Abstract
Late-onset noninfectious pulmonary complications (LONIPCs) occurring beyond 3 months after allogeneic stem cell transplantation (allo-SCT) have become recognized as life-threatening complications, and they reduce the recipient's quality of life. However, the pathogenesis and optimal treatment for LONIPCs are still unclear. In this study, we retrospectively analyzed the incidence and outcome of LONIPCs among allo-SCT recipients. Between October 1993 and September 2001, 96 patients underwent allo-SCT and 76 patients who survived and were free of disease for more than 3 months after SCT were enrolled. Among the 76 patients, 18 patients (23.7%) developed LONIPCs at a median interval of 227 days after allo-SCT (range, 91-1105 days). The patients with LONIPCs were subclassified into those with bronchiolitis obliterans (BO) (6 patients), with interstitial pneumonia (IP) (11 patients), or with both BO and IP (1 patient). The presence of extensive chronic graft-versus-host disease (GVHD) was significantly associated with the development of LONIPCs (P = .0008). Liver or skin involvement in chronic GVHD was not associated, but sicca syndrome was significantly associated with the development of LONIPCs (P < .0001). Most of the IP patients (58.3%) responded well to immunosuppressive treatment, while BO patients did not respond to the therapy. Eight of the 18 patients with LONIPCs died. The major cause of death was respiratory failure (62.5%). The relapse rate of primary malignant disease in the LONIPC patients was significantly lower than that of non-LONIPC patients (1 of 17 [5.9%] versus 16 of 52 [30.8%]; P = .0387). These results indicate that the development of LONIPCs was strongly associated with chronic GVHD and especially with sicca syndrome and the graft-versus-leukemia (GVL) effect. (Blood. 2003;102:4236-4242)
Introduction
With the introduction of new prophylactic antibiotics and infectious monitoring techniques, long-term survival and the possibility of cure become practical for many patients who undergo allogeneic stem cell transplantation (allo-SCT). However, pulmonary complications develop in 40% to 60% of the recipients, and these are recognized as a major cause of morbidity and mortality.1-4 In particular, late-onset noninfectious pulmonary complications (LONIPCs) occurring beyond 3 months after allo-SCT now have become recognized as life-threatening complications that reduce the recipient's quality of life (QOL).5-7 Palmas et al reported the incidence of LONIPCs was 10% after allogeneic bone marrow transplantations (allo-BMTs) and that 5 of 18 patients with LONIPCs died from progressive respiratory failure, with a median follow-up after LONIPC diagnosis of 13.5 months.5 Duncker et al also reported that the incidence was 14.8% and that 4 of 40 patients died from respiratory failure.6
Despite the severity of this disorder, the pathogenesis of LONIPCs is still unclear. It was said that its development was associated with the presence of chronic graft-versus-host disease (GVHD)5,6 and with the particular method of GVHD prophylaxis.8 Payne et al reported that cyclosporin A (CsA) protects against the development of obstructive airway disease after BMT in leukemic patients.8 It has been suggested that working alloreactive T cells cause LONIPCs in the context of chronic GVHD.5-7,9-12
The spectrum of LONIPCs is also undefined. Palmas et al proposed that the classification include bronchiolitis obliterans (BO), bronchiolitis obliterans with organizing pneumonia (BOOP), diffuse alveolar damage (DAD), lymphocytic interstitial pneumonia (LIP), and nonclassifiable interstitial pneumonia (NCIP).5 Afessa et al categorized LONIPCs as BO, BOOP, and idiopathic pneumonia syndrome (IPS).7 Because BO has several specific defining characteristics, as compared with the other disorders, LONIPCs were sometimes classified as BO and other conditions.7,9 Immunosuppressive therapy has been a mainstay treatment for LONIPCs, but BO is often resistant to this therapy.7,13,14
In this study we describe the results of a retrospective analysis of the risk factors for LONIPCs as well as the incidence, clinical aspects, and treatment outcome of these disorders.
Patients and methods
Patient selection
Clinical data for 96 patients who underwent allo-SCT at our institution between October 1993 and September 2001 were collected and reviewed retrospectively. Thirteen patients died and 7 patients relapsed within 3 months after allo-SCT, so they were excluded from the analysis. The 76 disease-free patients who survived more than 3 months after SCT were eligible for the study. Sixty-nine patients with hematologic malignancy were divided into 2 groups according to their disease status at the time of transplantation: a standard-risk group (n = 31) and a high-risk group (n = 38). The former group consisted of acute leukemia patients in first complete remission (CR) or chronic myeloid leukemia (CML) patients in the first chronic phase (CP), and the latter group consisted of patients beyond the first CR or the first CP and with myelodysplastic syndromes (MDS). Patients' disease status and characteristics are listed in Table 1.
Clinical features of 76 patients surviving more than 3 months after allo-SCT and 18 patients with LONIPCs
. | All patients, N = 76 . | . | Patients with LONIPCs, N = 18 . | . | ||
|---|---|---|---|---|---|---|
| Clinical feature . | n . | % . | n . | % . | ||
| Sex | ||||||
| Male | 52 | 68.4 | 12 | 66.7 | ||
| Female | 24 | 31.6 | 6 | 33.3 | ||
| Median age, y (range) | 31.9 (15-51) | — | 34.8 (15-50) | — | ||
| Diagnosis | ||||||
| AML | 20 | 26.3 | 6 | 33.3 | ||
| ALL | 18 | 23.7 | 3 | 16.7 | ||
| CML | 19 | 25.0 | 1 | 5.6 | ||
| NHL | 7 | 9.2 | 5 | 27.8 | ||
| AA | 3 | 3.9 | 1 | 5.6 | ||
| MDS | 5 | 6.6 | 2 | 11.1 | ||
| Others | 4 | 5.3 | 0 | 0 | ||
| Transplant source Related donor | ||||||
| BM | 9 | 11.8 | 1 | 5.6 | ||
| PBSCs | 18 | 23.7 | 6 | 33.3 | ||
| BM plus PBSCs | 1 | 1.3 | 0 | 0 | ||
| Unrelated donor | ||||||
| BM | 47 | 61.8 | 11 | 61.1 | ||
| CB | 1 | 1.3 | 0 | 0 | ||
| HLA compatibility | ||||||
| Full match | 59 | 77.6 | 14 | 77.8 | ||
| Mismatch | 17 | 22.4 | 4 | 22.3 | ||
| 1 locus mismatch | 9 | 11.8 | 3 | 16.7 | ||
| 2 loci mismatch | 8 | 10.5 | 1 | 5.6 | ||
| ABO compatibility | ||||||
| Match | 48 | 63.2 | 13 | 72.2 | ||
| Mismatch | 28 | 36.8 | 5 | 27.8 | ||
| Sex compatibility | ||||||
| Match | 47 | 61.8 | 11 | 61.1 | ||
| Mismatch | 27 | 38.2 | 7 | 38.9 | ||
| Conditioning regimen | ||||||
| TBI/TLI regimen | 35 | 46.1 | 11 | 61.1 | ||
| Non-TBI/TLI regimen | 41 | 53.9 | 7 | 38.9 | ||
| GVHD prophylaxis | ||||||
| CsA with or without MTX | 60 | 78.9 | 16 | 88.9 | ||
| FK506 plus MTX | 16 | 21.1 | 2 | 11.1 | ||
| Acute GVHD | ||||||
| Grade 0 | 22 | 28.9 | 5 | 27.8 | ||
| Grade I | 25 | 32.9 | 5 | 27.8 | ||
| Grade II | 22 | 28.9 | 7 | 38.9 | ||
| Grades III-IV | 7 | 9.2 | 1 | 5.6 | ||
| cGVHD | ||||||
| 0 | 22 | 28.9 | 2 | 11.1 | ||
| Limited | 13 | 17.1 | 1 | 5.6 | ||
| Extensive | 37 | 48.7 | 15 | 83.3 | ||
| NE | 4 | 5.3 | 0 | 0 | ||
| cGVHD/liver | ||||||
| − | 40 | 52.6 | 9 | 50.0 | ||
| + | 32 | 42.1 | 9 | 50.0 | ||
| cGVHD/skin | ||||||
| − | 37 | 48.7 | 8 | 44.4 | ||
| + | 35 | 46.1 | 10 | 55.6 | ||
| cGVHD/sicca syndrome | ||||||
| − | 43 | 56.6 | 3 | 16.7 | ||
| + | 33 | 43.3 | 15 | 83.3 | ||
. | All patients, N = 76 . | . | Patients with LONIPCs, N = 18 . | . | ||
|---|---|---|---|---|---|---|
| Clinical feature . | n . | % . | n . | % . | ||
| Sex | ||||||
| Male | 52 | 68.4 | 12 | 66.7 | ||
| Female | 24 | 31.6 | 6 | 33.3 | ||
| Median age, y (range) | 31.9 (15-51) | — | 34.8 (15-50) | — | ||
| Diagnosis | ||||||
| AML | 20 | 26.3 | 6 | 33.3 | ||
| ALL | 18 | 23.7 | 3 | 16.7 | ||
| CML | 19 | 25.0 | 1 | 5.6 | ||
| NHL | 7 | 9.2 | 5 | 27.8 | ||
| AA | 3 | 3.9 | 1 | 5.6 | ||
| MDS | 5 | 6.6 | 2 | 11.1 | ||
| Others | 4 | 5.3 | 0 | 0 | ||
| Transplant source Related donor | ||||||
| BM | 9 | 11.8 | 1 | 5.6 | ||
| PBSCs | 18 | 23.7 | 6 | 33.3 | ||
| BM plus PBSCs | 1 | 1.3 | 0 | 0 | ||
| Unrelated donor | ||||||
| BM | 47 | 61.8 | 11 | 61.1 | ||
| CB | 1 | 1.3 | 0 | 0 | ||
| HLA compatibility | ||||||
| Full match | 59 | 77.6 | 14 | 77.8 | ||
| Mismatch | 17 | 22.4 | 4 | 22.3 | ||
| 1 locus mismatch | 9 | 11.8 | 3 | 16.7 | ||
| 2 loci mismatch | 8 | 10.5 | 1 | 5.6 | ||
| ABO compatibility | ||||||
| Match | 48 | 63.2 | 13 | 72.2 | ||
| Mismatch | 28 | 36.8 | 5 | 27.8 | ||
| Sex compatibility | ||||||
| Match | 47 | 61.8 | 11 | 61.1 | ||
| Mismatch | 27 | 38.2 | 7 | 38.9 | ||
| Conditioning regimen | ||||||
| TBI/TLI regimen | 35 | 46.1 | 11 | 61.1 | ||
| Non-TBI/TLI regimen | 41 | 53.9 | 7 | 38.9 | ||
| GVHD prophylaxis | ||||||
| CsA with or without MTX | 60 | 78.9 | 16 | 88.9 | ||
| FK506 plus MTX | 16 | 21.1 | 2 | 11.1 | ||
| Acute GVHD | ||||||
| Grade 0 | 22 | 28.9 | 5 | 27.8 | ||
| Grade I | 25 | 32.9 | 5 | 27.8 | ||
| Grade II | 22 | 28.9 | 7 | 38.9 | ||
| Grades III-IV | 7 | 9.2 | 1 | 5.6 | ||
| cGVHD | ||||||
| 0 | 22 | 28.9 | 2 | 11.1 | ||
| Limited | 13 | 17.1 | 1 | 5.6 | ||
| Extensive | 37 | 48.7 | 15 | 83.3 | ||
| NE | 4 | 5.3 | 0 | 0 | ||
| cGVHD/liver | ||||||
| − | 40 | 52.6 | 9 | 50.0 | ||
| + | 32 | 42.1 | 9 | 50.0 | ||
| cGVHD/skin | ||||||
| − | 37 | 48.7 | 8 | 44.4 | ||
| + | 35 | 46.1 | 10 | 55.6 | ||
| cGVHD/sicca syndrome | ||||||
| − | 43 | 56.6 | 3 | 16.7 | ||
| + | 33 | 43.3 | 15 | 83.3 | ||
cGVHD indicates chronic GVHD; AML, acute myelogenous leukemia; ALL, acute lymphoblastic leukemia; NHL, non-Hodgkin lymphoma; and AA, aplastic anemia.
Infection prophylaxis and CMV monitoring
Oral decontamination was started from day-21 before the transplantation. Usually an antifungal drug, fluconazole or itraconazole, had been used until day 75 after transplantation. Amphotericin B was administered with an inhaler from day-21 to hematologic recovery. Trimethoprim-sulfamethoxazole (TMP-SMX) (TMP 160 mg, SMX 800 mg, 2 times per week) was also used to prevent Pneumocystis carinii pneumonia. Cytomegalovirus (CMV) infection was monitored weekly by CMV antigenemia. If CMV antigenemia tests became positive, patients were treated with ganciclovir.
Types of transplantation, prophylaxis, and treatment of GVHD
Fifty-six patients received allo-BMT, 18 received allogeneic peripheral blood stem cell transplantation (allo-PBSCT), and there was 1 unrelated cord blood transplantation (CBT) and 1 patient who received both BMT and PBSCT. T-cell-depleted allo-SCT was not performed. CsA or tacrolimus (FK506) plus short-term methotrexate (MTX) were used as GVHD prophylaxis. FK506 was used when the patient's type of HLA class I was different from the donor's. Acute and chronic GVHD were diagnosed and graded according to established criteria.15,16 Acute GVHD was treated with prednisolone (PSL). Extensive chronic GVHD was treated primarily with PSL and CsA or FK506. To estimate mouth/eye involvement (sicca syndrome), a Schirmer test and a secretion stimulant test of salivary glands were evaluated in all patients before and after allo-SCT.
Criteria and definition of LONIPCs and pulmonary disease classification
Routine pulmonary function tests (PFTs) were performed before transplantation and at about the first 3 months after transplantation. The criteria used to label PFTs were as follows: percent forced vital capacity (FVC) of the predicted value (FVC % predicted) less than 80% and forced expiratory volume of 1 second (FEV1)/FVC at least 70% as restricted pattern, FVC % predicted at least 80% and FEV1/FVC less than 70% as obstructive pattern, and FVC % predicted less than 80% and FEV1/FVC less than 70% as mixed pattern. On initial review of these 76 patients, those with clinical or radiologic signs and symptoms of lung disease and/or abnormalities in PFTs developing after the first 3 months of allo-SCT were considered. When these patients showed no evidence of an infectious cause, a diagnosis of LONIPCs was made. To exclude infection, standard culture and staining methods for bacterial, viral, and protozoan pathogens were employed. Bronchoalveolar lavage (BAL) and transbronchial lung biopsy (TBLB) were performed whenever possible. Open lung biopsies were not performed. Once a patient was identified as having LONIPCs, further disease classifications were made on the basis of clinical and radiologic findings, PFT, and pathological findings according to Palmas et al.5 LIP and NCIP were classified as IP.
Patients were classified as having BO if they showed a decline in FEV1 to less than 80% of the predicted value and FEV1/FVC less than 70%.14 IP was diagnosed if a patient showed bilateral diffuse parenchymal interstitial/alveolar infiltrates on chest x-ray and/or computed tomography (CT) with associated hypoxemia (PaO2 less than 70 mmHg, A-aDO2 more than 20 mmHg).
Statistical methods
The statistical analyses were performed on March 1, 2002. The end point for recording survival was the date the patient was last contacted before March 1, 2002. Logistic regression analysis was performed to investigate the influences of many factors, including disease status, donor source, sex, HLA compatibility, ABO blood type compatibility, conditioning regimen (including total body irradition/total lymphoid irradiation [TBI/TLI] regimen or non-TBI/TLI regimen), GVHD prophylaxis, and the presence of acute or chronic GVHD on the development of LONIPCs. The χ2 test and Fisher exact probability test were applied to between-groups analyses of categoric data utilizing Stat View software v. 4.51 (Abacus Concepts, Berkeley, CA). Overall survival was estimated by Kaplan-Meier product-limit estimates. The log-rank test was used to assess differences between the groups of patients with and without LONIPCs. A P value of less than .05 was considered to be statistically significant.
Results
Of the 76 patients who survived disease-free for more than 3 months after allo-SCT, 18 patients (23.7%) fulfilled the diagnostic criteria for LONIPCs (Tables 1 and 2). The median time to diagnosis of LONIPCs was 226.7 days after transplantation (range, 91-1105 days). The patients with LONIPCs were further subclassified as having BO (6 patients) or IP (12 patients). In 1 patient with IP, BO was diagnosed after the improvement of IP.
Clinical features of 18 patients with LONIPCs
. | . | . | . | GVHD . | . | . | Days from SCT to LONIPCs . | PFTs at onset of LONIPCs . | . | PFTs after treatment . | . | . | . | . | . | Mo of survival from LONIPCs . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case no. . | LONIPC category . | Primary disease . | TBI/TLI . | Acute . | Chronic . | Sicca . | . | FVC% predicted . | FEV1/FVC . | FVC% predicted . | FEV1/FVC . | Pattern of PFTs . | Treatment outcome . | HOT . | Causes of death . | . | Alive or dead . | ||||
| 1 | BO | AML | − | 2 | E | + | 185 | 91.3 | 69.6 | 96.9 | 72.2 | O | PR | − | NA | 25.5 | Alive | ||||
| 2 | BO | AML | − | 0 | E | + | 115 | 84.2 | 69.5 | 84.6 | 65.3 | O | NC | − | NA | 74.8 | Alive | ||||
| 3 | BO | CML | − | 2 | E | + | 150 | 83.8 | 66.9 | ND | ND | O | P | − | Respiratory failure | 4.2 | Dead | ||||
| 4 | BO | MDS | − | 2 | E | + | 336 | 87.3 | 71.8 | 83.5 | 67.1 | O | P | − | P carinii pneumonia | 5.1 | Dead | ||||
| 5 | BO | AML | + | 2 | E | + | 94 | 105.9 | 74.5 | 59.7 | 35.4 | O | P | + | Relapse | 6.3 | Dead | ||||
| 6 | BO | ALL | + | 1 | E | + | 240 | 114.1 | 56.9 | 101.8 | 41.3 | O | P | + | NA | 38.8 | Alive | ||||
| 7 | IP | NHL | − | 0 | E | − | 180 | 120.2 | 78.3 | 116.3 | 81.1 | N | CR | − | NA | 20.4 | Alive | ||||
| 8 | IP | NHL | + | 2 | O | + | 204 | 121.9 | 88.0 | 111.1 | 84.5 | N | CR | − | NA | 24.5 | Alive | ||||
| 9 | IP | MDS | − | 1 | E | + | 1105 | 100.7 | 90.2 | 111.4 | 90.0 | N | CR | − | NA | 15.9 | Alive | ||||
| 10 | IP | ALL | + | 0 | O | − | 117 | 111.8 | 87.5 | 117.1 | 82.3 | N | CR | − | NA | 44.5 | Alive | ||||
| 11 | IP | AA | + | 3 | E | + | 140 | 112.2 | 83.4 | 126.2 | 82.8 | N | CR | − | NA | 52.3 | Alive | ||||
| 12 | IP | NHL | + | 0 | E | + | 91 | 96.9 | 92.3 | ND | ND | N | P | − | Respiratory failure | 1.1 | Dead | ||||
| 13 | IP | NHL | + | 2 | E | + | 200 | 124.8 | 81.0 | ND | ND | N | P | − | Respiratory failure | 3.1 | Dead | ||||
| 14 | IP | NHL | + | 1 | E | + | 300 | 54.5 | 96.4 | 30.4 | 97.8 | R | P | + | NA | 45.8 | Alive | ||||
| 15 | IP/BO * | AML | − | 0 | E | + | 540 | 53.1 | 57.1 | 80.3 | 69.1 | M→O | CR | − | NA | 44.0 | Alive | ||||
| 16 | IP | AML | + | 1 | L | + | 144 | 59.6 | 77.9 | ND | ND | R | CR | − | 2nd malignancy (MDS) | 27.4 | Dead | ||||
| 17 | IP | ALL | + | 2 | E | − | 153 | 65.7 | 79.9 | ND | ND | R | P | − | Respiratory failure | 1.5 | Dead | ||||
| 18 | IP | AML | + | 1 | E | + | 160 | 57.4 | 98.7 | 57.2 | 99.0 | R | P | + | Respiratory failure | 45.7 | Dead | ||||
. | . | . | . | GVHD . | . | . | Days from SCT to LONIPCs . | PFTs at onset of LONIPCs . | . | PFTs after treatment . | . | . | . | . | . | Mo of survival from LONIPCs . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case no. . | LONIPC category . | Primary disease . | TBI/TLI . | Acute . | Chronic . | Sicca . | . | FVC% predicted . | FEV1/FVC . | FVC% predicted . | FEV1/FVC . | Pattern of PFTs . | Treatment outcome . | HOT . | Causes of death . | . | Alive or dead . | ||||
| 1 | BO | AML | − | 2 | E | + | 185 | 91.3 | 69.6 | 96.9 | 72.2 | O | PR | − | NA | 25.5 | Alive | ||||
| 2 | BO | AML | − | 0 | E | + | 115 | 84.2 | 69.5 | 84.6 | 65.3 | O | NC | − | NA | 74.8 | Alive | ||||
| 3 | BO | CML | − | 2 | E | + | 150 | 83.8 | 66.9 | ND | ND | O | P | − | Respiratory failure | 4.2 | Dead | ||||
| 4 | BO | MDS | − | 2 | E | + | 336 | 87.3 | 71.8 | 83.5 | 67.1 | O | P | − | P carinii pneumonia | 5.1 | Dead | ||||
| 5 | BO | AML | + | 2 | E | + | 94 | 105.9 | 74.5 | 59.7 | 35.4 | O | P | + | Relapse | 6.3 | Dead | ||||
| 6 | BO | ALL | + | 1 | E | + | 240 | 114.1 | 56.9 | 101.8 | 41.3 | O | P | + | NA | 38.8 | Alive | ||||
| 7 | IP | NHL | − | 0 | E | − | 180 | 120.2 | 78.3 | 116.3 | 81.1 | N | CR | − | NA | 20.4 | Alive | ||||
| 8 | IP | NHL | + | 2 | O | + | 204 | 121.9 | 88.0 | 111.1 | 84.5 | N | CR | − | NA | 24.5 | Alive | ||||
| 9 | IP | MDS | − | 1 | E | + | 1105 | 100.7 | 90.2 | 111.4 | 90.0 | N | CR | − | NA | 15.9 | Alive | ||||
| 10 | IP | ALL | + | 0 | O | − | 117 | 111.8 | 87.5 | 117.1 | 82.3 | N | CR | − | NA | 44.5 | Alive | ||||
| 11 | IP | AA | + | 3 | E | + | 140 | 112.2 | 83.4 | 126.2 | 82.8 | N | CR | − | NA | 52.3 | Alive | ||||
| 12 | IP | NHL | + | 0 | E | + | 91 | 96.9 | 92.3 | ND | ND | N | P | − | Respiratory failure | 1.1 | Dead | ||||
| 13 | IP | NHL | + | 2 | E | + | 200 | 124.8 | 81.0 | ND | ND | N | P | − | Respiratory failure | 3.1 | Dead | ||||
| 14 | IP | NHL | + | 1 | E | + | 300 | 54.5 | 96.4 | 30.4 | 97.8 | R | P | + | NA | 45.8 | Alive | ||||
| 15 | IP/BO * | AML | − | 0 | E | + | 540 | 53.1 | 57.1 | 80.3 | 69.1 | M→O | CR | − | NA | 44.0 | Alive | ||||
| 16 | IP | AML | + | 1 | L | + | 144 | 59.6 | 77.9 | ND | ND | R | CR | − | 2nd malignancy (MDS) | 27.4 | Dead | ||||
| 17 | IP | ALL | + | 2 | E | − | 153 | 65.7 | 79.9 | ND | ND | R | P | − | Respiratory failure | 1.5 | Dead | ||||
| 18 | IP | AML | + | 1 | E | + | 160 | 57.4 | 98.7 | 57.2 | 99.0 | R | P | + | Respiratory failure | 45.7 | Dead | ||||
HOT indicates home oxygen therapy; E, extensive type; O, obstructive; PR, partial remission; NC, no change; P, progressive; N, normal; CR, complete remission; R, restrictive; M, mixed; L, limited type; NA, not applicable; and ND, not done.
Case 15: BO was diagnosed after the improvement of IP.
Clinical presentation
Symptoms were similar among the subgroups and consisted of cough or dyspnea in all but 1 patient. Only 1 patient had no subjective symptoms and showed no radiologic abnormality at presentation. She showed a slight decline of O2 saturation from a measurement taken by pulse-oximeter during a routine visit to the outpatient clinic and was diagnosed as having BO after PFTs. Fever was present in 8 patients with IP and 1 patient with BO.
Chest radiography and computed tomography
The most common abnormality on chest radiography of IP patients was bilateral diffuse or patchy infiltrates. The computed tomography (CT) or high-resolution CT (HRCT) findings of IP patients included ground-glass opacities with or without pleural effusion and regions of consolidation. Some of them showed peripherally distributed patchy air space consolidation, ground-glass attenuation, or nodular opacities.
Three of the 6 patients with BO had normal chest radiographs at diagnosis. Specific findings for the other 3 patients included bronchi and bronchiolectasis, centrilobular opacities (2 with BO), and lung hypoattenuations (1 with BO). The HRCT results for these 3 BO patients indicated expiratory air trapping. Two BO patients got repetitious pneumothorax during progression of the lung disease.
Lung biopsies and pulmonary flow studies
BAL was performed for 13 patients (1 BO, 12 IP) without complications, and infection was excluded. TBLB was performed for 5 patients, and the findings were consistent with IP in all of them. Seven IP patients were not examined histologically, and their diagnosis was established by clinical and radiological findings.
Routine PFTs were examined in 66 patients, including 17 patients with LONIPCs. These examinations were carried out both before transplantation and at about 3 months after transplantation before the patients developed LONIPCs. Mean FVC % predicted for these patients 3 months after transplantation was slightly decreased compared with the baseline values, but this decrease was not statistically significant (Table 3). Mean FVC % predicted and FEV1/FVC at 3 months after transplantation were not significantly different between the patients who did (n = 17) or did not (n = 49) develop LONIPCs subsequently. Therefore, no obvious regimen-related toxicity in the lung was observed in the LONIPC patients before they developed LONIPCs. When they developed lung disease, PFTs were carried out for all patients with LONIPCs (Table 2). Four patients exhibited an obstructive pattern, which was consistent with the criteria for BO. Two patients with BO showed normal pattern at the onset of LONIPCs, but subsequently FEV1/FVC decreased to less than 70%. The PFT findings for the 12 patients with IP included restrictive change (4 patients, including 1 patient [patient no. 14] who had restrictive change because of a lung operation), mixed change (1 patient), and normal range (7 patients).
Pulmonary function tests before and at about 3 months after transplantation
. | Total, N = 66 . | . | Non-LONIPCs, N = 49 . | . | LONIPCs, N = 17 . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|
. | Before . | After . | Before . | After . | Before . | After . | P . | |||
| FVC % predicted | 105.6 | 99.6 | 105.6 | 102.2 | 105.6 | 92.3 | .725 | |||
| FEV1/FVC | 85.9 | 86.2 | 87.0 | 86.9 | 82.4 | 84.0 | .838 | |||
. | Total, N = 66 . | . | Non-LONIPCs, N = 49 . | . | LONIPCs, N = 17 . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|
. | Before . | After . | Before . | After . | Before . | After . | P . | |||
| FVC % predicted | 105.6 | 99.6 | 105.6 | 102.2 | 105.6 | 92.3 | .725 | |||
| FEV1/FVC | 85.9 | 86.2 | 87.0 | 86.9 | 82.4 | 84.0 | .838 | |||
Immunosuppressive treatment of LONIPCs
Once patients were identified as having LONIPCs, intensive immunosuppressive therapy was started with PSL 1 to 2 mg/kg and CsA or FK506. If the treatment with CsA plus PSL was not effective, CsA was changed to FK506. Azathioprine (AZT) was also added to refractory patients. Patients with IP often responded well to the immunosuppressive treatment (Table 2). Among 7 patients who had a normal pattern at the diagnosis of IP, 5 responded rapidly to intensive immunosuppressive treatment and achieved durable complete responses, and 1 patient relapsed after the tapering of steroids but responded to a second course of treatment using steroids, FK506, and AZT. However, 2 patients died of progressive respiratory failure, and we could not examine their subsequent PFTs. Among 4 IP patients with a mixed pattern, 2 patients improved with immunosuppressive treatment but another 2 patients died of progressive respiratory failure and we could not perform subsequent PFTs. For 1 patient with the improvement of clinical and radiologic findings (patient no. 15), the restrictive change improved but the obstructive change remained thereafter, with a persistent decrease of V50, V25, and maximal midexpiratory flow (MMEF), indicating middle or lower airway tract obstructions. These data indicate that the patient had both IP and BO. For another patient with a mixed pattern, clinical and radiologic changes improved with the treatment but the patient died of a second malignancy (patient no. 16).
On the other hand, most of the patients with BO (5 of 6) did not respond to the therapy, including 4 patients with progressive disease, and the value of FEV1/FVC subsequently decreased for these patients (Table 2).
Risk factors for developing LONIPCs
The presence of extensive chronic GVHD and high-risk disease were the variables significantly associated with the development of LONIPCs (P = .0042 and P = .0092, respectively). Among the target organs for development of chronic GVHD, liver or skin involvement was not associated with the development of LONIPCs, but sicca syndrome was strongly associated (P < .0001) (Table 4). Three of 31 standard-risk patients and 14 of 38 high-risk patients developed LONIPCs.
Risk factors for developing LONIPCs
. | All patients . | . | LONIPC patients . | . | . | ||
|---|---|---|---|---|---|---|---|
| Factor . | n . | % . | n . | % . | P . | ||
| Sex: male vs female | 52 of 76 | 68.4 | 12 of 18 | 66.7 | NS | ||
| Source | |||||||
| Related PBSCs vs BM | 18 of 27 | 66.7 | 6 of 7 | 85.7 | NS | ||
| Unrelated BM vs related BM | 47 of 56 | 83.9 | 11 of 12 | 91.7 | NS | ||
| HLA compatibility: mismatch vs full match | 17 of 76 | 22.4 | 4 of 18 | 22.2 | NS | ||
| Blood type mismatch: + vs − | 28 of 76 | 36.8 | 5 of 18 | 27.8 | NS | ||
| Sex mismatch: + vs − | 29 of 76 | 38.2 | 7 of 18 | 38.9 | NS | ||
| Conditioning: TBI/TLI vs non-TBI/TLI | 35 of 76 | 46.1 | 11 of 18 | 61.1 | NS | ||
| GVHD prophylaxis: CsA vs FK506 | 60 of 76 | 78.9 | 16 of 18 | 88.9 | NS | ||
| Acute GVHD: II-IV vs 0/I | 29 of 76 | 38.2 | 8 of 18 | 44.4 | NS | ||
| Chronic GVHD: extensive vs 0/limited | 37 of 76 | 48.7 | 15 of 18 | 83.3 | .0008 | ||
| Liver: + vs − | 32 of 76 | 42.1 | 9 of 18 | 50.0 | NS | ||
| Skin: + vs − | 35 of 76 | 46.1 | 10 of 18 | 55.6 | NS | ||
| Sicca syndrome: + vs − | 33 of 76 | 43.4 | 15 of 18 | 83.3 | <.0001 | ||
| Disease status: high vs standard | 38 of 69 | 55.1 | 14 of 17 | 82.4 | .0092 | ||
. | All patients . | . | LONIPC patients . | . | . | ||
|---|---|---|---|---|---|---|---|
| Factor . | n . | % . | n . | % . | P . | ||
| Sex: male vs female | 52 of 76 | 68.4 | 12 of 18 | 66.7 | NS | ||
| Source | |||||||
| Related PBSCs vs BM | 18 of 27 | 66.7 | 6 of 7 | 85.7 | NS | ||
| Unrelated BM vs related BM | 47 of 56 | 83.9 | 11 of 12 | 91.7 | NS | ||
| HLA compatibility: mismatch vs full match | 17 of 76 | 22.4 | 4 of 18 | 22.2 | NS | ||
| Blood type mismatch: + vs − | 28 of 76 | 36.8 | 5 of 18 | 27.8 | NS | ||
| Sex mismatch: + vs − | 29 of 76 | 38.2 | 7 of 18 | 38.9 | NS | ||
| Conditioning: TBI/TLI vs non-TBI/TLI | 35 of 76 | 46.1 | 11 of 18 | 61.1 | NS | ||
| GVHD prophylaxis: CsA vs FK506 | 60 of 76 | 78.9 | 16 of 18 | 88.9 | NS | ||
| Acute GVHD: II-IV vs 0/I | 29 of 76 | 38.2 | 8 of 18 | 44.4 | NS | ||
| Chronic GVHD: extensive vs 0/limited | 37 of 76 | 48.7 | 15 of 18 | 83.3 | .0008 | ||
| Liver: + vs − | 32 of 76 | 42.1 | 9 of 18 | 50.0 | NS | ||
| Skin: + vs − | 35 of 76 | 46.1 | 10 of 18 | 55.6 | NS | ||
| Sicca syndrome: + vs − | 33 of 76 | 43.4 | 15 of 18 | 83.3 | <.0001 | ||
| Disease status: high vs standard | 38 of 69 | 55.1 | 14 of 17 | 82.4 | .0092 | ||
NS indicates not significant.
Neither the use of TBI- or TLI-containing conditioning regimens, the source of stem cells, the type of GVHD prophylaxis, nor the presence of acute GVHD showed any significant association with the incidence of LONIPCs in our series. A specific risk factor for developing BO and IP was not detected.
Survival and the relationship between LONIPCs and graft- versus-leukemia effect
The median duration of follow-up after diagnosis of LONIPCs was 25 months (range, 1.1-74.8 months). Overall, 10 patients (55.6%) were alive at the time of this report. Among the 18 LONIPC patients, home oxygen therapy (HOT) was necessary for 4 patients (2 of 6 BO, 2 of 12 IP) because of progressive respiratory failure. Two of these patients were alive 38.8 and 45.8 months after diagnosis of LONIPCs, but the other 2 patients died of respiratory failure and relapse.
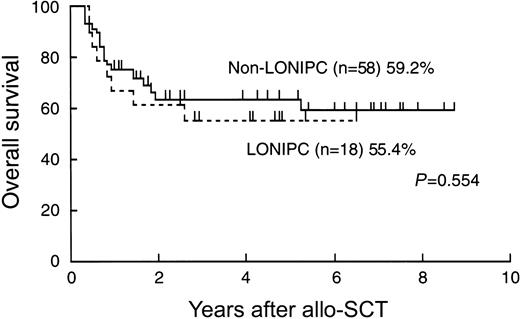
Eight LONIPC patients (44.4%) died. Times from the diagnosis of LONIPCs to death ranged from 1.1 to 45.7 months. The primary cause of death was progressive respiratory failure (5 patients). The causes of death for the other 3 patients were Pneumocystis carinii pneumonia, secondary malignancy, and relapse (Table 5). Overall survival of the LONIPC patients was not longer than that of non-LONIPC patients (Figure 1).
Causes of death with and without LONIPCs
. | Non-LONIPCs . | . | LONIPCs . | . | ||
|---|---|---|---|---|---|---|
| Causes of death . | Patients, N = 58 . | % . | Patients, N = 18 . | % . | ||
| Relapse | 12 | 66.7 | 1 | 12.5 | ||
| GVHD | 3 | 16.7 | 0 | 0 | ||
| TMA | 1 | 5.6 | 0 | 0 | ||
| Infection | 1 | 5.6 | 1 | 12.5 | ||
| Second malignancy | 1 | 5.6 | 1 | 12.5 | ||
| Respiratory failure due to LONIPCs | 0 | 0 | 5 | 62.5 | ||
. | Non-LONIPCs . | . | LONIPCs . | . | ||
|---|---|---|---|---|---|---|
| Causes of death . | Patients, N = 58 . | % . | Patients, N = 18 . | % . | ||
| Relapse | 12 | 66.7 | 1 | 12.5 | ||
| GVHD | 3 | 16.7 | 0 | 0 | ||
| TMA | 1 | 5.6 | 0 | 0 | ||
| Infection | 1 | 5.6 | 1 | 12.5 | ||
| Second malignancy | 1 | 5.6 | 1 | 12.5 | ||
| Respiratory failure due to LONIPCs | 0 | 0 | 5 | 62.5 | ||
TMA indicates thrombotic microangiopathy.
Overall survival of patients with and without LONIPCs, calculated using the Kaplan-Meier method.
Overall survival of patients with and without LONIPCs, calculated using the Kaplan-Meier method.
Among the 69 patients with hematologic malignancies, it was notable that the patients with LONIPCs had significantly lower relapse rates of primary malignant disease than did non-LONIPC patients (1 of 17 versus 16 of 52, P = .0387) (Table 6), although high-risk disease was a risk factor for developing LONIPCs. Because chronic GVHD is associated with the graft- versus-leukemia (GVL) effect, we analyzed the relapse rates for LONIPC and non-LONIPC patients with chronic GVHD (Table 6). Ten patients without LONIPCs relapsed, whereas only 1 patient with LONIPCs relapsed, although this difference was not statistically significant (P = .0565). Among the patients with extensive-type chronic GVHD, the patients with LONIPCs had significantly lower relapse rates than did non-LONIPC patients (1 of 14 versus 8 of 22, P = .0484).
Relapse rate is significantly lower in the patients with LONIPCs
. | Total no. of relapses . | Relapses in non-LONIPCs . | Relapses in LONIPCs . | P . |
|---|---|---|---|---|
| No. of patients | 17 of 69 | 16 of 52 | 1 of 17 | .0387 |
| Chronic GVHD | 11 of 46 | 10 of 31 | 1 of 15 | .0565 |
| Extensive-type chronic | ||||
| GVHD | 9 of 36 | 8 of 22 | 1 of 14 | .0484 |
. | Total no. of relapses . | Relapses in non-LONIPCs . | Relapses in LONIPCs . | P . |
|---|---|---|---|---|
| No. of patients | 17 of 69 | 16 of 52 | 1 of 17 | .0387 |
| Chronic GVHD | 11 of 46 | 10 of 31 | 1 of 15 | .0565 |
| Extensive-type chronic | ||||
| GVHD | 9 of 36 | 8 of 22 | 1 of 14 | .0484 |
Discussion
LONIPCs is a new disease entity that was first reported by Palmas et al in 1998.5 The pathogenesis of LONIPCs is still unclear, but the presence of chronic GVHD has been taken as a risk factor for developing LONIPCs. In this study, the presence of extensive chronic GVHD was significantly associated with the development of LONIPCs. Duncker et al showed the association of this pulmonary disorder with working alloreactive T cells in the context of chronic GVHD6 and that most LONIPC cases respond to immunosuppressive agents. Also, Cooke et al reported that alloreactive donor lymphocytes were associated with lung injury in an experimental murine model.17,18 Differences in minor histocompatibility antigens between the donor and recipient were suggested to be important stimuli in the pathogenesis of IPS.19,20
The categorization of this disease has not been defined. Similar to past reports, BO showed special characteristics in many clinical aspects of our series, including therapy resistance and progressive outcome. Yokoi et al reported that early histologic changes of BO included epithelial defects of the tracheae and bronchi and infiltration of inflammatory cells, and these transformed to a late phase in which airways were partially or completely obstructed.21 Consistent with this, the obstructive changes associated with PFT might lead to the terminal stage of pathological changes in BO. Therefore, it might be difficult to cure BO even at the time of initial diagnosis.7 In contrast, the patients with IP often responded well to immunosuppressive treatment, especially if they were in the early phase.
In this study, PFTs were performed before transplantation and at about 3 months after transplantation. Although the mean FVC % predicted was slightly decreased from the baseline value after transplantation, there was no significant difference between the patients who did or did not develop LONIPCs subsequently. Therefore, the PFT abnormalities in the LONIPC patients developed after the first 3 months after transplantation. Of the 6 patients with BO, 4 patients had progressive deterioration of disease despite intensive immunosuppressive treatment. Of the 12 patients with IP, 7 patients showed a normal pattern and 4 patients showed a restricted pattern during the development of IP. Five of these 7 patients with a normal pattern rapidly improved with intensive immunosuppressive treatment. However, 2 of 4 patients with a restricted pattern died of progressive respiratory failure. However, 1 patient with a mixed pattern, restrictive changes, and radiologic abnormalities improved rapidly with immunosuppressive treatment, but obstructive changes still remained thereafter. There was a persistent decrease of V50, V25, and MMEF, indicating middle or lower airway tract obstructions that were consistent with BO.
In this study, the presence of sicca syndrome was significantly associated with the development of LONIPCs (P < .0001), whereas liver or skin involvement of chronic GVHD was not associated. It is known that the minor salivary glands are a frequent target of chronic GVHD. In some recent reports, the histologic characteristics of the bronchial glands of LONIPC patients were similar to those seen in the salivary glands of chronic GVHD patients. Therefore, sicca syndrome and bronchial gland involvement in LONIPCs might be strongly associated with and caused by chronic GVHD. It is supposed that donor alloreactive lymphocytes attack the bronchial glands and make the respiratory tract dry, decreasing immunoglobulin secretion.22 In previous reports, other risk factors for LONIPCs associated with chronic GVHD were chronic myeloid leukemia, inclusion of busulfan in the conditioning regimen,23 and MTX and steroids given as GVHD prophylaxis,4,8,24,25 but these were not identified as risk factors here. HLA compatibility was not associated with the development of LONIPCs.
Among the patients with LONIPCs, the major cause of death was respiratory failure due to LONIPCs, and only 1 patient died after relapse of primary disease, whereas the primary cause of death in the patients without LONIPCs was relapse of the primary disease. The LONIPC patients showed significantly lower relapse rates than those seen for the non-LONIPC patients. Because chronic GVHD is associated with the GVL effect, we analyzed the relapse rates for patients with chronic GVHD, with or without LONIPCs. Among the patients with extensive-type chronic GVHD, the patients with LONIPCs had significantly lower relapse rates than did non-LONIPC patients. Furthermore, a high risk of primary disease was also a risk factor for developing LONIPCs, although the relapse rate was significantly lower for the LONIPC patients. These results indicate that patients with LONIPCs might achieve a strong GVL effect. In spite of a low relapse rate, the overall survival of LONIPC patients was similar to that of non-LONIPC patients, because most LONIPC patients died of progressive respiratory failure. If we can control the disease more temperately, a prolonged overall survival and better QOL of LONIPC patients may be expected.
We report here the result of a single-center study on the incidence, pathogenesis, and outcome of LONIPCs after the first allo-SCT. LONIPCs is an important cause of posttransplantation morbidity and mortality. The development of LONIPCs was strongly associated with the GVL effect. Risk reduction can be achieved by better prevention and control of chronic GVHD. Careful observation after allo-SCT of patients with extensive chronic GVHD, and especially those with sicca syndrome, is very helpful for early detection and treatment of LONIPCs, and this may lead to prolonged survival and better QOL for these patients. It would also be valuable to undertake a prospective analysis of lung complications following allo-SCT.
Prepublished online as Blood First Edition Paper, August 7, 2003; DOI 10.1182/blood-2002-10-3289.
Supported in part by grants from the special research program of the Chiba University Graduate School of Medicine, Japan.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Ms Megumi Ejiri for technical assistance. We also thank the members of the Division of Hematology, Department of Clinical Cell Biology, Chiba University Graduate School of Medicine, for providing excellent care of the patients and for valuable discussions.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal