1 (Stratagene, La Jolla, CA) and then as a BamHI-XbaI fragment into pVHExpress (a kind gift from Andrew Bradbury, Los Alamos National Laboratory, Los Alamos, NM),14 replacing the γ1 constant domains to yield pVHExpress α1H. The VH genes were inserted into pVHExpress and pVHExpress α1H, upstream of the γ1 or α1 constant regions, respectively, and the VL genes into pVKExpress upstream of the human κ gene.14
To generate the FcγRI x MSP119 diabody, an overlapping PCR-based strategy was used. First, the VH and VL regions of B10 and M22 were amplified. The 5′ VH primers incorporated a 5′ BssHII site in B10 VH and a sequence encoding the C-terminal-half of a 19-residue-“long” linker (RGSGGGGSGGRAGSGGGGS)15 in M22 VH. The 3′ primer for both VHs incorporated a sequence encoding a GGGGS linker,16 whereas the 5′ VL primers incorporated overlapping GGGGS linkers. The 3′ M22 VL primer included a sequence encoding the N-terminal-half of the long linker, whereas that for B10 VL included the sequence for a hexahistidyl tag and a XbaI site. The second round consisted of 2 overlap extension reactions coupling B10 VH to M22 VL, and M22 VH to B10 VL. Finally, the 2 fragments were ligated via an AscI site within the long-linker sequence, followed by insertion as a BssHII-XbaI fragment into pVHExpress, replacing the γ1 constant region.
Production of Abs and diabodies
Chinese hamster ovary (CHO)-K1 cells were transfected with corresponding heavy- and light-chain plasmids, or the diabody expression plasmid, and positive clones were selected.17 Clones secreting MSP119-specific IgG1, IgA1, or diabody were detected by enzyme-linked immunosorbent assay (ELISA) or immunoblotting using mouse antipolyhistidine (Sigma-Aldrich, Poole, Dorset, United Kingdom), goat antihuman IgG (Sigma-Aldrich), or goat antihuman IgA (Kirkegaard and Perry Laboratories, Gaithersburg, MD) Abs conjugated to horseradish peroxidase (HRP). From large-scale cultures, human IgG1 and mouse IgG2b were purified on Protein A-Sepharose (Amersham Biosciences, Little Chalfont, Bucks, United Kingdom), and human IgA1 and the diabody were affinity-purified on MSP119-Sepharose. The integrity of the proteins was verified on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels, and their expected sizes were calculated using ProtParam (European Molecular Biology Laboratory, http://www.expasy.ch).
Antigen binding studies
For immunofluorescence (IF) studies, washed erythrocytes infected with P yoelii YM and merozoites were fixed on slides in methanol-acetone (1:1, vol/vol) for 10 minutes. After blocking in phosphate-buffered saline (PBS)/5% (vol/vol) goat serum, slides were incubated with Abs at 1 μg mL-1 in blocking buffer for 1 hour, washed, then incubated for 1 hour with fluorescein isothiocyanate (FITC)-conjugated goat F(ab′)2 antihuman IgG1 or IgA1 (Caltag, Burlingame, CA) diluted 1:500 in blocking buffer. After washing, slides were mounted with antifade and examined by confocal microscopy (Zeiss LSM510). Binding was also analyzed by IF and flow cytometry using FITC-conjugated recombinant proteins.
Surface plasmon resonance analysis
Following optimization, glutathione-S-transferase (GST-MSP119) (0.5 μg) and GST (2 μg) were amine-coupled to test and control flow cells, respectively, on a CM5 chip (Biacore AB, Stevenage, United Kingdom). Abs (6.7 nM to 133 nM) were injected, and association and dissociation were observed. Data from a BIAcore X machine were analyzed using BIAevaluation 3.0 software.
Respiratory burst and phagocytosis assays
A chemiluminescence-based neutrophil respiratory burst assay was conducted on plates coated with MSP119 at 10 μg mL-1 as previously described.18 For phagocytosis assays, P yoelii merozoites were isolated from mouse blood (25%-30% parasitemia) using discontinuous Percoll gradients and lysis with 10% saponin.19 Merozoites (107) were incubated with 1.3 μM FITC-labeled Ab/diabody in 500 μL Krebs glucose saline (KGS) for 1 hour at 37°C. After 5 washes in KGS, 2.5 × 105 opsonized merozoites were incubated with 5 × 104 neutrophils (final volume, 500 μL) for 2 hours at 37°C with gentle shaking. Centrifugation through Ficoll removed unbound merozoites, and 5 × 103 cells were spun onto slides for fixing, staining, and microscopy. In duplicate experiments, 100 neutrophils were counted, scoring those with at least 3 internalized parasites as positive. In flow cytometry experiments, typically 10 000 neutrophils were counted. Gated cells were confirmed as neutrophils by reactivity with phycoerythrin-conjugated anti-CD16 Ab (Becton Dickinson).
Passive immunization and parasite challenge
Five female BALB/c mice from 8 weeks of age, bred under specific-pathogen-free conditions, were used per test. Abs and diabody (0.5 mg/injection) were administered intraperitoneally -1 day and +1 day with respect to parasite challenge and intravenously on the day of P yoelii YM challenge. Parasitized eythrocytes (104/mouse) were injected into the lateral tail vein at least 1 hour after Ab/diabody treatment. Parasitemia was assessed daily on Giemsa reagent-stained blood smears. Significant differences were determined in a paired t test (P < .01).
Results
Characterization of MSP119-specific human Abs and a diabody (hFcγRI × MSP119)
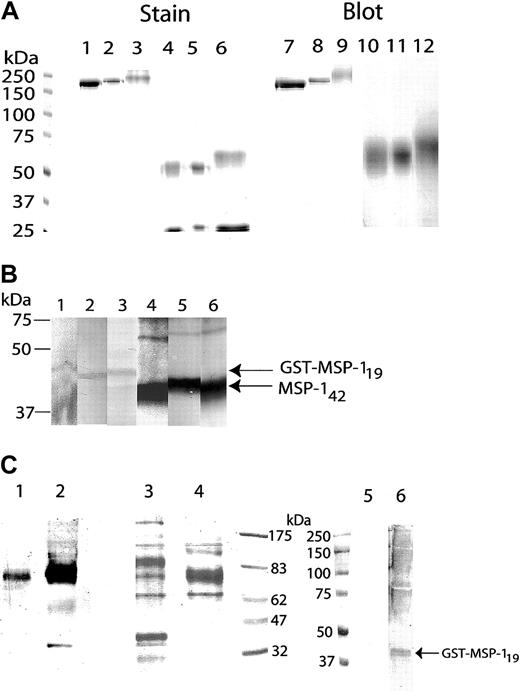
Abs and diabody, purified from CHO-K1 transfectant culture supernatants, contained polypeptides of the expected sizes on SDS-PAGE and the anticipated reactivities with isotype-specific Abs (Figure 1A). The Abs recognized a GST-MSP119 fusion protein and the approximately 42-kDa fragment of MSP1 (MSP142) in parasite extracts enriched for merozoites (Figure 1B). This reactivity was abolished under reducing conditions, because epitope integrity requires the presence of disulfide bonds.20
SDS-PAGE analysis of purified Abs and diabody. (A) Purified anti-MSP119 mouse IgG2b (lanes 1, 4, 7, and 10), human IgG1 (lanes 2, 5, 8, and 11), and human IgA1 (lanes 3, 6, 9, and 12) were subjected to SDS-PAGE under nonreducing (lanes 1-3 and 7-8) or reducing (lanes 4-5 and 10-12) conditions on 8.2% polyacrylamide gels, and stained with Coomassie blue (lanes 1-6) or immunoblotted (lanes 7-12). (B) The Abs detect recombinant GST-MSP119 fusion protein (lanes 1-3) or a fragment of MSP1 in an extract of P yoelii YM merozoites electrophoresed under nonreducing conditions (lanes 4-6) and transferred to nitrocellulose. Detecting Abs: lanes 1 and 4, mouse IgG2b; lanes 2 and 5, human IgG1; and lanes 3 and 6, human IgA1. (C) Purified FcγRI × MSP119 diabody was run under nonreducing (lanes 1 and 3) or reducing (lanes 2 and 4) conditions. Samples were either blotted and probed with anti-His (lanes 1 and 2) or stained with Coomassie blue (lanes 3 and 4). The diabody detected a blot of recombinant GST-MSP119 fusion protein run under nonreducing conditions (lane 6). Secondary anti-His Ab did not cross-react with the fusion protein alone (lane 5).
SDS-PAGE analysis of purified Abs and diabody. (A) Purified anti-MSP119 mouse IgG2b (lanes 1, 4, 7, and 10), human IgG1 (lanes 2, 5, 8, and 11), and human IgA1 (lanes 3, 6, 9, and 12) were subjected to SDS-PAGE under nonreducing (lanes 1-3 and 7-8) or reducing (lanes 4-5 and 10-12) conditions on 8.2% polyacrylamide gels, and stained with Coomassie blue (lanes 1-6) or immunoblotted (lanes 7-12). (B) The Abs detect recombinant GST-MSP119 fusion protein (lanes 1-3) or a fragment of MSP1 in an extract of P yoelii YM merozoites electrophoresed under nonreducing conditions (lanes 4-6) and transferred to nitrocellulose. Detecting Abs: lanes 1 and 4, mouse IgG2b; lanes 2 and 5, human IgG1; and lanes 3 and 6, human IgA1. (C) Purified FcγRI × MSP119 diabody was run under nonreducing (lanes 1 and 3) or reducing (lanes 2 and 4) conditions. Samples were either blotted and probed with anti-His (lanes 1 and 2) or stained with Coomassie blue (lanes 3 and 4). The diabody detected a blot of recombinant GST-MSP119 fusion protein run under nonreducing conditions (lane 6). Secondary anti-His Ab did not cross-react with the fusion protein alone (lane 5).
The His-tagged diabody was purified on GST-MSP119-Sepharose columns. On SDS-PAGE, the single polypeptide had an apparent molecular mass of approximately 75 kDa (Figure 1C), and was recognized by antihexahistidyl Abs. The His-tag may be somewhat buried in the undenatured protein since antihexahistidyl Ab reactivity was strongest with the reduced protein, and attempts to purify diabody by nickel affinity chromatography proved unsuccessful. The diabody preparation contained a contaminant of approximately 35 kDa, similar to that observed by others.15 Fast protein liquid chromatography (FPLC) analysis (not shown), indicated the diabody to have a solution size of about 50 kDa to 60 kDa, in line with the predicted molecular mass of 54 kDa, and implying that the diabody runs aberrantly on SDS-PAGE. Immunoblotting showed that the diabody bound the MSP119 fusion protein (Figure 1C).
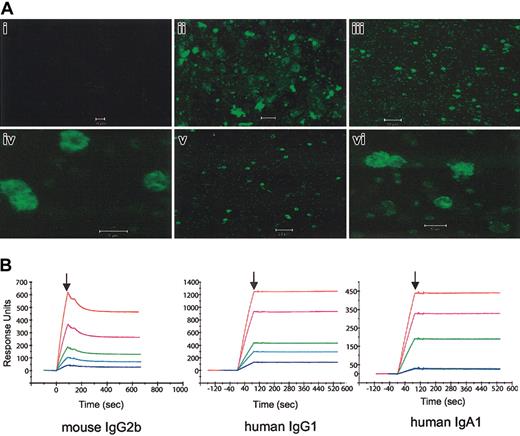
By indirect immunofluorescence (Figure 2A), the IgG1, IgA1, and diabody produced characteristic patterns of MSP1 reactivity in schizonts, merozoites, and ring-stage parasites. IgG1 or IgA1 specific for an irrelevant antigen did not react with parasites. Flow cytometry revealed that diabody also bound granulocytes and a cell fraction containing monocytes (not shown).
Immunofluorescence and SPR analysis. (A) Immunofluorescence patterns of Abs or diabodies reactive with MSP119 on methanol-acetone-fixed smears of merozoites and erythrocytes infected with P yoelii YM. The smears were incubated with human IgG1 (iii,iv), human IgA1 (v,vi) and the FcγRI × MSP119 diabody (ii). No specific fluorescence was detected with an irrelevant FITC-labeled Ab (i) or with the secondary F(ab′)2 anti-Ig Ab alone (not shown). Original magnification: panels i-iii, v, × 40; iv, vi, × 100. (B) SPR association and dissociation curves of Ab binding to MSP119 immobilized on a CM5 sensor chip. Abs were injected into flow at time 0, and replaced with buffer at the points indicated by vertical arrows.
Immunofluorescence and SPR analysis. (A) Immunofluorescence patterns of Abs or diabodies reactive with MSP119 on methanol-acetone-fixed smears of merozoites and erythrocytes infected with P yoelii YM. The smears were incubated with human IgG1 (iii,iv), human IgA1 (v,vi) and the FcγRI × MSP119 diabody (ii). No specific fluorescence was detected with an irrelevant FITC-labeled Ab (i) or with the secondary F(ab′)2 anti-Ig Ab alone (not shown). Original magnification: panels i-iii, v, × 40; iv, vi, × 100. (B) SPR association and dissociation curves of Ab binding to MSP119 immobilized on a CM5 sensor chip. Abs were injected into flow at time 0, and replaced with buffer at the points indicated by vertical arrows.
Importantly, surface plasmon resonance (SPR) analysis revealed no reduction in affinity for MSP119 for the chimeric IgG1 or IgA1 when compared with the parental mouse IgG2b (Figure 2B). The engineered Abs appeared to have greater association and slower dissociation rates than the IgG2b. The extremely slow dissociation rates, although precluding accurate calculation of kinetic rate constants, indicate high-affinity interactions. Diabody binding to MSP119 was also detectable by SPR, although its affinity was predicted to be less than that of mouse IgG2b.
Abs and diabody trigger neutrophil nicotinamide adenine dinucleotide phosphate oxidase activation through FcR cross-linking
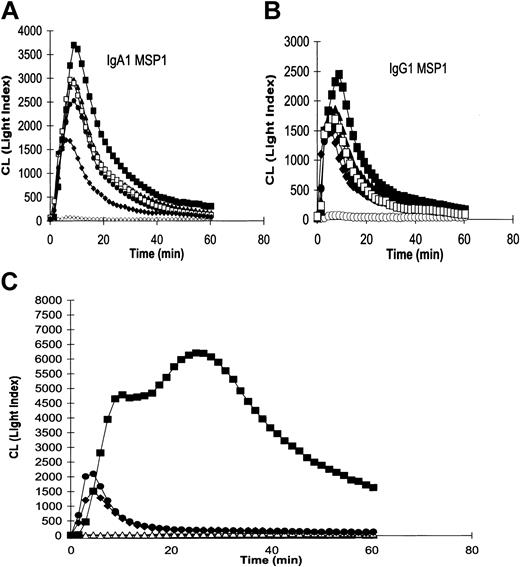
We assessed the ability of these novel reagents to elicit an oxidative burst in human neutrophils (Figure 3). When attached to MSP119-coated plates at equimolar concentrations, IgA1 was consistently more efficient at inducing a respiratory burst than IgG1 (Figure 3A-B). The mouse IgG2b anti-MSP119 was able to elicit a weak burst, presumably through lower-affinity interactions with human FcγR. Recombinant IgG1 and IgA1 Abs recognizing an irrelevant antigen, 3-nitro-4-hydroxy-5-iodophenylacetate (NIP), were unable to induce a burst on MSP119-coated plates. Importantly, the diabody was approximately 3-fold more effective at inducing neutrophil oxidative bursts than IgG1 when coated at equimolar concentration (3.8 × 10-7M; Figure 3C). The response to the diabody was biphasic and sustained at higher levels over longer periods.
Stimulation of neutrophil NADPH oxidative bursts using Abs attached to GST-MSP119-coated microtiter plates. Chemiluminescence (CL; arbitary units) was induced by (A) anti-MSP119 human IgA, or (B) antihuman MSP119 human IgG1 at (▪) 1 × 10-6 M, (▴) 5 × 10-7 M, (•) 1 × 10-7 M, and (□) 5 × 10-8 M. Isotype controls (○) IgA1-NIP or IgG1-NIP, or (♦) MSP119 specific mouse IgG2b at 1 × 10-6 M. (C) Diabody or Ab at 1 × 10-6 M; (▪), FcγRI × MSP119; (•), IgG1-MSP119; (♦), IgG2b-MSP119; (□), IgG1 isotype control; ▵, no Ab or diabody. In each case the experiments were performed 4 times with neutrophils from different donors with similar results.
Stimulation of neutrophil NADPH oxidative bursts using Abs attached to GST-MSP119-coated microtiter plates. Chemiluminescence (CL; arbitary units) was induced by (A) anti-MSP119 human IgA, or (B) antihuman MSP119 human IgG1 at (▪) 1 × 10-6 M, (▴) 5 × 10-7 M, (•) 1 × 10-7 M, and (□) 5 × 10-8 M. Isotype controls (○) IgA1-NIP or IgG1-NIP, or (♦) MSP119 specific mouse IgG2b at 1 × 10-6 M. (C) Diabody or Ab at 1 × 10-6 M; (▪), FcγRI × MSP119; (•), IgG1-MSP119; (♦), IgG2b-MSP119; (□), IgG1 isotype control; ▵, no Ab or diabody. In each case the experiments were performed 4 times with neutrophils from different donors with similar results.
Neutrophil phagocytosis of merozoites opsonized with Abs
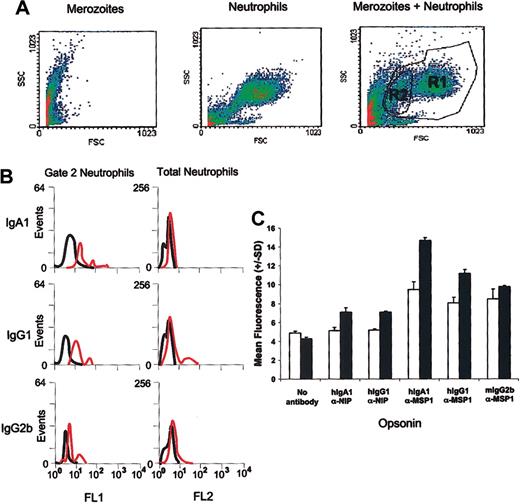
The binding to human neutrophils of merozoites opsonized with Ab was studied by flow cytometry (Figure 4). The relative characteristics of purified merozoites and neutrophils were ascertained by forward and side scatter analysis. The average size of neutrophils increased when cultured with merozoites in the absence of Ab, and 2 discrete neutrophil populations were observed using anti-CD16 as a marker (Figure 4A). Cells in one subpopulation (gated as R2) most likely represented degranulating cells, since they displayed reduced size and granularity. Binding of Ab-coated merozoites, in particular those coated in IgA1, was significantly greater than uncoated controls (Figure 4B-C). Importantly, neutrophils in gate R2 were particularly efficient at binding opsonized merozoites (Figure 4B-C).
Binding of merozoites to neutrophils. (A) Forward and side scatter flow cytometry analysis of merozoites, neutrophils, or a mixture showing gating of different CD16+ neutrophil populations. (B) Binding of merozoites opsonized with FITC-labeled MSP119-specific human IgG1, human IgA1, or mouse IgG2b (red traces), to total neutrophils (R1) or neutrophils in gate 2 (R2). Binding of merozoites opsonized with an isotype-matched control Ab shown by the black trace. (C) Mean fluorescence intensities of opsonized merozoites binding to R1-(□) or R2-gated neutrophils (▪). Error bars indicate standard deviation from the mean.
Binding of merozoites to neutrophils. (A) Forward and side scatter flow cytometry analysis of merozoites, neutrophils, or a mixture showing gating of different CD16+ neutrophil populations. (B) Binding of merozoites opsonized with FITC-labeled MSP119-specific human IgG1, human IgA1, or mouse IgG2b (red traces), to total neutrophils (R1) or neutrophils in gate 2 (R2). Binding of merozoites opsonized with an isotype-matched control Ab shown by the black trace. (C) Mean fluorescence intensities of opsonized merozoites binding to R1-(□) or R2-gated neutrophils (▪). Error bars indicate standard deviation from the mean.
By confocal microscopy, fluorescein-labeled merozoites could only be detected within neutrophil phagosomes after opsonization with Abs specific for MSP119 (Figure 5). Giemsa staining revealed that MSP119-specific Ab was very effective at agglutinating merozoites. Residual bodies containing hemozoin, an insoluble crystalline product of parasite digestion of hemoglobin, are coated with MSP1 (A.A.H. et al, unpublished data, January 2002), are detectable together with individual merozoites within discrete phagosomal compartments. The anti-MSP119 mouse IgG2b appeared slightly less effective than the human Abs at mediating phagocytosis, although the difference was not significant. No phagocytosis was observed with either the diabody or IgG1 or IgA1 anti-NIP controls.
Neutrophil phagocytosis of merozoites. Confocal fluorescence microscopy of phagocytosis of labeled merozoites opsonized with FITC-labeled MSP119-specific Abs by human neutrophils. Labelled merozoites are arrowed. For percentage phagocytosis, the mean and standard deviations of 2 experiments are shown. Original magnification, × 100.
Neutrophil phagocytosis of merozoites. Confocal fluorescence microscopy of phagocytosis of labeled merozoites opsonized with FITC-labeled MSP119-specific Abs by human neutrophils. Labelled merozoites are arrowed. For percentage phagocytosis, the mean and standard deviations of 2 experiments are shown. Original magnification, × 100.
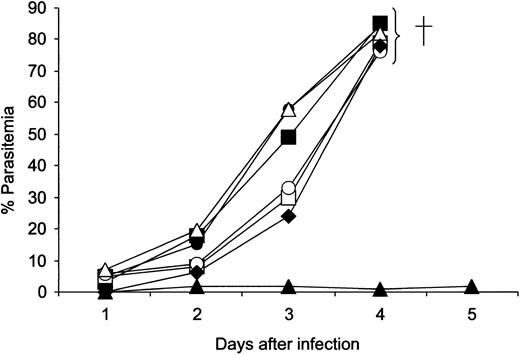
Suppression of parasitemia by mouse IgG2b anti-MSP119 but not the engineered anti-MSP119 reagents
In vivo experiments in normal mice demonstrated that, of the anti-MSP119 Abs and diabody, only the mouse IgG2b effectively suppressed a lethal blood stage challenge infection (Figure 6). No parasites were detectable in these mice 6 months after the challenge infection.
Course of P yoelii YM parasite infection in mice. Groups of 5 BALB/c mice injected with mouse IgG2b anti-MSP119 (▴), human IgG1anti-MSP119 (□), human IgA1 anti-MSP119 (○), human IgG1anti-NIP (▪), human IgA1 anti-NIP (•), FcγRI × MSP119 diabody (♦), and PBS ▵. Each point represents the geometric mean parasitemia of mice in each group at the time after parasite challenge. All mice in the mouse IgG2b group survived for 6 months; all the mice in the other groups with high parasitemia were killed on either day 4 or day 5.
Course of P yoelii YM parasite infection in mice. Groups of 5 BALB/c mice injected with mouse IgG2b anti-MSP119 (▴), human IgG1anti-MSP119 (□), human IgA1 anti-MSP119 (○), human IgG1anti-NIP (▪), human IgA1 anti-NIP (•), FcγRI × MSP119 diabody (♦), and PBS ▵. Each point represents the geometric mean parasitemia of mice in each group at the time after parasite challenge. All mice in the mouse IgG2b group survived for 6 months; all the mice in the other groups with high parasitemia were killed on either day 4 or day 5.
Although none of the engineered reagents could prevent a lethal infection, they did cause a significant reduction in parasitemia on both days 2 and 3 after challenge (P < .01) when compared with mice treated with isotype controls. None of the mice receiving control reagents survived beyond day 4.
Discussion
As a preliminary step toward the development of passive immune therapy for the control of malaria, we have generated Ab reagents with specificity for an epitope on MSP19 from P yoelii, for use in this murine model of malaria.20,21 These reagents were generated through cloning of mouse Ig variable domains from a hybridoma, and their engraftment onto human constant region genes, for expression in mammalian cells.
The engineered Abs recognize parasites in infected erythrocytes and can trigger potent FcR-mediated neutrophil effector mechanisms. Human IgA1 directed against MSP119 was consistently the most efficient Ab at mediating neutrophil phagocytosis of merozoites. Highly effective killing mechanisms mediated by FcαR have been demonstrated against numerous pathogens and this receptor is now considered a trigger molecule with therapeutic potential.22,23 FcαR is expressed endogenously on monocytes and neutrophils, the most populous FcR-positive leukocytes in blood and potent effectors of merozoite killing.9,24 FcαR on Kupffer22 and dendritic cells25 make it a potential initiator of MSP119 antigen presentation. Triggering FcαR inhibits tumor necrosis factor α (TNF-α) and interleukin 6 (IL-6) synthesis by human monocytes,26 which is desirable since a positive correlation exists between high levels of these cytokines and poor prognosis in severe malaria.27 This study argues for a re-examination of IgA's role in human malarial infections, particularly because IgA and FcαR in mice differ from their human counterparts.7 Interestingly, naturally occurring Plasmodium-specific IgA is found in humans living in endemic areas, with high titres in serum28 and breast milk.29
Despite the abilities of the engineered Abs to trigger phagocytosis in vitro, and the fact that the human and mouse Abs possess comparable affinities for MSP119, only the mouse IgG2b was able to protect mice from a lethal parasite infection in vivo. We believe that the failure of the engineered Abs to protect mice is most likely due to the inability of their human Fc regions to effectively trigger murine effector mechanisms. The inability of human IgA1 to protect mice in vivo may be explained by the lack of a murine homolog for human FcαR (CD89) and the questionable ability of IgA to fix complement. Direct demonstration of a role for the Fcα region of IgA in mediating protection in vivo awaits studies in mice transgenic for human FcαR and complement genes, as planned by our laboratory in the future. The reasons for the lack of effect with human IgG1 are less clear, but may rest in poor complement activation. Complement can play a role in killing malarial parasites, in a manner dependent on the presence of specific Abs and phagocytic cells, particularly neutrophils.30,31 Intriguingly, complement plays a central role in passive protection by human IgG1 antipneumococcal Abs in mice.32 This result suggests that our IgG1 may well activate complement in vivo but that merozoites are in some way resistant to this activation. Human IgG1 has been shown to bind to murine FcγR.33,34 Possibly, recruitment of mouse FcγR by human IgG1 did occur but preferentially triggered an inhibitory signal rather than an activatory one. Without further dissection of the molecules involved, it is difficult to reconcile the reasons for the lack of protection offered by the human Abs.
Regardless of the mechanisms involved, the inability of the human IgA1 and IgG1 versions of anti-MSP119 to protect indicate that, for this epitope at least, mere blocking of MSP119 function by some form of steric hindrance is insufficient to bring about protection. However, the specific epitope recognized appears to be critical since Ab fragments with different specificities have been shown to reduce parasitemia.35,36
The unique Abs described here will permit delineation of human immune effector mechanisms in vivo, in experiments using mice transgenic for human FcR and complement genes. Such in vivo experiments are not possible with human malaria parasites. Hence, recombinant human Abs engineered as described here will be useful in correlating particular epitopes on MSP119 with protective immunity, as an aid to vaccine design, and may form the bases of effective neutralization assays. Until now, work on human malaria parasites has made use of Abs purified from immune sera in neutralization tests. This situation is not optimal since sera contain a mixture of Abs, some with inappropriate specificities (such as blocking Abs37 ) and the potential to trigger inhibitory FcR through intracellular immunoreceptor tyrosine inhibition motif (ITIM) signaling. Reagents such as those detailed here offer reproducible standards for neutralization assays.
Passive therapy with inhibitory recombinant Abs may complement traditional vaccine development. Selective targeting of invariant inhibitory epitopes on MSP119 overcomes potential problems of antigenic polymorphism. Moreover, by careful choice of Ab class it should be possible to target pertinent antigens to antigen-presenting cells, providing a “vaccine effect” to limit parasite proliferation on reinfection.23 In addition, passive immunotherapy does not require complex delivery systems or adjuvants, although the latter have been shown to bolster protective immunity.38
One potential impediment to using parasite-specific IgG for malaria therapy might be competition with IgG of the nonspecific hypergammaglobulinemia frequently generated by parasites as a smokescreen to evade immunity.27 These Abs may compete for binding to FcγR and could explain why large doses of specific Ab are required for parasite neutralization both in vivo and in vitro.39 A further problem with parasite-specific IgG is that its Fc region may bind to FcγR on platelets, B cells, or other noncytotoxic cells resulting in the inadvertent triggering of inhibitory FcγRIIb, which might benefit the parasite much as it has been shown in other systems to favor tumor cell growth.40
We have developed a bispecific single-chain diabody that may overcome some of these shortcomings. By coupling variable domains recognizing MSP119 to variable domains recognizing human FcγRI, via a flexible peptide linker, we have generated a bispecific diabody designed to bind FcγRI outside its natural high-affinity binding site for IgG, thus preventing interference by irrelevant IgG. We found that simultaneous engagement of MSP119 and FcγRI by the diabody induced a potent neutrophil respiratory burst. The biphasic response was approximately 3-fold higher and of longer duration than the oxidative burst derived from equimolar amounts of Ab, suggesting that lower concentrations would be required in vivo to derive a similar therapeutic effect. The biphasic nature of the diabody response may result from either the reagent's monovalency and hence poor capacity to cluster antigen with concomitant FcγRI clustering, or an inability of the diabody, unlike IgG, to cluster both FcγRI and FcγRIII on the neutrophil surface. These limitations are likely to lead to an activation profile with different kinetics from that of bivalent IgG, as observed by others.41
In contrast to IgG1, the diabody was unable to induce phagocytosis of live merozoites. It is known that activation of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase by chemicals42 and Abs22 can occur in the absence of phagocytosis. Given that neutrophils normally express very low levels of FcγRI (< 2000 receptors/cell),43 compared with FcγRII (∼30 000-60 000 receptors/cell) or FcγRIII (∼100 000-200 000 receptors/cell), these results suggest that fewer receptors need to be cross-linked for the induction of a respiratory burst, than would be required for phagocytosis. The reduced affinity and monovalency for FcγRI would contrive to reduce the diabody's overall avidity, and may explain the absence of phagocytosis. Reagents that can kill malaria parasites without phagocytosis may be advantageous. During severe malaria a lethal burden may be placed on both the spleen and liver where parasites and infected erythrocytes are removed by phagocytosis.44 Thus, therapies that avoid unwanted liver and spleen pathology, yet kill parasites, may be very desirable. A novel bispecific Ab has recently been developed for the treatment of P falciparum.45 This reagent targeted the CD3 antigen of human T cells to MSP119. In cooperation with T cells, the bispecific Ab induced a significant increase in merozoite phagocytosis and growth inhibition of P falciparum in vitro. The potential for bispecific Abs in controlling malaria infection therefore appears considerable.
Given increasing problems with resistance to previously reliable drugs such as chloroquine, a vaccine against malaria has become the ultimate goal. Unfortunately, its development has been beset with problems and alternative strategies to treat malaria have become of paramount importance. Here, as a first step toward Ab-based therapies, we describe novel parasite-specific reagents that could also form the bases for assays aimed at obtaining in vitro correlates of protection in the development of optimal MSP119- based vaccines.
Prepublished online as Blood First Edition Paper, July 10, 2003; DOI 10.1182/blood-2003-02-0583.
Supported by a Wellcome Trust Advanced Training Fellowship (056638/Z/99/Z/144/IM/JL) to R.J.P.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Andrew Bradbury and Irene Ling for kindly providing expression vectors and recombinant MSP119, respectively.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal