Abstract
Human T-cell leukemia virus type 1 (HTLV-1) infection profoundly alters T-cell gene expression, and the dysregulated synthesis of cytokines could influence the course and pathologic consequences of infection. In the process of screening T-cell lines for T helper 1 (Th1) and Th2 cytokine mRNAs, we observed that interleukin-13 (IL-13) mRNA was highly expressed in HTLV-1-infected, IL-2-dependent T-cell lines. IL-9 and interferon gamma (IFN-γ) mRNAs were also expressed at high levels in chronically infected cell lines. IL-5 mRNA was detected in 60% of the HTLV-1-infected cell lines, but mRNAs for IL-4, IL-10, IL-2, and IL-15 were either below detection limits or did not correlate with HTLV-1 infection. Transcriptional activation of the IL-13 promoter by the HTLV-1 Tax trans-regulatory protein was demonstrated in Jurkat T cells transiently transfected with an IL-13 promoter-reporter plasmid. The clinical relevance of these observations was demonstrated by immunofluorescent staining and flow cytometry of lymphocytes obtained from HTLV-1-infected patients. These studies revealed that IL-13 production was directly related to the level of Tax expression in the infected CD4+ T cells soon after in vitro culture. As IL-13 plays key roles in tumor immunosurveillance, asthma, and central nervous system inflammation, it may contribute to the pathophysiology of HTLV-1-associated diseases. (Blood. 2003;102:4130-4136)
Introduction
Human T-cell leukemia virus type 1 (HTLV-1) is a retrovirus that infects about 10 to 20 million people worldwide with endemic foci in Southern Japan, the Caribbean, Central and South Africa, and South America. Less than 5% of HTLV-1-infected individuals develop either adult T-cell leukemia (ATL) or a chronic inflammatory disease of the central nervous system termed HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP). HTLV-1 also has been implicated in a broader spectrum of diseases including uveitis, infective dermatitis, polymyositis, arthropathy, and Sjögren syndrome.1-3 HTLV-1-associated diseases may be accompanied by pulmonary disorders and immunosuppression, and it is noteworthy that patients with acute ATL generally succumb to opportunistic infections.4 The majority of HTLV-1 proviruses are found in CD4+ and in CD8+ T cells,5-7 and there is a clonal expansion of HTLV-1-infected CD4+ T cells.8
HTLV-1 infection of primary T cells in vitro results in the infrequent establishment of chronically infected T-cell lines that continuously proliferate in the absence of antigenic stimulation, but most require interleukin-2 (IL-2) for growth.9-12 A small number of HTLV-1-infected T-cell lines also have been established that do not require IL-2, but these do not closely resemble patient-derived HTLV-1-infected T cells.13 The viral trans-regulatory protein, Tax, is the primary effector of T-cell transformation. In addition to its role in activating virus transcription, Tax alters the expression or function of signal transduction proteins, cell cycle regulators, and transcription factors.14 Targets of the Tax protein also include cytokine promoters,15-19 and dysregulation of cytokine expression in HTLV-1-infected individuals may play an important role in the course of disease.
Although expression patterns of a number of cytokines have been studied in HTLV-1-infected cells, IL-13 has not been examined in this context. Because IL-13 is a key mediator of various immune functions,20-22 including the inhibition of tumor immunosurveillance23,24 and the pathophysiology of asthma,25,26 we asked whether its expression is altered in HTLV-1-infected T cells. IL-13 mRNA was expressed at high levels in 10 independent, chronically infected T-cell lines, but not uninfected controls. Transient transfections of Jurkat T cells revealed that the IL-13 promoter was transcriptionally activated by ectopic expression of the HTLV-1 Tax regulatory protein. Peripheral blood lymphocytes (PBLs) from asymptomatic HTLV-1 carriers, HAM/TSP patients, and uninfected donors were cultured ex vivo, stained with fluorescently labeled antibodies to CD4, Tax, and IL-13 proteins, and analyzed by flow cytometry. IL-13 was detected only in those CD4+ T cells that also expressed Tax protein. Indeed, there was a positive correlation between the levels of Tax expression and IL-13 synthesis. Therefore, we propose that the activation of IL-13 expression in HTLV-1-infected T cells may contribute to the pathophysiology of HTLV-1-associated diseases.
Materials and methods
Cell lines
The HTLV-1-transformed (IL-2-independent) T-cell lines, MT-2,11 HuT-102,9 C8166,12 and C10-MJ,10 have been described elsewhere. The HTLV-1-immortalized (IL-2-dependent) T-cell lines, 1657, FS, SP, A212, and EG, were generously provided by Dr Renu Lal (Centers for Disease Control [CDC], Atlanta, GA).27 The HTLV-1-immortalized cell lines, MS-9, MS-64, MS-68, and MS-74, were established by coculture of activated human peripheral blood mononuclear cells (PBMCs) with 293T cells transfected with the HTLV-1 provirus clone, pHTLV-X1MT.28 The cell line, DCH-4, was established by transduction of activated, primary human CD4+ T cells with a lentivirus vector encoding an HTLV-1 Tax-enhanced yellow fluorescent protein fusion. The 3 plasmids used to produce recombinant lentivirus included (1) a packaging plasmid, pCMVΔ8.2; (2) a transfer vector, pHRtaxyfp; and (3) an envelope expression plasmid, pCMV-VSV-G.29 Noninfected T-cell lines, Jurkat and Kit 225, served as negative controls. The cell lines, Kit225, 1657, FS, SP, A212, EG, MS-9, MS-64, MS-68, MS-74, and DCH-4, were maintained in RPMI-1640 medium supplemented with 10% fetal calf serum (FCS), 2 mM glutamine, 100 IU/mL penicillin, 100 μg/mL streptomycin, and 100 U/mL recombinant human IL-2 (rhIL-2) (Roche Applied Science, Indianapolis, IN). Jurkat, MT-2, HUT102, C8166, and C10-MJ T-cell lines were maintained in complete RPMI-1640 medium without rhIL-2. PBMCs were isolated from buffy coats of leukopheresed blood from healthy volunteers by Ficoll-Hypaque density centrifugation. CD4+ and CD8+ T-cell subsets were isolated from PBMCs by negative selection using magnetic beads (Stem Cell Technologies, Vancouver, BC) according to the manufacturer's instructions. Purified CD4+ and CD8+ T cells were activated with anti-CD3/anti-CD28 antibody-coated beads (Dynal Biotech, Lake Success, NY) and maintained in RPMI-1640 complete medium with rhIL-2.
Plasmids
The HTLV-1 Tax expression plasmid, pRSTax-1C, and the luciferase reporter plasmids, pGL3-basic and pGL3-CMV, were described previously.30 The lentivirus vectors were originally obtained from Didier Trono and are described elsewhere.31 The human IL-13 promoter (-939 to +48) was provided by David Lewis (Stanford University School of Medicine)32 and was subcloned into the pGL3-basic reporter plasmid (Promega, Madison, WI). A series of IL-13 promoter fragments with nested 5′-deletions were constructed by polymerase chain reaction (PCR) amplification and cloned into the pGL3-basic plasmid. These 5′-deletion constructs are designated as pD3397 (-357 to +48); pD3402 (-155 to +48); and pD3403 (-67 to +48), where the numbers in parentheses indicate the IL-13 promoter termini relative to the RNA start site.
RNA preparation and analyses
Total cellular RNAs were isolated from cell lines using Qiagen RNeasy purification kits (Qiagen, Alameda, CA). The Multiprobe RNase protection assays (RPAs) were performed with 5 to 10 μg total RNA according to the manufacturer's directions (Pharmingen, San Diego, CA) with the following modifications. Hybridization probes were synthesized with (33P)-uridine triphosphate (UTP) (70-80 μC per reaction) using the Pharmingen In Vitro Transcription kit. Following incubation, yeast tRNA and EDTA (ethylenediaminetetraacetic acid) were added and products were purified on Amersham-Pharmacia G25 Microspin columns (Piscataway, NJ). To each sample RNA, 0.5 to 1.0 × 106 cpm of probe, was added in a final hybridization volume of 10 to 20 μL (at least 50% Pharmingen hybridization buffer). A master cocktail, containing 200 μL Ambion RNAse inactivation/precipitation reagent III (Ambion, Austin, TX), 50 μL ethanol, 5 μg yeast tRNA, and 1 μL Ambion GycoBlue coprecipitate, was added to each RNA sample to precipitate the protected RNA. After adding the individual RNAse-treated samples to 250 μL of the inactivation/precipitation cocktail, the samples were mixed well, placed at -70°C for 15 minutes, and subjected to centrifugation at 14 000 rpm for 15 minutes. The supernatants were decanted and the pellet was suspended in 3 μL Pharmingen sample buffer.
Real-time reverse transcription (RT)-PCR for HTLV-1 pX-tax/rex mRNA was performed on a Prism 7700 sequence detector (Perkin Elmer/Applied Biosystems, Foster City, CA) using the following primers: ex2/6950, 5′-ACCAACACCATGGCCCA-3′; 3′-7170, 5′-GAGTCGAGGGATAAGGAAC-3′. The TaqMan probe was 5′FAM-CCTTTCATTCACGACTAACTGC-TAMRA-3′. The reaction conditions were 50°C for 2 minutes, 95°C for 10 minutes to activate the DNA polymerase, then 50 cycles of 15 seconds at 94°C and 1 minute at 60°C. All reaction volumes were 25 μL, including 5 μL cDNA template and 20 μL master mix (Perkin Elmer/Applied Biosystems), 100 ng of each primer, TaqMan probe, and water. Calibration curves were derived by running 10-fold serial dilutions of cDNA plasmid over the range of 2.5 × 100 to 2.5 × 107 copies. Cellular cDNA samples were run at several dilutions. Each assay included duplicate wells for each dilution of calibration plasmids, triplicate wells for each cellular cDNA, and controls that included nonspecific plasmid DNA, TLE (10 mM Tris, pH 7.4; 0.1 mM EDTA), and water, all in duplicate. The threshold cycle values (Ct) were used to plot the calibration curve. All standard curves had a coefficient of variation of at least 0.97. The copy numbers were normalized to the human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) values measured in separate real-time PCR assays with the GAPDH kit (Perkin Elmer/Applied Biosystems) and calibration DNA standards from Serologicals (Norcross, GA). All copy numbers derived are the result of at least 3 determinations.
ELISA assays for secreted IL-13 protein
HTLV-1-infected T-cell lines were suspended in fresh medium at 1 × 106 cells per milliliter and were cultured for 24 hours; supernatants were then collected for cytokine enzyme-linked immunosorbent assays (ELISAs). The assay was performed by the National Cancer Institute-Frederick core facility using IL-13 ELISA kit (R&D Systems, Minneapolis, MN).
Transfections and luciferase assays
Jurkat T cells (1 × 106 cells) were transfected with 4 μg luciferase reporter plasmid DNA and 1 μg pRS-Tax1C or empty expression vector DNA using DMRIE-C reagent (Invitrogen, Carlsbad, CA). After 48 hours, transfected cells were harvested, washed with phosphate-buffered saline (PBS), and suspended in 200 μL lysis buffer (Promega). Luciferase activities were measured with 100 μL cell lysate in the Promega luciferase assay system according to the manufacturer's protocol.
Flow cytometry
PBMCs were obtained from 3 patients with a clinical diagnosis of HAM/TSP, 3 asymptomatic HTLV-1 carriers, and 1 healthy individual at St Mary's Hospital, London, United Kingdom. PBMCs were prepared as previously described.19 Briefly, PBMCs were isolated from whole blood on Histopaque-1077 (Sigma, St Louis, MO) density gradient and washed twice with PBS. PBMCs were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS), 2 mM glutamine, 100 IU/mL penicillin, and 100 μg/mL streptomycin. For the intracellular protein staining, PBMCs were cultured in complete medium with 20 nM Concanamycin A (Sigma) present throughout the culture period and 10 μg/mL Brefeldin A (Sigma) for at least 4 hours prior to harvest. After harvesting, cells were fixed with 4% paraformaldehyde in PBS (pH 7.4) for 5 minutes at 37°C. Fixed cells were washed with PBS and then suspended in PBS containing 7% normal goat serum (PNGS; Sigma). The fixed cells were stained with phycoerythrin (PE)-cyanine 5.1 (PC5)-labeled anti-CD4 and PE and Texas Red (ECD)-labeled anti-CD8 antibodies for 20 minutes at room temperature and then washed with PNGS. To prevent nonspecific binding, Fc receptor (FCR)-blocking reagent (human immunoglobulin G [IgG] from Miltenyi Biotec [Bergisch Gladbach, Germany]) was used in all procedures. Intracellular cytokine staining was performed by suspending cells in PNGS containing 0.2% saponin (PS), followed by washing with PS. Cells were then suspended in PS containing an IgG3 antitax monoclonal antibody (mAb) (Lt-4)33 or an isotype control mAb (Southern Biotechnology, Birmingham, AL) and a PE-conjugated IL-13 mAb (Pharmingen) or its respective isotype control mAb for 20 minutes at room temperature. Cells were washed twice with PS, stained with fluorescein isothiocyanate (FITC)-labeled goat F (ab′)2 anti-mouse IgG3 (Southern Biotechnology) in PS for 20 minutes, and then washed twice with PS. Cells were suspended in 1 mL PBS and analyzed by flow cytometry on a Coulter EpicsXL (Beckman Coulter, Brea, CA) using Coulter Expo 32 software.
Results
Cytokine gene expression profiles in HTLV-1-infected cell lines
In order to identify HTLV-1-induced alterations in cytokine gene expression, we initially examined cytokine mRNA expression profiles in a panel of diverse HTLV-1-infected T-cell lines. Of the 9 IL-2-dependent, HTLV-1-infected T-cell lines examined, 5 were CD4+ (MS-9, 1657, FS, SP, and A212) and 4 were CD8+ (MS-68, MS-64, EG, and MS-78). An additional CD4+ T-cell line was examined (DCH-4), which was immortalized by transduction with a recombinant lentivirus vector encoding a Tax-YFP fusion protein. Included in the panel were 4 IL-2-independent HTLV-1-infected T-cell lines (MT-2, HuT-102, C8166, and C10-MJ). Used as controls were 2 uninfected T-cell lines (Jurkat and Kit 225) and primary activated CD4+ and CD8+ T cells.
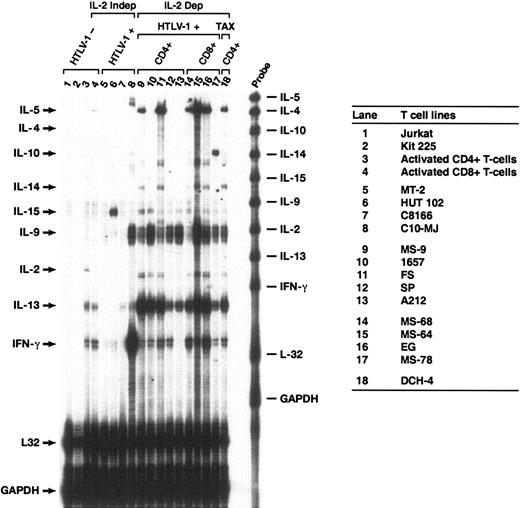
There were 9 cytokine mRNAs (IL-2, IL-4, IL-5, IL-9, IL-10, IL-13, IL-14, IL-15, and interferon gamma [IFN-γ]) analyzed with a multiprobe RNase protection assay (RPA) using total cellular RNA from each cell culture (Figure 1). None of the cytokine mRNAs surveyed were expressed at detectable levels in the uninfected Jurkat and Kit 225 T-cell lines (Figure 1, lanes 1-2). Activated CD4+ and CD8+ T cells (lanes 3-4) both expressed IL-13 and IFN-γ mRNAs; CD4+ T cells expressed IL-2 mRNA and CD8+ T cells expressed IL-5 mRNA at low but detectable levels.
Multiprobe RNase protection analysis of cytokine mRNAs expressed in HTLV-1-infected and uninfected T cells. Total RNA was prepared from the cell lines shown in the right-hand panel. Lanes 1-4: uninfected T-cell lines and activated CD4+ and CD8+ T cells. Lanes 5-8: chronically infected T-cell lines that do not require IL-2 for growth. Lanes 9-17: HTLV-1-immortalized T-cell lines that require IL-2 for growth. Lane 18: T-cell line immortalized with a lentivirus vector encoding a Tax-YFP fusion protein. Cell surface phenotypes of the IL-2-dependent, HTLV-1-immortalized cells were CD4+ (lanes 9-13,18) or CD8+ (lanes 14-17). The probes used for RNase protection are shown on the right side of the gel and protected fragments are indicated by arrows on the left side. Probes for L32 and GAPDH mRNA were included as controls for mRNA quantity and quality. The figure shows results from a typical experiment, which was performed at least 3 times.
Multiprobe RNase protection analysis of cytokine mRNAs expressed in HTLV-1-infected and uninfected T cells. Total RNA was prepared from the cell lines shown in the right-hand panel. Lanes 1-4: uninfected T-cell lines and activated CD4+ and CD8+ T cells. Lanes 5-8: chronically infected T-cell lines that do not require IL-2 for growth. Lanes 9-17: HTLV-1-immortalized T-cell lines that require IL-2 for growth. Lane 18: T-cell line immortalized with a lentivirus vector encoding a Tax-YFP fusion protein. Cell surface phenotypes of the IL-2-dependent, HTLV-1-immortalized cells were CD4+ (lanes 9-13,18) or CD8+ (lanes 14-17). The probes used for RNase protection are shown on the right side of the gel and protected fragments are indicated by arrows on the left side. Probes for L32 and GAPDH mRNA were included as controls for mRNA quantity and quality. The figure shows results from a typical experiment, which was performed at least 3 times.
The cytokine mRNA expression patterns among the IL-2-independent, HTLV-1-transformed T cells (MT-2, HuT-102, C8166, and C10-MJ) were highly variable, and, with several exceptions, barely detectable levels of cytokine mRNAs were expressed (Figure 1, lanes 5-8). The HuT-102 cell line (lane 6) expressed IL-15 mRNA at high levels as previously reported.34 In contrast to the other 3 cell lines, C10-MJ cells expressed IL-9 and IFN-γ mRNAs in large amounts (lane 8). IL-13 mRNA was detected at low levels in C8166 and C10-MJ cells but not in MT-2 and HuT-102 cells.
Among the chronically infected, IL-2-dependent T-cell lines, the cytokine mRNA expression patterns were more consistent than the IL-2-independent cell lines (Figure 1, lanes 9-18 compared with lanes 5-8). IL-13 mRNA expression was highly active in all of the IL-2-dependent, HTLV-1-infected T-cell lines compared with IL-2-independent counterparts and uninfected controls. IFN-γ mRNA was produced in all but one of the IL-2-dependent cell lines (A212), and IL-9 was strongly activated in all cell lines with the exception of MS-68. IL-5 and IL-14 mRNAs were detected in 3 of 6 HTLV-1-infected CD4+ T-cell lines (MS-9, FS, and DCH-4) and in 3 of 4 CD8+ cell lines (MS-68, MS-64, and EG). IL-4 mRNA expression was not activated in any of these samples, and IL-10 mRNA expression was activated only in MS-78 cells (lane 17). In general, the IL-2-dependent, HTLV-1-immortalized cell lines displayed activation of IL-13, IL-9, and IFN-γ mRNA synthesis, sometimes accompanied by activation of IL-5 expression. The cytokine expression profiles appeared to be independent of whether the infected cells were CD4+ or CD8+. The cytokine mRNA expression patterns were independent of IL-2 added to the growth medium. In particular, IL-13 mRNA expression levels were identical when HTLV-1-infected T-cell lines were grown either in the presence or in the absence of IL-2 for 16 hours (data not shown). Conversely, treatment of MT2 or HUT102 cells with IL-2 did not activate IL-13 mRNA synthesis.
Secretion of IL-13 protein in HTLV-1-infected T cells
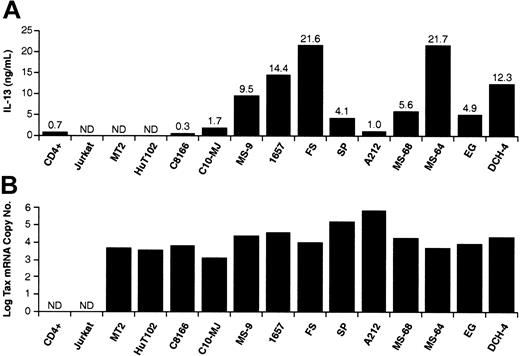
We next determined IL-13 protein levels in supernatants from the T-cell lines by ELISA (Figure 2A). IL-13 protein was not detected in supernatants from Jurkat cells but was produced at low levels from activated CD4+ T cells. Among the HTLV-1-positive cell lines, IL-13 was not detected in MT2 and HuT-102 cell cultures. Low levels of IL-13 were detected in C8166, C10/MJ cells, and A212 cultures. All of the other HTLV-1-infected cell lines produced IL-13 at levels ranging between 4 and 21 ng/mL. These protein levels corresponded to IL-13 mRNA band intensities in Figure 1. We also examined the levels of HTLV-1 Tax mRNA expressed in these cell lines by quantitative real-time PCR analysis (Figure 2B). Tax mRNA was detected only in HTLV-1-infected cell lines and correlated with Tax protein levels.28 In cell lines, IL-13 production was activated only in Tax-positive cells, but IL-13 levels were not proportional to amounts of Tax mRNA expressed. The latter may reflect the complex nature of IL-13 gene expression that may involve Tax-mediated alterations of cellular transcription factor activity as well as DNA and chromatin modifications.
IL-13 protein secretion and Tax mRNA levels in HTLV-1-infected and uninfected T-cell lines. (A) T cells were suspended in fresh media at 1 × 106 cells per milliliter and incubated for 24 hours. Culture medium was then collected and IL-13 protein was quantified by ELISA. (B) HTLV-1 Tax mRNA was quantified by TaQman RT-PCR analysis. Tax cDNA copy numbers are expressed as the log10 of the Tax cDNA copy number relative to 1 × 105 copies of GAPDH cDNA. IL-13 protein and Tax mRNA levels are the mean of at least 3 determinations with standard deviations of less than 10% of the mean value.
IL-13 protein secretion and Tax mRNA levels in HTLV-1-infected and uninfected T-cell lines. (A) T cells were suspended in fresh media at 1 × 106 cells per milliliter and incubated for 24 hours. Culture medium was then collected and IL-13 protein was quantified by ELISA. (B) HTLV-1 Tax mRNA was quantified by TaQman RT-PCR analysis. Tax cDNA copy numbers are expressed as the log10 of the Tax cDNA copy number relative to 1 × 105 copies of GAPDH cDNA. IL-13 protein and Tax mRNA levels are the mean of at least 3 determinations with standard deviations of less than 10% of the mean value.
HTLV-1 Tax transactivates the IL-13 promoter
The ability of Tax to activate the IL-13 promoter was examined by cotransfecting Jurkat T cells with luciferase reporter plasmids controlled by the IL-13 promoter in combination with a Tax expression plasmid (Figure 3). IL-13 reporter plasmids containing promoter sequences 357 bp (pD3397) or 155 bp (pD3402) upstream of the RNA start site were activated approximately 18-fold by Tax (Figure 3B). The IL-13 promoter fragment in pD3403 contains only 67 bp upstream of the RNA start site and was not activated by ectopic expression of Tax (Figure 3B). The region of the IL-13 promoter between -67 and -155 contains NFAT and GATA3 binding sites that are essential for promoter activity in activated T cells32,35,36 and when mutated individually diminished Tax activation by approximately 60% and 80%, respectively (D.D., unpublished observation, June 2003).
HTLV-1 Tax trans-activates the IL-13 promoter in transiently transfected Jurkat T cells. (A) Luciferase reporter plasmids containing the human IL-13 promoter element extending 357 bp (pD3397), 155 bp (pD3402), or 67 bp (pD3403) upstream of the RNA start site are shown. Locations of the promoter-proximal NFAT and GATA3 sites are indicated. (B) Jurkat T cells were cotransfected with the indicated IL-13 promoter reporter plasmids and either a Tax expression plasmid (▪) or an empty expression vector (□). Luciferase activity was determined in cell extracts prepared 48 hours after transfection and is expressed as the percent of the activity obtained in cells transfected with pCMV-luc. The data represent the mean of 5 independent transfection experiments with standard deviations indicated by error bars.
HTLV-1 Tax trans-activates the IL-13 promoter in transiently transfected Jurkat T cells. (A) Luciferase reporter plasmids containing the human IL-13 promoter element extending 357 bp (pD3397), 155 bp (pD3402), or 67 bp (pD3403) upstream of the RNA start site are shown. Locations of the promoter-proximal NFAT and GATA3 sites are indicated. (B) Jurkat T cells were cotransfected with the indicated IL-13 promoter reporter plasmids and either a Tax expression plasmid (▪) or an empty expression vector (□). Luciferase activity was determined in cell extracts prepared 48 hours after transfection and is expressed as the percent of the activity obtained in cells transfected with pCMV-luc. The data represent the mean of 5 independent transfection experiments with standard deviations indicated by error bars.
IL-13 expression in CD4+ T cells from HTLV-1-infected individuals
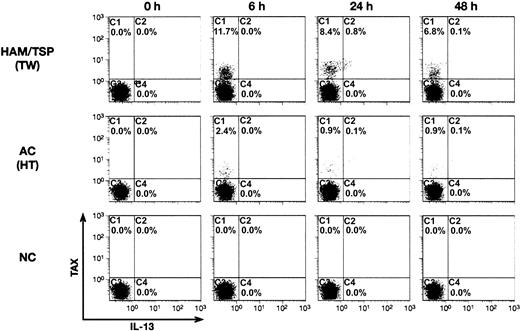
We next asked whether IL-13 expression is activated in T cells from HTLV-1-infected individuals. To this end, PBMCs were collected from one healthy donor, 3 asymptomatic HTLV-1 carriers, and 3 HAM/TSP patients. PBMCs were cultured for 24 hours in medium containing Concanamycin A (to inhibit cytotoxic T-lymphocyte [CTL] activity) and Brefeldin A (to inhibit IL-13 secretion), but not IL-2 or mitogens. Cells were then fixed and stained for cell surface markers with fluorescent dye-conjugated anti-CD4 and anti-CD8 antibodies followed by intracellular staining for Tax and IL-13. Stained cells were analyzed by 4-color flow cytometry; Tax and IL-13 expression were examined in cells gated for CD4. Tax protein was not detected in the CD4+ T cells from the HTLV-negative, healthy donor (Figure 4). In contrast, Tax protein was detected in CD4+ T cells from both HAM/TSP patients and asymptomatic carriers. The relative number of Tax-expressing CD4+ T cells varied from 8.36% to 38.1% among the HAM/TSP patient samples and correlated with provirus load, which ranged from 2.8% to 41.4% (Table 1). Compared with the HAM/TSP samples, CD4+ T cells from asymptomatic carriers had lower provirus loads (1.3% to 1.6%) and fewer Tax-expressing CD4+ T cells (0.44% to 4.24%). IL-13 protein was expressed in HTLV-1-infected CD4+ T cells from both HAM/TSP patients and asymptomatic HTLV-1 carriers (Figure 4; Table 1). IL-13 protein was expressed exclusively in cells that also expressed Tax; in fact, there was a direct correlation between the intensity of staining for Tax and expression of IL-13. This was most apparent in lymphocytes from the HAM/TSP patient designated TBI (Figure 4). The IL-13 signal was observed with 2 different anti-IL-13 mAbs and could be blocked by addition of exogenous IL-13; no IL-13 signal was detected with a matched isotype control mAb (data not shown).
Activation of IL-13 synthesis in CD4+ T cells infected with HTLV-1 in vivo. Blood was drawn and PBMCs were prepared from 3 HAM/TSP patients, 3 asymptomatic carriers (AC), and 1 HTLV-1-negative donor (NC). Cells were cultured for 24 hours in medium containing brefeldin A and concanamycin A but lacking mitogens. Cells were then stained with fluorescent-tagged antibodies directed against cell surface CD4+ or intracellular IL-13 and HTLV-1 Tax proteins and analyzed by flow cytometry. Fluorescence intensities of Tax versus IL-13 are plotted for cells gated as CD4+. Neither Tax nor IL-13 signals were observed using matched isotype control antibodies. Patient designations are shown in the upper-right corner of each quadrant.
Activation of IL-13 synthesis in CD4+ T cells infected with HTLV-1 in vivo. Blood was drawn and PBMCs were prepared from 3 HAM/TSP patients, 3 asymptomatic carriers (AC), and 1 HTLV-1-negative donor (NC). Cells were cultured for 24 hours in medium containing brefeldin A and concanamycin A but lacking mitogens. Cells were then stained with fluorescent-tagged antibodies directed against cell surface CD4+ or intracellular IL-13 and HTLV-1 Tax proteins and analyzed by flow cytometry. Fluorescence intensities of Tax versus IL-13 are plotted for cells gated as CD4+. Neither Tax nor IL-13 signals were observed using matched isotype control antibodies. Patient designations are shown in the upper-right corner of each quadrant.
IL-13 and Tax expression in lymphocytes from HTLV-1-infected donors
. | Percent of PBMCs . | . | . | |
|---|---|---|---|---|
| Patients . | Tax* . | IL-13† . | Proviral load‡ . | |
| HAM/TSP | ||||
| TW § | 8.36 | 0.76 | 2.8 | |
| TBI ∥ | 11.8 | 3.60 | 12.3 | |
| TBL ∥ | 36.8 | 0.30 | 41.4 | |
| AC | ||||
| HBI § | 0.44 | 0.18 | 1.5 | |
| HT § | 0.83 | 0.12 | 1.3 | |
| HN § | 4.24 | 0.12 | 1.6 | |
. | Percent of PBMCs . | . | . | |
|---|---|---|---|---|
| Patients . | Tax* . | IL-13† . | Proviral load‡ . | |
| HAM/TSP | ||||
| TW § | 8.36 | 0.76 | 2.8 | |
| TBI ∥ | 11.8 | 3.60 | 12.3 | |
| TBL ∥ | 36.8 | 0.30 | 41.4 | |
| AC | ||||
| HBI § | 0.44 | 0.18 | 1.5 | |
| HT § | 0.83 | 0.12 | 1.3 | |
| HN § | 4.24 | 0.12 | 1.6 | |
Percentage of Tax-positive cells in CD4 + T lymphocytes.
Percentage of Tax- and IL- 13-positive cells in CD4+ T lymphocytes.
Provirus load is expressed as percent of PBMCs containing HTLV-1 provirus.
Cells were cultivated in the presence of 10 μg/mL Brefeldin A for 6 hours.
Cells were cultivated in the presence of 10 μg/mL Brefeldin A for 24 hours.
Kinetics of Tax and IL-13 expression in ex vivo cultures of CD4+ T cells
It has been reported previously that fresh PBMCs from HTLV-1-infected patients express very low levels of virus, but for unknown reasons virus expression becomes detectable soon after the start of in vitro culture.19 We exploited this phenomenon to examine the kinetics of Tax and IL-13 protein expression in PBMCs from an uninfected donor, an asymptomatic HTLV-1 carrier, and a HAM/TSP patient. Tax and IL-13 expression patterns in CD4+ T cells were determined by intracellular staining and flow cytometry at 6, 24, or 48 hours of in vitro culture (Figure 5). The percentage of total CD4+ T cells did not vary during the time course. Neither Tax protein nor IL-13 was detected in T cells from the healthy donor at any time point. Tax expression was not detected in CD4+ T cells from the HAM/TSP patient or the asymptomatic carrier at the start of in vitro culture (Figure 5, 0 hour) but increased rapidly thereafter, reaching a peak at 6 hours, and then declined. As expected, the number of Tax-expressing cells was greater in the HAM/TSP sample compared with the asymptomatic carrier, but the expression kinetics were similar. These data are in agreement with a previous report that showed that Tax expression reached a maximum at 6 to 12 hours after in vitro culture and then decreased by 50% during the next 12 to 36 hours.19 IL-13 protein expression lagged behind that of Tax and reached a maximum at the 24-hour time point and then decreased rapidly during the next 24 hours (Figure 5). The kinetics of IL-13 expression also indicate the specificity of the IL-13 signal, since a nonspecific signal would be observed at all time points. In summary, the number of IL-13-positive cells was proportional to Tax expression, and IL-13 expression was temporally related to Tax accumulation. The time lapse between peak Tax and IL-13 accumulation could be related to the induction of cellular factors needed to activate the IL-13 promoter.
Kinetics of IL-13 and Tax expression during in vitro culture of PBMCs from HTLV-1-infected individuals. PBMCs prepared from a HAM/TSP patient (TW; top), an HTLV-1-positive asymptomatic carrier (HT; middle), and an HTLV-1-negative healthy control (NC; bottom) were stained and analyzed by flow cytometry as in Figure 4. PBMCs were stained and analyzed immediately after preparation (0 hour) or incubated in medium containing Brefeldin A and Concanamycin A for the time periods indicated at the top of the figure (6, 24, and 48 hours) prior to analysis. Fluorescence intensities of Tax (y-axis) and IL-13 (x-axis) are plotted for CD4+ T cells.
Kinetics of IL-13 and Tax expression during in vitro culture of PBMCs from HTLV-1-infected individuals. PBMCs prepared from a HAM/TSP patient (TW; top), an HTLV-1-positive asymptomatic carrier (HT; middle), and an HTLV-1-negative healthy control (NC; bottom) were stained and analyzed by flow cytometry as in Figure 4. PBMCs were stained and analyzed immediately after preparation (0 hour) or incubated in medium containing Brefeldin A and Concanamycin A for the time periods indicated at the top of the figure (6, 24, and 48 hours) prior to analysis. Fluorescence intensities of Tax (y-axis) and IL-13 (x-axis) are plotted for CD4+ T cells.
Discussion
IL-13 expression was induced in the HTLV-1-infected CD4+ T cells shortly after lymphocytes from infected patients were placed in culture. The percentage of cells expressing HTLV-1 Tax was proportional to provirus load, which was highest in HAM/TSP patients. IL-13 expression correlated with Tax protein levels and was not detected in uninfected cells. HTLV-1 induction of IL-13 synthesis was also observed in 10 independent, chronically infected T-cell lines. This group of HTLV-1-infected cell lines included both CD4 single-positive and CD8 single-positive T cells. Because IL-13 has been shown to elicit a variety of biologic effects on lymphoid and nonlymphoid cells,20 it is tempting to speculate that HTLV-1 has conscripted it to mediate functions necessary for virus propagation or for survival of the infected cell.
The overlap between HTLV-1-associated disease manifestations and the known consequences of IL-13 expression is compelling. For example, recent reports that IL-13 acts as an inhibitor of tumor immunosurveillance23,24 raises the possibility that its expression by HTLV-1-infected T cells could influence the antiviral immune response and could underlie the immunosuppression seen in acute HTLV-1-associated disease.2,4,37,38 HTLV-1-infected individuals often present with pulmonary disease,4,39,40 and IL-13 is known to be a critical factor in the pathophysiology of asthma.25,26 It was recently reported that IL-13-producing T cells were elevated in the relapse phase of multiple sclerosis, a neurodegenerative disease similar to HAM/TSP.41 In that study, HAM/TSP patients did not show changes in the number of IL-13-producing T cells, but this may reflect the shorter in vitro culture period used. It seems unlikely that IL-13 is solely responsible for HTLV-1-associated diseases, but in concert with other cytokines and chemokines produced by HTLV-1-infected cells, it may be an important contributor. The possible therapeutic application of IL-13 antagonists, in the context of HTLV-1-associated diseases, should be contemplated.
Analyses of the HTLV-1-infected patient samples and chronically infected cell lines suggested that Tax is involved in activating the IL-13 promoter. This was supported by the activation of an IL-13 promoter-reporter plasmid by ectopic expression of Tax in transiently transfected Jurkat T cells. Although these studies revealed that the promoter-proximal region containing NFAT and GATA3 binding sites was essential for Tax trans-activation,32,35,36 regulation of the endogenous IL-13 promoter is likely to be more complex. For example, MT2 and HuT-102 cells, which express HTLV-1 Tax, did not express IL-13. It is likely that the IL-13 promoter in MT-2 cells is silenced by DNA methylation, since the IL-13 promoter-reporter plasmid was active in transfected MT-2 cells and endogenous IL-13 mRNA synthesis was induced after treatment of the cells with 5-aza-cytidine (H.-K.C., unpublished observation, May 2002). Further analysis of the role of cellular cofactors and effects of Tax on chromatin structure and DNA methylation at the IL-13 promoter will be very informative.
Although IL-13 was induced in chronically infected cells, other T helper 2 (Th2) cytokines were not coordinately activated. For example, mRNAs for IL-4 and IL-10 were not generally detected in cells that produced IL-13. Although the IL-5 promoter shares transcription control elements with the IL-13 promoter,32,35,36 the former gene was activated in only 60% of the cell lines that produced IL-13. Although the IL-13 gene is located on human chromosome 5 in a cluster with IL-3, IL-4, IL-5, and GM-CSF genes, IL-4 and IL-5 were not coordinately activated by HTLV-1. IL-9 and IFN-γ were consistently activated in the HTLV-1-infected cell lines, in agreement with previous studies.19,42,43 Although a number of cytokines have been reported to be activated by HTLV-1 infection or Tax expression, we observed a rather limited and specific pattern of cytokine gene activation in HTLV-1-infected cell lines. We suggest that Tax-mediated activation of IL-13 expression is specific and biologically relevant.
IL-13 may act as an autocrine activator of leukemic cell proliferation in Hodgkin lymphoma, since the Reed-Sternberg cells express the IL-13 receptor and secrete IL-13 protein.44-46 Although the IL-13 receptor, a heterodimer composed of IL-13Rα1 and IL-4Rα chains, is not normally expressed in T cells,47 the possibility existed that it was induced in HTLV-1-infected T cells. RNase protection analysis revealed that the HTLV-1-infected T cells that expressed IL-13 mRNA (Figure 1) did not express detectable amounts of IL-13Rα1 mRNA; they did, however, express IL-4Rα mRNA (H.-K.C., unpublished observation, April 2002). It was reported that IL-13 treatment of CD4+ T cells and T-cell lines resulted in the phosphorylation of signal transducer and activator of transcription 6 (STAT6).48,49 This paradoxical observation suggested that the HTLV-1-infected cell lines that produce IL-13 might exhibit constitutive STAT6 phosphorylation by an autocrine mechanism independent of IL-13Rα. We observed that STAT6 was neither constitutively activated nor was it responsive to exogenous IL-13 treatment in the HTLV-1-infected T-cell lines, MS-9 and MS-68 (D.D. and G.H., unpublished observation, December 2002). However, STAT6 was activated in these cells in response to IL-4 treatment. Therefore, it is unlikely that IL-13 acts as an autocrine activator of HTLV-1-infected T cells or that IL-13-producing cells are selected during the course of establishing the chronically infected T-cell lines.
It will be of interest in the future to define the biologic functions of IL-13 with respect to HTLV-1 infection and to explore the relationship between IL-13 expression and the course and severity of HTLV-1-associated diseases.
Prepublished online as Blood First Edition Paper, August 14, 2003; DOI 10.1182/blood-2003-04-1043.
Supported by the Wellcome Trust (United Kingdom) (C.R.M.B., G.P.T., and P.K.C.G.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We would like to thank Michael Sanford for assistance with RPAs and Richard Frederickson for graphics.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal