Abstract
The antiangiogenic factor thrombospondin 1 (TSP-1) binds with high affinity to several heparin-binding angiogenic factors, including fibroblast growth factor 2 (FGF-2), vascular endothelial growth factor (VEGF), and hepatocyte growth factor/scatter factor (HGF/SF). The aim of this study was to investigate whether TSP-1 affects FGF-2 association with the extracellular matrix (ECM) and its bioavailability. TSP-1 prevented the binding of free FGF-2 to endothelial cell ECM. It also promoted the mobilization of matrix-bound FGF-2, generating a TSP-1/FGF-2 complex. The region of TSP-1 responsible for these activities was located within the 140-kDa antiangiogenic and FGF-2 binding fragment, whereas the 25-kDa heparin-binding fragment was inactive. Matrix-released FGF-2/TSP-1 complex had a reduced ability to bind to and induce proliferation of endothelial cells. TSP-1 depleted the ECM laid by FGF-2-overproducing tumor cells of its FGF-2-dependent mitogenic activity for endothelial cells. Besides FGF-2, TSP-1 also inhibited VEGF and HGF/SF binding to the ECM and mobilized them from the ECM. Our study shows that TSP-1 acts as a scavenger for matrix-associated angiogenic factors, affecting their location, bioavailability, and function. (Blood. 2003; 102:4399-4406)
Introduction
Angiogenesis, sprouting of new blood vessels from pre-existing ones, is a crucial event in physiologic and pathologic processes, including tumor growth and metastasis.1 Angiogenesis is a complex process, regulated by pro- and antiangiogenic factors, that involves dynamic interaction between a variety of cells, growth factors, and the extracellular matrix (ECM).2 As in other morphogenic processes, the ECM acts not just as a structural support, but also as a direct modulator of cellular functions. Matrix components and bioactive fragments released by limited proteolysis can directly regulate endothelial cell functions.3 In addition, the matrix contributes to angiogenesis by acting as a reservoir for angiogenic factors. Growth factors are stored in the matrix through binding to heparansulfate proteoglycans (HSPGs). Binding to HSPGs is reversible,4 and biologically active factors can be released from the matrix by different agents.5,6
Fibroblast growth factor 2 (FGF-2, basic FGF) is the most extensively studied example of matrix-stored angiogenic factor.5,7 As do other members of the FGF family, FGF-2 has a high affinity for the glycosaminoglycan heparin and for HSPGs. Cell-surface HSPGs are required for the formation of an active FGF/FGF receptor signaling complex,8 for internalization, and hence for internalization-dependent activities such as endothelial cell proliferation.7,9 Conversely, matrix-associated HSPGs allow the storage of FGF-2 within the ECM.10 Matrix-associated FGF-2 can then act locally, or be released as a soluble, biologically active factor.6 Mobilization of active FGF-2 from the matrix is an important mechanism of induction of angiogenesis. Biologically active FGF-2 is released by heparin,11 matrix-degrading proteases,12 heparanase,13 and the FGF-binding protein (FGF-BP).14 Conversely, other endogenous or pharmacologic agents that prevent FGF-2 interaction with HSPGs exert an antiangiogenic activity, as in the case of platelet factor 4 (PF-4),15 a soluble syndecan ectodomain,16 suramin,17 chemically modified heparins, and heparin-mimicking polyanionic compounds.18-20
Other important angiogenic factors, including vascular endothelial growth factor (VEGF) and hepatocyte growth factor/scatter factor (HGF/SF), bind to HSPGs that again contribute to growth factor binding to cell receptors and to the ECM.21-23
Thrombospondin 1 (TSP-1) is the most studied member of a family of at least 5 related proteins.24,25 TSP-1 is a matricellular molecule, a modular glycoprotein composed of multiple active domains, that modulates the cell response to the environment by concurrently binding to cell receptors, matrix components, proteolytic enzymes, and soluble factors.24,25 Soluble TSP-1 is a major secretion product of stimulated platelets. In addition, it is secreted by different cell types, including endothelial cells, fibroblasts, and inflammatory cells. TSP-1 is the first endogenous inhibitor of angiogenesis to be identified,26-28 although its role in angiogenesis is complex due to the presence of both pro- and antiangiogenic domains on the TSP-1 molecule.29 The antiangiogenic activity of TSP-1 has been located in a 140-kDa TSP fragment that also retains the antineoplastic activity of TSP-1.26,27,30
We previously reported that TSP-1, through its 140-kDa antiangiogenic fragment, binds with high affinity to FGF-2, affecting its binding to HSPGs on the surface of endothelial cells.31 TSP-1 also binds to other angiogenic factors, including the viral product Tat,32 VEGF,33 and HGF/SF.23 We designed the present study to investigate whether TSP-1 affected the association of endogenous and exogenous FGF-2 with subendothelial ECM, ultimately affecting the angiogenic factor's bioavailability and biologic activity.
Materials and methods
Reagents
TSP-1 was purified from the supernatant of thrombin-stimulated fresh human platelets.27 Fragments were produced by digesting TSP-1 with thrombin (20 U/mL) for 20 hours at 37°C. Digestion was stopped by 2 mM phenylmethylsulfonyl fluoride and digestion products were separated by chromatography on heparin-Sepharose (Amersham Pharmacia Biotech, Uppsala, Sweden) as described.27 The TSP-1 mimetic peptide, DI-TSP, based on the sequence of the second type I repeat,34 was kindly provided by J. Henkin (Abbott Laboratories, Abbott Park, IL). Its sequence is Ac-Sar-Gly-Val-D-Ile-Thr-Nva-Ile-Pro-Arg-ethylamide (where Sar and Nva are sarcosine and L-norvaline, respectively). Human recombinant FGF-2 was kindly provided by Dr F. Bertolero (Pharmacia-Upjohn, Nerviano, Italy). VEGF was from R&D Systems (Minneapolis, MN) and HGF/SF from ICN Biomedicals (Eschwege, Germany). Anti-TGFβ antibodies were from R&D Systems.
Labeling of angiogenic factors
The labeling reagent, biotinamidocaproate-N-hydroxysulfo-succinimide ester (Sigma, St Louis, MO), was incubated with FGF-2, VEGF, and HGF/SF at a molar ratio of 13:1 (biotinylation reagent to angiogenic factor), for 30 minutes at room temperature. The biotinylated factors were then isolated by chromatography on heparin-Sepharose. Gelatin was added at a final concentration of 0.05%. Biotin-labeled FGF-2 was detected as a single band of the same molecular weight of unlabeled FGF-2 in Western blot analysis. It maintained the ability to bind to heparin with high affinity and to TSP-1 with a dissociation constant (Kd) identical to 125I-labeled FGF-2. In preliminary experiments, 125I-labeled FGF-2 and biotin-labeled FGF-2 bound to ECM with the same affinity, in a heparin-dependent manner. Biotin-labeled FGF-2 was biologically active, as it stimulated human umbilical vein endothelial cell (HUVEC) proliferation at a degree comparable to unlabeled FGF-2.
Cell culture
Bovine aortic endothelial cells (BAECs) were cultured in Dulbecco modified Eagle medium (DMEM) with 10% fetal bovine serum (FBS).31 Tet-FGF2/HEC-1-B, stable transfectants generated from the human endometrial adenocarcinoma HEC-1-B cell line and expressing FGF-2 under the control of a tetracycline-responsive promoter,35 were cultured in DMEM supplemented with l-glutamine, 10% FBS, 1% nonessential amino acids, 1% sodium piruvate, and 30 μg/mL hygromycin. In this model, tetracycline or its analog doxycycline, at concentrations higher than 1 ng/mL, switched off FGF-2 expression.35
Preparation of subendothelial matrix
ECM from endothelial cells was prepared as described.11 Briefly, BAECs were grown in 96-well plates. Three to 6 days after reaching confluency, cells were washed with phosphate-buffered saline (PBS), and dissolved by incubation with 0.5% Triton X-100, 0.0375% NH3 in PBS, for 3 minutes at room temperature. The exposed ECM was washed 4 times with PBS to remove cellular debris and detergents, and immediately used. In some experiments, matrix laid by HUVECs29 was used, prepared as described for BAECs.
Binding of growth factors to the matrix
The method described by Bashkin et al11 was used, with some modifications. ECM was washed with 0.1% gelatin in PBS (PBS-gelatin). Biotin-labeled FGF-2 (1-4 ng/well), VEGF (30-60 ng), or HGF/SF (5-7 ng) in PBS-gelatin were added to the matrix alone or together with TSP-1, its fragments, DI-TSP peptide, or heparin in a final volume of 40 μL/well. After a 3-hour incubation at room temperature, wells were washed twice with PBS-gelatin to remove the unbound factors, and the bound factor was quantified by adding peroxidase-conjugated ExtrAvidin (Sigma) followed by 1.2-phenylenediamine dihydrocloride (OPD; Dako, Glostrup, Denmark) as a chromogen. The reaction was stopped with 0.5 M H2SO4 and absorbance at 490 nm was measured.
In some experiments, the ECM was preincubated with TSP-1 (220 nM, 40 μL per well) for 2 hours at room temperature and washed before the addition of labeled FGF-2. Control experiments were also performed with 125I-labeled FGF-2, prepared as previously described.31
Mobilization of growth factors from the matrix
The method described by Bashkin et al11 was partly modified. After washing the ECM with PBS-gelatin, 40 μL per well of PBS-gelatin containing biotin-labeled FGF-2 (2-8 ng/well), VEGF (15-75 ng), or HGF/SF (7-11 ng) was applied to the matrix and incubated for 3 hours at room temperature. After washing with PBS-gelatin to remove the unbound factors, wells were incubated for 3 hours at room temperature with 40 μL PBS-gelatin containing or not containing TSP-1, its fragments, DI-TSP peptide, or heparin. The amount of angiogenic factors released from the ECM was determined by Western blot analysis of the supernatant. Samples were resolved in sodium dodecyl sulfate (SDS), 15% (FGF-2 and VEGF) or 8% (HGF/SF) polyacrylamide gel electrophoresis (PAGE) under reducing conditions and blotted to a nitrocellulose membrane. The amount of biotin-labeled angiogenic factors was assessed using peroxidase-conjugated ExtrAvidin and a chemiluminescence detection kit (ECL; Amersham Pharmacia). Following densitometric analysis, the amount of released factors is expressed as the percentage of total bound angiogenic factor (labeled factor present in the matrix solubilized with Laemmli buffer after incubation with the growth factor). Control experiments were performed with 125I-labeled FGF-2.
Analysis of the TSP-1/FGF-2 complex
The supernatant containing FGF-2 released from the matrix by TSP-1, obtained as described in the preceding paragraph, was analyzed as follows.31
Chemical cross-linking. The TSP-1/FGF-2 complex was cross-linked as previously described.31 The samples containing biotin-labeled FGF-2 released from the matrix were treated with disuccinimidyl suberate (DSS; Pierce, Rockford, IL) at a final concentration of 1 mM and incubated for 15 minutes at room temperature. Samples were resolved by SDS-PAGE with 7% polyacrylamide gels under reducing conditions, followed by Western blot.
Gel filtration chromatography. Unlabeled FGF-2 (1 μg per well) was allowed to bind to the matrix and released by TSP-1 (220 nM) or by 2 M NaCl. The samples containing mobilized FGF-2 were then loaded onto an S200 column (Amersham Pharmacia). The column was eluted with PBS at a flow rate of 0.3 mL/min, and 225 μL fractions were collected. Each fraction was spotted onto a nitrocellulose membrane and subjected to Western blot analysis with anti-FGF-2 antiserum.31
Real-time biomolecular interaction assay
A BIAcore X apparatus (BIAcore, Piscataway, NJ) was used. Surface plasmon resonance (SPR) was exploited to measure changes in refractive index caused by the ability of purified TSP-1 to bind to human recombinant FGF-2 immobilized to a BIAcore sensorchip. For this purpose, 25 μg/mL FGF-2 was allowed to react with a flow cell of a CM5 sensorchip that was previously activated with 50 μL of a mixture of 0.2 M N-ethayl-N′-(3-diethylaminopropyl)carbodiimide hydrochloride (EDC) and 0.05 M N-hydroxysuccinimide (NHS). These experimental conditions allowed the immobilization of 7365 resonance units (RU), corresponding to approximately 0.41 pmol FGF-2. Similar results were obtained for the immobilization of bovine serum albumin (BSA), used as a negative control and for blank subtraction. Increasing concentrations of TSP-1 in 10 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), 150 mM NaCl, 3.4 mM EDTA (ethylenediaminetetraacetic acid), 0.005% surfactant P20, pH 7.4 (HBS) were then injected over the BSA or FGF-2 surfaces for 4 minutes and then washed until dissociation was observed. The SPR signal was expressed in terms of RU.
Binding of FGF-2 to endothelial cells
FGF-2 binding to endothelial cells was investigated essentially as described.31 Subconfluent BAECs in 96-well plates were incubated for 30 minutes at 4°C in cold serum-free DMEM with 0.15% gelatin and 25 mM HEPES (DMEM-gelatin). Supernatants, containing mobilized FGF-2, obtained as described in “Mobilization of growth factors from the matrix”, were added, and incubated for 2 hours at 4°C. After washes with cold DMEM-gelatin to remove unbound FGF-2, cells were lysed with NaOH 0.2 N. Lysates were spotted onto a nitrocellulose membrane and analyzed by Western blot.
Endothelial cell proliferation assay
BAECs (3000 cells per 100 μL) were seeded in a 96-well plate in 2% FBS-containing medium. Twenty-four hours later, 40 μL samples released from the matrix (see “Mobilization of growth factors from the matrix”) were added. After 72 hours, cells were fixed and stained with 0.5% crystal violet in 20% methanol, then rinsed and dried. The stain was eluted with an ethanol and 0.1 M sodium citrate (1:1) solution and absorbance at 540 nm was read.
To study the effect of TSP-1 on endogenous FGF-2, the Tet-FGF2/HEC-1-B model35 was used. Tet-FGF2/HEC-1-B cells were treated or not for 3 days with tetracycline or its analog doxycycline (2 ng/mL) to modulate FGF-2 expression. Cells (5000 cells per well) were plated in 96-well plates (with or without tetracycline) and incubated for 3 days. The matrix deposited by these cells, which contained high (untreated cells) or low FGF-2 (tetracycline-treated cells), was prepared as in “Preparation of subendothelial matrix” and incubated with a 40-μL solution of PBS-gelatin containing or not containing TSP-1 for 3 hours at room temperature. The sample from each well, containing released FGF-2, was then plated on endothelial cells to assess the proliferative activity. In parallel, the remaining matrix was tested for ability to support cell proliferation. BAECs (2500 cells per 100 μL) were plated over the treated matrix in medium with 2% FBS, and the cell number was evaluated after 72 hours.
Statistical analysis
Statistical differences were assessed by ANOVA (α ≤ 0.05) followed by Bonferroni post-hoc test. Comparisons were significant when the P value was less than or equal to .0167 (3 groups) or less than or equal to .0083 (4 groups).
Results
Inhibition of FGF-2 binding to the ECM by TSP-1
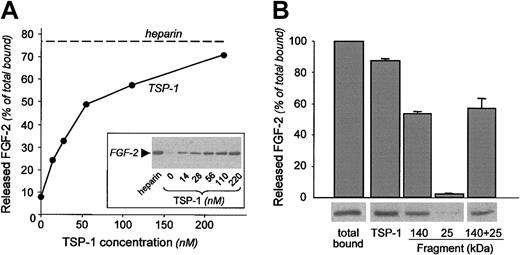
We investigated whether TSP-1 might affect the binding of free FGF-2 to the subendothelial matrix laid by endothelial cells. Biotin-labeled FGF-2 was plated onto the ECM alone or together with TSP-1 (14 nM-220 nM). TSP-1 reduced FGF-2 binding to the matrix, with an IC50 (concentration that causes 50% inhibition of binding) of 17 nM (Figure 1A). TSP-1 prevented FGF-2 binding to matrix of different origins, including matrix laid by BAECs, HUVECs, and the reconstituted basement membrane Matrigel, at a similar extent (Figure 1A). Heparin (10 μg/mL), used as reference inhibitor of FGF-2 binding to HSPGs in the ECM11 caused more than 85% inhibition of FGF-2 binding to the ECM. Control experiments showed that the same results were obtained using 125I-labeled FGF-2 in place of biotin-labeled FGF-2 and that albumin, laminin, type IV collagen, and von Willebrand factor were unable to inhibit FGF-2 binding to the ECM (not shown).
Effect of TSP-1 and TSP-1 fragments on FGF-2 binding to the ECM. (A) Biotin-labeled FGF-2 (1-2 ng/well) was added to the matrix laid by BAEC (•), HUVECs (▪), or to Matrigel (▴) in the presence of different concentrations of soluble TSP-1 (continuous lines). To test the effect of matrix-bound TSP-1 (dotted line) the matrix was pretreated with 220 nM TSP-1 and washed before the addition of labeled FGF-2. (B) Biotin-labeled FGF-2 was incubated on the ECM with different concentrations of TSP-1 (⋄) or its 140-kDa fragment (▪), its 25-kDa fragment (▵), or the 2 fragments together (•). After a 3-hour incubation, the matrix was washed to remove unbound FGF-2 and the amount of matrix-bound FGF-2 was evaluated using the ExtrAvidin-peroxidase/OPD system. Data (mean ± SD of triplicates) are expressed as the percentage of control binding (bound FGF-2 in the absence of inhibitor). Results are from one experiment representative of at least 3 experiments.
Effect of TSP-1 and TSP-1 fragments on FGF-2 binding to the ECM. (A) Biotin-labeled FGF-2 (1-2 ng/well) was added to the matrix laid by BAEC (•), HUVECs (▪), or to Matrigel (▴) in the presence of different concentrations of soluble TSP-1 (continuous lines). To test the effect of matrix-bound TSP-1 (dotted line) the matrix was pretreated with 220 nM TSP-1 and washed before the addition of labeled FGF-2. (B) Biotin-labeled FGF-2 was incubated on the ECM with different concentrations of TSP-1 (⋄) or its 140-kDa fragment (▪), its 25-kDa fragment (▵), or the 2 fragments together (•). After a 3-hour incubation, the matrix was washed to remove unbound FGF-2 and the amount of matrix-bound FGF-2 was evaluated using the ExtrAvidin-peroxidase/OPD system. Data (mean ± SD of triplicates) are expressed as the percentage of control binding (bound FGF-2 in the absence of inhibitor). Results are from one experiment representative of at least 3 experiments.
TSP-1 might affect FGF-2 binding to the matrix by 2 potential mechanisms: competition with FGF-2 for binding to HSPGs or direct binding and sequestration of FGF-2. To check the first hypothesis, the ECM was preincubated with TSP-1 before the addition of labeled FGF-2. In 2 experiments, preincubation of the ECM with TSP-1, at the maximal effective concentration of 220 nM, did not affect FGF-2 binding (Figure 1A). This suggests that TSP-1 does not occupy FGF-2-binding sites on matrix HSPGs, thus pointing to a mechanism of sequestration. To verify this possibility, we tested the activity of the 2 main TSP-1 fragments. Binding of labeled FGF-2 to the ECM was prevented by the antiangiogenic, carboxy-terminal 140-kDa fragment that contains the binding site for FGF-2,31 whereas the amino-terminal, 25-kDa fragment that contains the main heparin-binding site of TSP-1 was not active and did not increase the activity of the 140-kDa fragment when tested together (Figure 1B). The 140-kDa fragment contains known antiangiogenic sequences, located in the second and third type I repeat. To test the involvement of this sequence, we used the antiangiogenic TSP-1 mimetic peptide DI-TSP.34 The peptide did not affect the binding of FGF-2 to TSP-1, and did not prevent the binding of FGF-2 to the ECM (Table 1), indicating that this site of TSP-1 is not involved in FGF-2 recognition or in the inhibition of FGF-2 binding to the matrix.
Effect of DI-TSP peptide on FGF-2 binding to TSP-1 and on FGF-2 interaction with the matrix
Condition . | Concentration . | Inhibition of FGF-2 binding to TSP-1, % of control . | Inhibition of FGF-2 binding to ECM, % of control . | Mobilization of matrix-bound FGF-2, % of bound FGF-2 . |
|---|---|---|---|---|
| Vehicle | — | 100.0 ± 6.0 | 100.0 ± 3.3 | 29.6 ± 0.3 |
| TSP-1 | 100 nM | 13.7 ± 6.5 | 14.8 ± 2.3 | 74.0 ± 1.2 |
| DI-TSP | 10 μM | 90.0 ± 3.2 | 104.0 ± 6.4 | 30.4 ± 0.3 |
| DI-TSP | 1 μM | 84.0 ± 1.7 | 104.1 ± 1.0 | NT |
| DI-TSP | 100 nM | 87.0 ± 21.1 | 101.4 ± 6.4 | NT |
Condition . | Concentration . | Inhibition of FGF-2 binding to TSP-1, % of control . | Inhibition of FGF-2 binding to ECM, % of control . | Mobilization of matrix-bound FGF-2, % of bound FGF-2 . |
|---|---|---|---|---|
| Vehicle | — | 100.0 ± 6.0 | 100.0 ± 3.3 | 29.6 ± 0.3 |
| TSP-1 | 100 nM | 13.7 ± 6.5 | 14.8 ± 2.3 | 74.0 ± 1.2 |
| DI-TSP | 10 μM | 90.0 ± 3.2 | 104.0 ± 6.4 | 30.4 ± 0.3 |
| DI-TSP | 1 μM | 84.0 ± 1.7 | 104.1 ± 1.0 | NT |
| DI-TSP | 100 nM | 87.0 ± 21.1 | 101.4 ± 6.4 | NT |
Labeled FGF-2 was incubated with immobilized TSP-1 alone (vehicle) or in the presence of soluble TSP-1 or DI-TSP peptide, used as competitors, as described. 31 Data are the percentage of control binding, without competitors (vehicle). The ability of TSP-1 and DI-TSP peptide to affect FGF-2 binding to the matrix and to mobilize FGF-2 from the matrix were assayed as described in “Materials and methods.”—indicates not applicable; NT, not tested.
Mobilization of matrix-bound FGF-2 by TSP-1
Binding of FGF-2 to HSPGs in the matrix is a dynamic process, and molecules that modulate this interaction are known to mobilize FGF-2.6,11 We investigated whether TSP-1 affected this process and mobilized matrix-associated FGF-2. Subendothelial matrix was preincubated with labeled FGF-2 before the addition of different concentrations (14 nM-220 nM) of soluble TSP-1. Western blot analysis of the released material revealed that TSP-1 enhanced FGF-2 mobilization from the matrix, up to a degree comparable to heparin (Figure 2A).
Effect of TSP-1 and TSP-1 fragments on the release of FGF-2 from the ECM. Biotin-labeled FGF-2 (2-4 ng/well) was incubated onto the matrix for 3 hours and washed to remove unbound factor. The matrix was exposed to (A) different concentrations of TSP-1 (•) or 100 μg/mL heparin (used as a control; dotted line); (B) TSP-1 or its fragments (170 nM). The amount of mobilized FGF-2 present in the supernatant was analyzed by Western blot. Following densitometric analysis of the Western blot, released FGF-2 is expressed as the percentage of total FGF-2 bound to the matrix (mean ± SD). Data are from one experiment representative of at least 2 experiments.
Effect of TSP-1 and TSP-1 fragments on the release of FGF-2 from the ECM. Biotin-labeled FGF-2 (2-4 ng/well) was incubated onto the matrix for 3 hours and washed to remove unbound factor. The matrix was exposed to (A) different concentrations of TSP-1 (•) or 100 μg/mL heparin (used as a control; dotted line); (B) TSP-1 or its fragments (170 nM). The amount of mobilized FGF-2 present in the supernatant was analyzed by Western blot. Following densitometric analysis of the Western blot, released FGF-2 is expressed as the percentage of total FGF-2 bound to the matrix (mean ± SD). Data are from one experiment representative of at least 2 experiments.
Control experiments showed that (1) similar findings were obtained with 125I-labeled FGF-2 in place of biotin-labeled FGF-2; (2) albumin, laminin, type IV collagen, and von Willebrand factor were unable to mobilize FGF-2 from the ECM; and (3) TSP-1 mobilized FGF-2 also from matrix laid by HUVECs and from Matrigel, to a similar extent (data not shown).
In agreement with its ability to affect FGF-2 binding to the ECM, the 140-kDa fragment significantly mobilized matrix FGF-2 from the matrix, whereas the heparin-binding, 25-kDa fragment was inactive, and did not increase FGF-2 mobilization by the 140-kDa fragment when tested together (Figure 2B). The DI-TSP peptide did not cause FGF-2 mobilization (Table 1).
Collectively, these findings suggested a mechanism of sequestration of FGF-2 by TSP-1, through the formation of a TSP-1/FGF-2 complex. To verify that FGF-2 released from the matrix by TSP-1 was indeed present as a complex with TSP-1, the material released from the ECM was analyzed by gel filtration chromatography, and the obtained fractions were examined for immunoreactivity with anti-FGF-2 antibodies in dot-blot analysis.31 FGF-2 released from the matrix by a 2 M NaCl wash, eluted with a retention volume of 5.5 mL, similar to control, unbound FGF-2 (Figure 3A). In contrast, FGF-2 released from the matrix by incubation with TSP-1 eluted with the void volume of the column (Figure 3A), suggesting the presence of a high-molecular-weight FGF-2/TSP-1 complex.
FGF-2 is released from the matrix by TSP-1 as an FGF-2/TSP-1 complex. ECM was incubated with FGF-2 and then exposed to TSP-1. The samples, containing FGF-2 released from the matrix, were then analyzed as follows. (A) Gel filtration chromatography. FGF-2 released from the ECM by TSP-1 (continuous line) or by 2 M NaCl (dashed line) were loaded on an S200 column and the eluted fractions were analyzed by dot-blot analysis using anti-FGF-2 antiserum. Vo = void volume, elution volume for TSP-1; FGF-2, elution of control, free FGF-2. (B) Chemical cross-linking. Samples containing biotin-labeled FGF-2 released from the matrix by TSP-1 or heparin, or that was spontaneously released (control) were treated with the cross-linking agent DSS and analyzed by Western blot. Arrows indicate the high-molecular-weight bands consistent with a TSP-1/FGF-2 complex; arrowhead, free FGF-2; and asterisk, the edge of the separating gel. Molecular weight markers (in kDa) are on the left.
FGF-2 is released from the matrix by TSP-1 as an FGF-2/TSP-1 complex. ECM was incubated with FGF-2 and then exposed to TSP-1. The samples, containing FGF-2 released from the matrix, were then analyzed as follows. (A) Gel filtration chromatography. FGF-2 released from the ECM by TSP-1 (continuous line) or by 2 M NaCl (dashed line) were loaded on an S200 column and the eluted fractions were analyzed by dot-blot analysis using anti-FGF-2 antiserum. Vo = void volume, elution volume for TSP-1; FGF-2, elution of control, free FGF-2. (B) Chemical cross-linking. Samples containing biotin-labeled FGF-2 released from the matrix by TSP-1 or heparin, or that was spontaneously released (control) were treated with the cross-linking agent DSS and analyzed by Western blot. Arrows indicate the high-molecular-weight bands consistent with a TSP-1/FGF-2 complex; arrowhead, free FGF-2; and asterisk, the edge of the separating gel. Molecular weight markers (in kDa) are on the left.
To confirm the presence of the TSP-1/FGF-2 complex, samples containing biotin-labeled FGF-2 released from the ECM by TSP-1 were cross-linked using DSS and probed in a Western blot. Although not quantitative (because of inefficiency in the process of cross-linking and in blotting of high-molecular-weight proteins), this technique provides qualitative analysis of molecular complexes. Figure 3B shows the presence of a biotin-labeled high-molecular-weight band that barely entered the gel, compatible with a complex between trimeric TSP-1 (450 kDa) and FGF-2 (18 kDa) and a band with an apparent molecular weight of approximately 200 kDa, compatible with a complex between monomeric TSP-1 (180 kDa) and FGF-2, as previously described.31 These high-molecular-weight bands were present only in the sample containing FGF-2 mobilized by TSP-1, and not in samples containing FGF-2 spontaneously released, released by heparin, and in TSP-1-treated matrix in the absence of labeled FGF-2 (Figure 3B).
The hypothesis of sequestration of FGF-2 by TSP-1 implies that FGF-2 binds with a similar affinity to TSP-1 and HSPGs. TSP-1/FGF-2 interaction was characterized by real-time biomolecular interaction assay. FGF-2 was immobilized onto a BIAcore CM5 sensorchip and increasing concentrations of TSP-1 were injected over the FGF-2 surface. After blank subtraction, sensorgrams were used to calculate kinetic parameters: association rate constant (kon) was 1.89 × 104 M-1s-1 and dissociation rate constant (koff) was 2.05 × 10-4 s-1. Thus, FGF-2/TSP-1 interaction occurs with a dissociation constant (Kd) = koff/kon equal to 11 nM. This value is similar to that previously determined with different experimental approaches.31 This finding indicates that FGF-2 affinity for TSP-1 as well as the other kinetics parameters calculated by BIAcore are comparable to those of FGF-2 for HSPG,36,37 in keeping with our hypothesis of sequestration.
Activity of FGF-2 released from the matrix by TSP-1
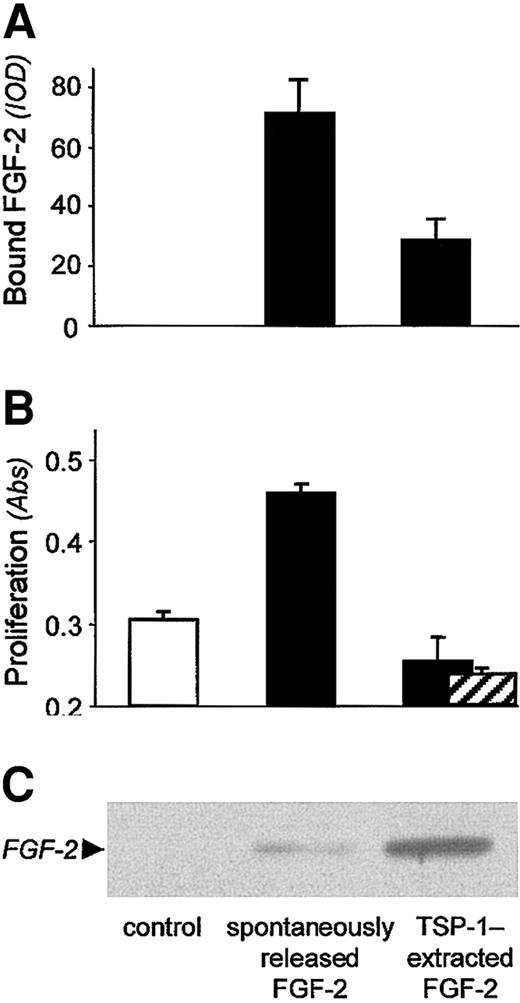
We next investigated whether TSP-1-mobilized FGF-2 retained its ability to bind to endothelial cells and stimulate their proliferation. The sample released from a matrix pretreated with labeled FGF-2 and then exposed to plain buffer (spontaneously released FGF-2) was analyzed in Western blot (Figure 4C), and, by comparison with a scale of known amounts of FGF-2 loaded on the same gel, it was calculated to be approximately 0.5 ng/mL to 3 ng/mL. Spontaneously released FGF-2 bound endothelial cells (Figure 4A) and stimulated endothelial cell proliferation (Figure 4B) at a higher degree than the control sample (obtained from a matrix not treated with FGF-2, P ≤ .0001). In contrast, FGF-2 released from the ECM by TSP-1 was more abundant than the spontaneously released factor (Figure 4C): it was calculated to be approximately 5 ng/mL to 10 ng/mL, namely an optimal concentration for stimulation of proliferation. Nonetheless, its ability to bind to endothelial cells was poor (Figure 4A) and it failed to induce endothelial cell proliferation above the control (for both assays, P ≤ .01 compared with spontaneous release, Figure 4B). This finding thus shows that the matrix-derived FGF-2/TSP-1 complex is biologically inactive in terms of induction of endothelial cell proliferation.
Functional analysis of FGF-2 released from the matrix by TSP-1. Subendothelial matrix was preincubated with biotin-labeled FGF-2 (▪) or not preincubated (□; control), and then treated with TSP-1 or plain buffer (spontaneously released FGF-2). Released material was then analyzed by Western blot (C) and, in parallel, plated on endothelial cells to measure FGF-2 binding to cell surface (A) and ability to induce cell proliferation (B), as described in “Materials and methods.” (A) Samples were added to BAECs and incubated for 2 hours at 4°C. After washes, cells were lysed and the amount of bound FGF-2 was analyzed by dot-blot analysis. Data (mean ± SD of triplicates) are expressed as arbitrary units of optical density (IOD). (B) Samples were added to BAECs and the number of proliferated cells was evaluated 72 h later. Anti-TGFβ antibodies did not revert the lack of proliferative activity of FGF-2 released from the ECM by TSP-1 (▨). Data (mean ± SD of triplicates) are expressed as absorbance.
Functional analysis of FGF-2 released from the matrix by TSP-1. Subendothelial matrix was preincubated with biotin-labeled FGF-2 (▪) or not preincubated (□; control), and then treated with TSP-1 or plain buffer (spontaneously released FGF-2). Released material was then analyzed by Western blot (C) and, in parallel, plated on endothelial cells to measure FGF-2 binding to cell surface (A) and ability to induce cell proliferation (B), as described in “Materials and methods.” (A) Samples were added to BAECs and incubated for 2 hours at 4°C. After washes, cells were lysed and the amount of bound FGF-2 was analyzed by dot-blot analysis. Data (mean ± SD of triplicates) are expressed as arbitrary units of optical density (IOD). (B) Samples were added to BAECs and the number of proliferated cells was evaluated 72 h later. Anti-TGFβ antibodies did not revert the lack of proliferative activity of FGF-2 released from the ECM by TSP-1 (▨). Data (mean ± SD of triplicates) are expressed as absorbance.
We next investigated whether the observed inhibition could be explained by a direct inhibitory effect of TSP-1 on endothelial cells, mediated by the TSP-1 type I repeats, through CD36 and TGFβ, 2 known mediators of TSP-1 inhibitory activity. A possible role of CD36 in this system is ruled out by the fact that this TSP-1 receptor is not expressed by endothelial cells derived from large vessels.38 Moreover, antibodies against TGFβ did not revert the lack of proliferative activity of the TSP-1/FGF-2 complex (Figure 4B), ruling out the involvement of this pathway in our system. Finally, the antiangiogenic peptide, DI-TSP, based on the sequence of the type I repeat, did not affect BAEC proliferation (104 ± 0.4% of control, at 10 μM), excluding the involvement of this TSP-1 region.
Effect of TSP-1 on endogenous FGF-2-containing matrix
The experiments described thus far were performed using exogenously added, labeled FGF-2. To validate the relevance of these findings in a more physiologic setting, we investigated the effect of TSP-1 on endogenous FGF-2, produced and stored in the matrix by FGF-2-producing tumor cells. Tet-FGF2/HEC-1-B tumor cells, in which FGF-2 production is under the control of a tetracycline-responsive promoter (Tet-off system),35 were used as a source of FGF-2-enriched ECM.
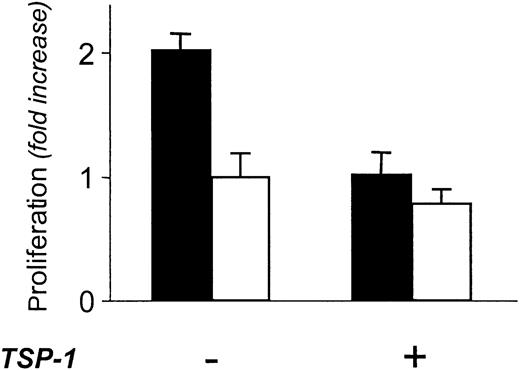
FGF-2-containing matrix is able to support endothelial cell proliferation.39 Accordingly, the matrix produced by Tet-FGF2/HEC-1-B cells in the absence of tetracycline (Figure 5, black columns) stimulated the proliferation of endothelial cells seeded on it. The stimulation was significantly higher (P ≤ .0001) than that induced by the matrix laid by tumor cells in which FGF-2 expression was down-regulated following exposure to tetracycline (Figure 5, white columns), the difference in mitogenic activity between the 2 conditions being ascribable only to the different FGF-2 expression. When the matrix produced by FGF-2-overexpressing cells (no tetracycline) was pretreated with TSP-1 (220 nM) before endothelial cell seeding, its proliferative activity was reduced (P ≤ .001) to the levels of low FGF-2-producing cells (Figure 5). This finding confirms that TSP-1 is able to deplete the matrix of the associated growth factors also when FGF-2 is physiologically embedded in the matrix, depriving it of the ability to directly support endothelial cell proliferation.
Effect of TSP-1 on the proliferative ability of the matrix laid by the FGF-2-overproducing Tet-FGF2/HEC-1-B endometrial adenocarcinoma cells. Matrix laid by Tet-FGF2/HEC-1-B cells treated with tetracycline (low FGF-2; □) or not treated (high FGF-2; ▪) was prepared as described in “Materials and methods,” and exposed or not to 220 nM TSP-1. Endothelial cells were plated on the treated matrix. The number of proliferated cells was evaluated 72 hours later. Cell proliferation is expressed as fold increase in cell number compared with control matrix (low FGF-2). Results (mean ± SD) are from one experiment representative of 2 experiments.
Effect of TSP-1 on the proliferative ability of the matrix laid by the FGF-2-overproducing Tet-FGF2/HEC-1-B endometrial adenocarcinoma cells. Matrix laid by Tet-FGF2/HEC-1-B cells treated with tetracycline (low FGF-2; □) or not treated (high FGF-2; ▪) was prepared as described in “Materials and methods,” and exposed or not to 220 nM TSP-1. Endothelial cells were plated on the treated matrix. The number of proliferated cells was evaluated 72 hours later. Cell proliferation is expressed as fold increase in cell number compared with control matrix (low FGF-2). Results (mean ± SD) are from one experiment representative of 2 experiments.
Modulation of VEGF and HGF/SF interaction with the ECM
Besides FGF-2, TSP-1 also binds to the angiogenic factors VEGF33 and HGF/SF.23 Both VEGF and HGF/SF are HSPG-binding factors and are found to be associated with the ECM.21-23 We therefore investigated whether TSP-1 modulated also VEGF and HGF/SF binding to and release from the matrix.
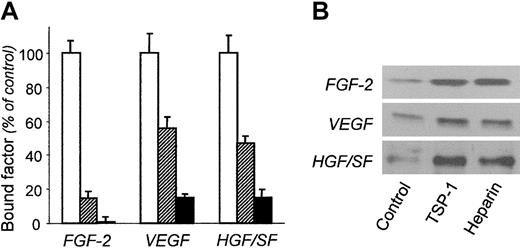
TSP-1 caused a significant (P ≤ .001) reduction of the binding of biotin-labeled VEGF and HGF/SF to the ECM (Figure 6A). As in the case of FGF-2, preincubation of the ECM with TSP-1 before the addition of the angiogenic factors did not alter VEGF and HGF/SF binding to the ECM (not shown), again suggesting that inhibition was not due to competition between TSP-1 and VEGF or HGF/SF for binding to matrix HSPGs.
Effect of TSP-1 on the interaction of VEGF and HGF/SF with the subendothelial matrix. (A) Biotin-labeled FGF-2, VEGF, or HGF/SF was incubated on the ECM alone (control; □) or in the presence of 220 nM TSP-1 (▨) or 100 μg/mL heparin (▪). The amount of matrix-bound angiogenic factors, measured using the ExtrAvidin-peroxidase/OPD system is expressed as the percentage of control binding (mean ± SD of triplicate values). (B) After preincubation with FGF-2, VEGF, or HGF/SF, the ECM was treated with buffer (control), 220 nM TSP-1, or 100 μg/mL heparin. The samples released from the matrix were analyzed by Western blot. Results are from one experiment representative of at least 2 experiments.
Effect of TSP-1 on the interaction of VEGF and HGF/SF with the subendothelial matrix. (A) Biotin-labeled FGF-2, VEGF, or HGF/SF was incubated on the ECM alone (control; □) or in the presence of 220 nM TSP-1 (▨) or 100 μg/mL heparin (▪). The amount of matrix-bound angiogenic factors, measured using the ExtrAvidin-peroxidase/OPD system is expressed as the percentage of control binding (mean ± SD of triplicate values). (B) After preincubation with FGF-2, VEGF, or HGF/SF, the ECM was treated with buffer (control), 220 nM TSP-1, or 100 μg/mL heparin. The samples released from the matrix were analyzed by Western blot. Results are from one experiment representative of at least 2 experiments.
The ability of TSP-1 to mobilize matrix-bound VEGF and HGF/SF was also investigated. Western blot analysis of samples from matrix pretreated with labeled VEGF or HGF/SF and then with TSP-1 showed that TSP-1 (220 nM) increased factor release from the matrix up to a degree comparable with heparin (Figure 6B).
Discussion
The interaction of heparin-binding angiogenic factors with the ECM is instrumental in the regulation of the growth factor location, storage, and activity, and compounds that modulate this interaction are known to affect the whole process of angiogenesis. This study shows that TSP-1, a known modulator of angiogenesis, prevents the association of FGF-2 with the matrix and causes the mobilization of matrix-bound FGF-2 in a biologically inactive form, therefore acting as a scavenger for the growth factor.
Binding of FGF-2 to the ECM is mostly mediated by HSPGs present in the matrix. The finding that TSP-1 prevents the binding of FGF-2 to the matrix, and hence to matrix HSPGs, extends our previous observation that TSP-1 inhibits the binding of FGF-2 to HSPGs present on the surface of endothelial cells.31 It is interesting to note that only soluble TSP-1 was able to affect FGF-2 binding to the matrix, whereas matrix-bound TSP-1 (matrix preincubated with TSP-1 before the addition of FGF-2) had no effect. This finding further confirms the concept that the activity of TSP-1 is extremely dependent on its physical status: cell adhesion, spreading, chemotaxis/haptotaxis, and proliferation are differently (sometimes even antagonistically) regulated by soluble and immobilized TSP-1.27,40,41 This is conceivably due to differences in the molecular conformation between soluble and bound TSP-1, with consequent changes in the relative availability of the multiple TSP-1 active domains.24
TSP-1 inhibition of FGF-2 binding to the ECM might occur through 2 possible mechanisms: direct binding and sequestration of the growth factor by TSP-1, or competition with FGF-2 for binding to matrix HSPGs (as both TSP-1 and FGF-2 bind to HSPGs). Three lines of evidence suggest that inhibition of FGF-2 binding to the matrix is not caused by a competition with TSP-1 for binding to HSPGs: (1) preincubation of the matrix with TSP-1 before the addition of FGF-2, to allow TSP-1 binding to exposed HSPG, did not reduce the amount of bound FGF-2, as would be expected if TSP-1 masked the HSPG binding sites for FGF-2; (2) the 25-kDa fragment of TSP-1 containing the main heparin-binding domain was inactive in preventing FGF-2 binding that was instead inhibited by the 140-kDa fragment. This finding suggests that the high affinity HSPG binding site of TSP-1 is not involved in FGF-2 mobilization, although we cannot completely rule out the involvement of sequences in the type I repeat of TSP-1 described to interact with heparin with low affinity, whose actual biologic relevance is still debated25,42,43 ; and (3) TSP-1 binds to FGF-2 with high affinity, similar to the affinity of FGF-2 for heparin. Altogether, this evidence supports the hypothesis that TSP-1 binding to FGF-2 might sequester the growth factor, preventing its binding to the matrix.
It could be hypothesized that in normal conditions, the levels of soluble TSP-1 are too low to affect the interaction of FGF-2 with the much more abundant HSPGs in the matrix. However, in conditions in which high amounts of soluble TSP-1 are released (eg, following platelet activation), the increased TSP-1 concentrations conceivably become effectual in competing with HSPGs, hence displacing FGF-2 from its storage sites. This hypothesis of in vivo scenario warrants further investigation.
Binding of FGF-2 with HSPGs is reversible and characterized by a fast dissociation rate.4 The association of FGF-2 with the matrix is therefore a dynamic process, and molecules that prevent the association of FGF-2 to HSPGs or that degrade the matrix can induce a rapid release of FGF-2, free to diffuse in the soluble phase.6,11,44 ECM-bound FGF-2 is mobilized by matrix-degrading enzymes, such as the heparan sulfate-degrading endoglycosidases heparanase13 or the proteolytic enzymes urokinase, stromelysin, collagenase, and plasmin.12,45 Alternatively, matrix FGF-2 is mobilized by molecules that bind to FGF-2 as in the case of heparin and heparin-mimicking compounds,18 a soluble syndecan-1 ectodomain,16 and, as shown in this study, by TSP-1.
The functional consequences of FGF-2 mobilization depend on the mechanisms of action of the releasing agent. FGF-2 is released by heparanase or proteases as a complex with HS fragments that present the growth factor to its tyrosine kinase receptor, hence potentiating its activity.12,13 Similarly, FGF-BP releases active FGF-2, and potentiates its binding to the cell receptor, contributing to the onset of angiogenesis in pathologic conditions, and particularly in tumor angiogenesis, where it can determine the acquisition of angiogenic competence (“angiogenic switch”) in tumor cells.14 In contrast, FGF-2 released from the matrix by TSP-1 is not able to induce endothelial cell proliferation, indicating that TSP-1 mobilization of FGF-2 results in the release of a nonactive form of the growth factor. This finding suggests that, in vivo, the local relative concentrations of TSP-1 versus other FGF-2-mobilizing agents might determine whether FGF-2 is mobilized in an active or inactive form.
Our hypothesis of sequestration of the growth factor by TSP-1 is supported by the finding that more than 90% of mobilized FGF-2 was present as a complex with TSP-1 rather than as a free molecule, as shown by gel filtration chromatography and chemical cross-linking analysis. Moreover, we have shown that the extracted complex has a reduced ability to bind to endothelial cells, further supporting the hypothesis of sequestration of the growth factor by TSP-1. The fact that CD36 is not expressed in endothelial cells from large vessels, together with the lack of activity of the type I repeat-mimetic DI-TSP peptide and of anti-TGFβ antibodies, suggest that these molecular pathways, known to mediate the direct antiangiogenic activity of TSP-1, are not involved in our system. Therefore, although other mechanisms might still be involved, sequestration of FGF-2 by TSP-1 is conceivably the mechanism of the lack of activity of mobilized FGF-2.
Another platelet degranulation, antiangiogenic product, PF-4, binds to FGF-2 and mobilizes matrix-bound FGF-2, modulating its bioavailability.46 However, the ability of PF-4 to release soluble FGF-2 has been proposed to depend on a competition with FGF-2 for binding to HSPGs, rather than to sequestration of the growth factor and formation of a complex.15,47 Therefore, in this respect TSP-1 acts in a unique way, by sequestering the growth factor through the formation of a complex that hampers FGF-2 binding to endothelial cell HSPGs, and ultimately its activity on endothelial cells.
Inhibition of matrix-derived FGF-2 activity by TSP-1 was observed also in the model of FGF-2-overproducing Tet-FGF2/ HEC-1-B tumor cells,35 in which endogenous FGF-2 is physiologically embedded within the ECM. Since matrix-bound FGF-2 is able to promote endothelial cell adhesion and proliferation,48-50 this model was used to study the effect of TSP-1 on the activity of endogenous, matrix-associated FGF-2. Exposure to TSP-1 deprived the matrix of its FGF-2-dependent proliferative ability for endothelial cells, confirming that TSP-1 mobilizes FGF-2 also when the growth factor is physiologically embedded in a matrix. In addition, these findings indicate that the final inhibitory effect of TSP-1 is dual: on one side it mobilizes matrix-associated growth factor in a sequestered, inactive form, and on the other side, it reduces the proliferative activity of the matrix itself, by depletion of the stored growth factors.
Besides FGF-2, TSP-1 also prevented the binding of 2 other heparin-binding angiogenic factors, VEGF and HGF/SF, to the ECM, and mobilized the 2 factors when already bound to the matrix. These findings therefore indicate that the effect of TSP-1 is not restricted to FGF-2, but it might represent a more general endogenous mechanism to control the bioavailability of different matrix-binding angiogenic factors.
TSP-1 binds to VEGF and HGF/SF, as well as FGF-2, and in all cases binding is inhibited by heparin, suggesting similarities in terms of functional consequences. In agreement with our findings, TSP-1 was reported to inhibit the proangiogenic activity of VEGF and HGF/SF,23,33 and to prevent the binding of VEGF to endothelial cell surface HSPGs.33 Moreover, in a recent study, TSP-1 has been described to affect the availability of VEGF for its receptor, though in this case, the effect was not imputed to a direct interaction of TSP-1 with VEGF, but to an indirect mechanism involving suppression of matrix metalloproteinase-9 activation.51 In another study, the TSP type I repeats present in connective tissue growth factor (CTGF) were reported to bind to VEGF and to prevent growth factor binding to endothelial cells.52 It is worth noting that the TSP domain of CTGF binds to the exon 7-coded region of VEGF165, namely in the region mainly involved in binding to HSPGs.21,52 Altogether, these studies further support our hypothesis for a role of TSP-1 in binding the heparin-binding region of angiogenic factors, consequently affecting their interaction with HSPGs and activity.
Different molecular mechanisms contribute to the antiangiogenic activity of TSP-1. Interaction of sequences in the type I repeat of TSP-1 with CD36, leading to the activation of a Fyn-caspase-3-P38MAPK cascade, is considered the main mechanism of the inhibition of endothelial cell motility and induction of apoptosis.53 There is also evidence that the interaction of the carboxy-terminal region of TSP-1 with CD47, by regulating integrin activity, can contribute to the antiangiogenic activity of TSP-1.24 Other mechanisms propone indirect regulation of endothelial cell function by TSP-1, through alteration of ECM organization and modulation of protease activity.24,51 In line with the concept of a role for TSP-1, as well as other matricellular proteins, in controlling the interplay among cells, matrix, and soluble factors, our study indicates a new activity for TSP-1. We propose that modulation of angiogenic factor interaction with the matrix, and hence bioavailability, is a mechanism that might contribute to the antiangiogenic activity of TSP-1.
Prepublished online as Blood First Edition Paper, August 28, 2003; DOI 10.1182/blood-2003-03-0893.
Supported by grants from the Italian Association for Cancer Research (AIRC) to G.T. and M.P. B.M. is the recipient of a fellowship from the Italian Foundation for Cancer Research (FIRC).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful to A. Bugatti for the BIAcore analysis, and to F. Sangalli for assistance with the chromatography experiments.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal