Abstract
Natural killer (NK) cells are composed of subsets characterized by the expression of inhibitory or activating receptors, or both, specific for different major histocompatibility complex (MHC) class I determinants. We have previously shown that inhibitory receptor blockade of syngeneic NK cells was an effective means of ex vivo purging of leukemia-contaminated bone marrow and that the transplantation of mice with the purged bone marrow cells (BMCs) resulted in long-term, relapse-free survival. We have extended the investigation to assess the antitumor effects mediated by NK cells H2-allogeneic to tumor cells. We demonstrate that various tumor cell lines are more susceptible to lysis by H2-allogeneic NK cells than by syngeneic NK cells in vitro even though comparable percentages of Ly49 NK cells were present. Using allogeneic NK cells to purge leukemia-contaminating BMCs before transplantation resulted in a higher proportion of mice with long-term survival than using syngeneic NK cells. Allogeneic NK cells did not suppress hematopoietic reconstitution as measured by granulocyte/monocyte-colony-forming unit (CFU-GM), complete blood count (CBC), and donor chimerism at various days after transplantation. Inhibitory receptor blockade of allogeneic NK cells also significantly increased these antitumor effects at lower NK/tumor ratios compared with those of syngeneic NK cells. These results demonstrate that H2-allogeneic NK cells mediate more potent antitumor effects than syngeneic NK cells without adverse hematologic effects and thus may be useful in cancer therapy. (Blood. 2003;102:4067-4075)
Introduction
Natural killer (NK) cells constitute a subset of lymphocytes, which play an important role in the first line of immune defense. In the past couple of decades, NK-cell biology has been more defined, and many studies have demonstrated the important role that major histocompatibility complex (MHC) class I plays in NK-cell development and functions.1-5 Studies examining the role of MHC class I in NK-cell-mediated cytotoxicity against tumor targets and lymphoblasts have shown that the targets lacking MHC class I expression or bearing MHC class I molecules that are “non-self” to NK cells are more susceptible to NK-cell-mediated lysis than the targets expressing self class I.6-10 These data support the missing-self hypothesis, which postulates that NK cells search for self-MHC class I and that the failure of finding self-MHC class I results in the failure of inactivation of NK cells and thus target cell lysis.11 Additional studies have identified inhibitory and activating receptors that characterize NK-cell subsets and are specific for MHC class I determinants. In mice, the inhibitory and activating receptors belong to C-type lectin families and include Ly49 and CD94/NKG2 receptors specific for classical and nonclassical MHC class I, respectively.12-16 Human counterparts are composed of killer immunoglobulin-like receptors (KIRs) belonging to immunoglobulin superfamily and CD94/NKG2 receptors.17-20 Biochemical and functional studies have demonstrated that cross-linking of inhibitory receptors on human and mouse NK-cell subsets by their specific ligands results in the transduction of inhibitory signals through the binding of tyrosine phosphatases by immunoreceptor tyrosine-based inhibitory motifs (ITIMs) and the inactivation of NK-cell functions.21-23 Thus, interrupting the binding of specific MHC class I ligand by inhibitory receptors without eliminating the subsets expressing the receptors using F(ab′)2 fragments of antibodies to the receptors is one way to minimize inactivating the NK-cell subset, and thus increasing NK activity.24,25
The increase in NK-cell activity can also be achieved by cross-linking activating receptors.26-30 Ly49- and KIR-activating receptors in mice and humans, respectively, associate with an adaptor protein, DAP-12, which contains the immunoreceptor tyrosine-based activation motif (ITAM), through which an activation signal is induced with the binding of MHC class I ligand by the activating receptors.31-33 More recently, the NKG2D-activating receptor has been described. It associates with the DAP-10 adaptor protein,34,35 and the activation signal is induced through the interaction of MHC class I-related molecules: Rae-1 and H60 in mice,36 and MICA/MICB and ULBP in humans.37,38 It has been shown that though these ligands are not expressed on normal cells studied to date, their expression can be up-regulated on tumor cells and virally infected cells or induced by stress.38-41 Furthermore, binding of inducible ligands by NKG2D activating receptors increases NK-cell activity against the target cells even though they express self-MHC class I at a normal level.39,42
Because of the ability of NK cells to mediate cytotoxic effects against virally infected cells and some tumor targets without prior sensitization and to produce multiple cytokines, which can affect antitumor effects and other immune functions, NK cells have been exploited in various ways to improve the effectiveness of cancer therapy.43-45 Bone marrow transplantation (BMT) and stem cell transplantation are among the treatments used for neoplastic and nonneoplastic diseases; however, several serious obstacles, most of which are fatal, hamper their efficacy. One of the obstacles to be resolved is relapse from the original tumor contaminating the bone marrow when autologous BMT is used as cancer therapy.46,47 Different physical, clinical, and immunologic means have been used to purge the contaminating tumor cells ex vivo, including the use of activated autologous NK cells to overcome relapse.48-52
In our previous report investigating the possibility of syngeneic NK cells as purging agents in a murine leukemia model, we demonstrated that blocking inhibitory receptors specific for class I on a subset of syngeneic NK cells during ex vivo purging of C1498 murine leukemia contaminating the bone marrow graft significantly increased the extent of tumor cell elimination by syngeneic NK cells.53 We also demonstrated that transplanting tumor-contaminating BMCs purged with syngeneic NK cells with inhibitory receptor blockade resulted in an increased proportion of long-term survivors without affecting hematopoietic cell recovery.53 To determine the extent of antitumor effects mediated by allogeneic NK cells in vitro, we compared cytotoxicity by allogeneic and syngeneic NK cells against that of various tumor targets. In addition, we used a mixture of BMCs and C1498 murine leukemia cells cultured with syngeneic or allogeneic NK cells in a syngeneic BMT model to investigate the effects of ex vivo purging of tumor-contaminated BMCs by allogeneic NK cells in vivo. In this report, we demonstrate that allogeneic NK cells, with and without the inhibitory receptor blockade, mediate more powerful antitumor effects than syngeneic NK cells against tumor in vitro and in vivo without suppressive alloreactivity against BMC.
Materials and methods
Mice
C57BL/6 (B6, H2b), C57BL/10 (B10, H2b), C.B-17 severe combined immunodeficient scid/scid (SCID, H2d), and B6-Ly5.2 congenic mice were obtained from the Animal Production Area (National Cancer Institute, Frederick, MD [NCI-Frederick]). B10.D2 (H2d) congenic mice were purchased from Jackson Laboratory (Bar Harbor, ME). Breeding pairs of B6 SCID (H2b) mice were generously provided by Dr Robert H. Wiltrout (NCI-Frederick), and mice were bred in the NCI-Frederick animal facility. All mice were kept under specific pathogen-free conditions and were used at 8 to 14 weeks of age.
Antibodies
Fluorescein isothiocyanate (FITC)-antimouse CD45.1 and CD45.2 and phycoerythrin (PE)-antimouse CD45.1 antibodies were purchased from PharMingen (San Diego, CA). F(ab′)2 fragments of normal mouse immunoglobulin (IgG) were purchased from Jackson ImmunoResearch (West Grove, PA). F(ab′)2 fragments of anti-Ly49C/I (5E6, mouse IgG2a) and anti-Ly49G2 (4D11, rat IgG2a) monoclonal antibodies (mAbs) were prepared as previously described.24 The purity of F(ab′)2 fragments was determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).24
Cell lines
C1498 (H2b) murine leukemia, EL4 (H2b) murine T-cell lymphoma, and P815 (H2d) murine mastocytoma were obtained from American Type Culture Collection (Rockville, MD), and murine renal carcinoma cell line (RENCA, H2d) was generously provided by Dr Robert Wiltrout (NCI-Frederick). For in vitro experiments using C1498, frozen stocks were thawed every 2 months. For in vivo experiments using C1498, frozen stocks were thawed 7 to 14 days before in vivo administration and were kept in the log phase of growth until use; this growth condition was critical for consistent 100% lethality at the dose used in vivo. Minimum lethal dose (MLD) was 1 × 104 and 1 × 105 in lethally irradiated54 and immunocompetent mice,55 respectively.
Adherent lymphokine-activated killer and SCID NK-cell cultures
To generate adherent lymphokine-activated killer (ALAK) cells, splenocytes and BMCs from B6, BALB/c, B10, and B10.D2 mice were depleted of B cells using nylon-wool columns followed by T-cell depletion using anti-Thy1.2 mAb (30H12) and rabbit-complement lysis. The cells were cultured in NK-cell medium (RPMI 1640 supplemented with 10% fetal bovine serum [FBS; Gemini Bio-Products, Woodland, CA], 100 U/mL penicillin/streptomycin, 2 mM L-glutamine, 10 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], 1 mM nonessential amino acids, 1 mM sodium pyruvate, 2.5 × 10-5 M 2-mercaptoethanol [ME], and 1 μg/mL indomethacin [Sigma, St Louis, MO]) containing 5000 IU/mL recombinant human interleukin-2 (rhIL-2; Developmental Therapeutics Program, NCI-Frederick) at 1 to 2 × 106 cells/mL for 5 to 7 days at 37°C, 5% CO2. At day 3 or 4, nonadherent cells were transferred to new flasks, and all cells were fed with 50% conditioned medium, 50% new NK-cell medium, and rhIL-2. Only the adherent cells (ALAK) were used for experiments. They were composed of more than 90% NK1.1+CD3-, 3% to 5% NK1.1+CD3+, and less than 1% NK1.1-CD3+ cells. In some experiments, splenocytes and BMCs from B6 SCID or C.B-17 SCID mice were cultured in NK-cell medium containing rhIL-2 (5000 IU/mL) at 0.5 × 106 cells/mL for splenocytes or 1 × 106 cells/mL for BM cells for 5 to 7 days. IL-2-activated splenocytes and BMCs from B6 SCID mice were more than 97% NK1.1+.
Cytotoxicity and tumor-colony assays
C1498, EL4, and RENCA cells were labeled with 150 μCi (5.55 MBq) sodium chromate (51Cr; Amersham, Piscataway, NJ) per sample for 60 to 90 minutes. Two thousand labeled cells were incubated with activated NK cells in 96-well, round-bottomed plates in triplicate at different effector-to-target (E/T) ratios for 4 hours. Cytotoxicity level was determined by counting the radioactivity released in culture media collected at 100 μL/well on a TriLux scintillation counter (Perkin Elmer, Boston, MA). For tumor-colony assays, P815 tumor cells were cultured with IL-2-activated NK cells from B6 SCID or C.B-17 SCID mice in the presence of 2500 IU/mL rhIL-2 for 20 to 24 hours, after which the tumor cells were cultured for another 5 days in colony assay medium (Iscove modified Dulbecco medium [IMDM] containing 15% FBS, 100 U/mL penicillin/streptomycin, 2 mM l-glutamine, 5 × 10-5 M 2-ME, and 1.1% methylcellulose [wt/vol]) at 50 cells per 35-mm Petri dish (in triplicate) based on the cell numbers at the initiation of coculture, and tumor colonies were counted at day 5 on a stereo microscope (Nikon, Melville, NY).
NK/tumor cocultures and proliferation assay
B10 and B10.D2 ALAK cells were pretreated with 25 μg/mL F(ab′)2 fragments of normal mouse IgG, 5E6, or 4D11 antibody and then were cultured with 3 × 104 C1498 or P815 cells at an E/T ratio of 10:1 or 50:1, respectively, in the presence of 2500 IU/mL rhIL-2 in 48-well plates for 20 to 24 hours. Cocultured cells were washed, resuspended in 10% complete medium (RPMI 1640 supplemented with 10% FBS, 100 U/mL penicillin/streptomycin, 2 mM l-glutamine, 10 mM HEPES, 1 mM nonessential amino acids, 1 mM sodium pyruvate, and 5 × 10-5 M 2-ME), and replated in 96-well, flat-bottomed plates at 3000, 300, or 30 tumor cells per well in triplicate based on the cell numbers at the initiation of coculture. At various days of culture, cells were pulsed with 1 μCi (0.037 MBq) [3H]-thymidine per well overnight and harvested onto glass fiber filtermats. The level of [3H]-thymidine incorporation was determined by using a TriLux scintillation counter.
Ex vivo purging of tumor and survival studies
For in vivo studies, 3.6 × 107 BMCs from B6 mice and 3.6 × 105 C1498 cells were cultured with B10 or B10.D2 ALAK cells at an ALAK/BMC/tumor ratio of 100:100:1 in IMDM supplemented with 10% FBS, 5 × 10-5 M 2-ME, and 100 U/mL penicillin/streptomycin (10% IMDM) with 5000 IU/mL rhIL-2 for 24 hours. Cocultured cells were harvested and injected into B6 recipients irradiated with a single exposure of irradiation from a cesium Cs 137 source (2.12 Gy/min) at 8 Gy (10 mice per group per experiment) at 3 × 106 BMCs, 3 × 106 NK cells, and 3 × 104 C1498 cells cells per mouse (intravenously) based on the cell numbers at the initiation of cocultures. Additionally, aliquots of the cocultured cells were transferred into 10% IMDM for proliferation assays as described in “NK/tumor cocultures and proliferation assay,” except for using 2 × 103 C1498 cells per well based on the cell number at the initiation of the cocultures. For in vivo experiments investigating the effects of inhibitory receptor blockade of allogeneic ALAK cells, 3 × 105 to 7.5 × 105 B10.D2 ALAK cells were pretreated with 250 μg normal mouse IgG, 5E6, or 4D11 F(ab′)2 for 2 hours in the presence of 5000 IU/mL rhIL-2. ALAK cells were cultured further with B6 BMCs contaminated with C1498 cells (ALAK/BMC, 10:100 or 25:100; ALAK/C1498, 10:1 or 25:1; final concentration of antibody at 25 μg/mL) for 24 hours. Irradiated B6 recipients (8-10 mice per group) were then injected with the cocultured cells as described above. Mice were monitored for survival.
Hematopoietic reconstitution studies
For experiments assessing the effects of allogeneic ALAK cells on BMC, 5 to 6 × 107 B10 or B10.D2 ALAK cells were cultured with BMCs prepared from B6-Ly5.2 mice at a BMC/ALAK ratio of 1:1 in 10% IMDM in the presence of 5000 IU/mL rhIL-2 for 24 or 48 hours. Cocultured cells were harvested and resuspended in Dulbecco phosphate-buffered saline (DPBS) and injected into B6 recipients (15 mice per group) that were irradiated at 8 Gy at 3 × 106 BMCs and 3 × 106 NK cells per mouse (intravenously) based on cell numbers at the initiation of the coculture. At various days after BMT, splenocytes, BMCs, and peripheral blood samples were collected from 3 mice per group. Then 5 × 105 splenocytes and 5 × 104 BMCs were cultured in colony-assay media containing 10 ng/mL recombinant murine granulocyte-macrophage colony-stimulating factor (rmGM-CSF) (Amgen, Thousand Oaks, CA) and 10 ng/mL rmIL-3 (Developmental Therapeutics Program, NCI-Frederick) in 35-mm Petri dish in triplicate in a humidified atmosphere at 37°C with 5% CO2. At day 7, monocyte/granulocyte colonies were enumerated on a stereomicroscope. Peripheral blood samples were collected in EDTA (ethylenediaminetetraacetic acid)-treated Microtainer tubes (BD-Pharmingen, San Diego, CA), and CBCs were determined by analyzing the blood samples on a Hemavet Multispecies Hematology Analyzer (CDC Technologies, Oxford, CT). The level of donor chimerism was determined at day 29 or 90 by staining splenocytes and BMCs with PE-CD45.1 and FITC-CD45.2 and analyzing on FACScan (Becton Dickinson, San Jose, CA).
Statistics
Unpaired t test with Welch correction was used for in vitro assays, and log-rank test was used for survival studies.
Results
Tumor cells are more susceptible to cytotoxicity mediated by allogeneic than syngeneic NK cells in vitro
It is possible that allogeneic NK cells mediate increased cytotoxicity against various tumors because of the lack of self-MHC class I on the tumor cells. However, in the heterogeneous population of allogeneic NK cells, there may be a subset(s) bearing the inhibitory receptors specific for MHC class I expressed by the tumor cells, and thus a clear difference between cytotoxicity mediated by syngeneic and allogeneic NK cells against the same tumor target may not be observed. To determine whether NK cells allogeneic to various tumor targets mediate more cytotoxicity than syngeneic NK cells, activated NK cells were generated by culturing splenocytes and BMCs—depleted of T cells, B cells, and monocytes—from B6 (H2b), BALB/c (H2d), B10 (H2b), and B10.D2 (H2d) mice in rhIL-2 for 5 to 7 days. The adherent population of lymphokine-activated killer cells from such cultures was essentially NK cells (more than 90% NK1.1+CD3-, 3%-5% NK1.1+CD3+, and less than 1% NK1.1-CD3+); thus the term ALAK is used throughout this report to refer to activated NK cells from immunocompetent mice. Tumor cells with variable degrees of sensitivity to NK-cell-mediated lysis were tested in a 4-hour 51Cr-release assay. When EL4 (H2b) and RENCA (H2d) cells are exposed to ALAK cells derived from B6 or BALB/c mice in 4-hour cytotoxicity assays, ALAK cells allogeneic to the tumors mediated significantly more cytotoxicity than that mediated by syngeneic ALAK cells (Figure 1A). When ALAK cells from B10 or B10.D2 were used against C1498 (H2b), B10.D2 ALAK cells allogeneic to C1498 killed the tumor cells to a greater extent than H2-matched B10 ALAK cells (Figure 1B), indicating that the increased killing by allogeneic NK cells is not restricted to particular strains of mice. On the other hand, when NK cells from B6 SCID or C.B-17 (H2d) SCID mice were used as effectors against NK-resistant P815 (H2d) cells, no killing could be observed either by H2-matched or allogeneic SCID NK cells at effector-to-target (E/T) ratios up to 50:1 in a 4-hour assay (data not shown). However, when the E/T ratio was increased to 100:1 and the culture period to 24 or 48 hours before tumor colony assays (Figure 2A), allogeneic NK cells inhibited the tumor growth significantly, whereas H2-matched NK cells had no effect (Figure 1C). These results indicate that allogeneic NK cells mediate more effective cytotoxicity than syngeneic NK cells toward various tumor lines in vitro.
NK/ALAK cells allogeneic to the tumor targets mediate cytotoxicity superior to that of syngeneic or H2-matched NK/ALAK cells. IL-2-activated ALAK cells were prepared from B6, BALB/c, B10, and B10.D2 mice, and IL-2-activated NK cells were prepared from B6 SCID or C.B-17 SCID mice, as described in “Materials and methods.” (A-B) 51Cr-labeled RENCA (H2d) or EL4 (H2b) cells were cultured with B6 or BALB/c ALAK cells, and 51Cr-labeled C1498 cells were cultured with B10 or B10.D2 ALAK cells in triplicate for 4 hours, after which the level of cytotoxicity was measured, as described in “Materials and methods.” (C) P815 (H2d) cells were cocultured with C.B-17 SCID or B6 SCID NK cells for 24 hours, and the inhibition of tumor growth was determined by colony assay, as described in “Materials and methods.” Representative data and SEMs from 4 independent experiments are shown. *P < .01 versus no NK and C.B-17 SCID NK groups.
NK/ALAK cells allogeneic to the tumor targets mediate cytotoxicity superior to that of syngeneic or H2-matched NK/ALAK cells. IL-2-activated ALAK cells were prepared from B6, BALB/c, B10, and B10.D2 mice, and IL-2-activated NK cells were prepared from B6 SCID or C.B-17 SCID mice, as described in “Materials and methods.” (A-B) 51Cr-labeled RENCA (H2d) or EL4 (H2b) cells were cultured with B6 or BALB/c ALAK cells, and 51Cr-labeled C1498 cells were cultured with B10 or B10.D2 ALAK cells in triplicate for 4 hours, after which the level of cytotoxicity was measured, as described in “Materials and methods.” (C) P815 (H2d) cells were cocultured with C.B-17 SCID or B6 SCID NK cells for 24 hours, and the inhibition of tumor growth was determined by colony assay, as described in “Materials and methods.” Representative data and SEMs from 4 independent experiments are shown. *P < .01 versus no NK and C.B-17 SCID NK groups.
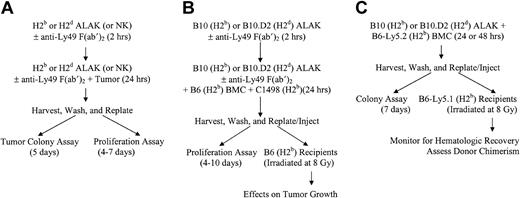
Schematic representations of cocultures, in vitro assays, and BMT. NK or ALAK cells with or without pretreatment with F(ab′)2 fragments of anti-Ly49 antibodies were cultured with C1498 or P815, BMCs contaminated with C1498, or BMCs alone for 24 hours before further use, as described in “Materials and methods.” (A) In vitro colony or proliferation assays. (B) Ex vivo purging protocol for BMCs contaminated with C1498 and BMT. (C) Adaptation of ex vivo purging protocol to examine the effects on BMCs.
Schematic representations of cocultures, in vitro assays, and BMT. NK or ALAK cells with or without pretreatment with F(ab′)2 fragments of anti-Ly49 antibodies were cultured with C1498 or P815, BMCs contaminated with C1498, or BMCs alone for 24 hours before further use, as described in “Materials and methods.” (A) In vitro colony or proliferation assays. (B) Ex vivo purging protocol for BMCs contaminated with C1498 and BMT. (C) Adaptation of ex vivo purging protocol to examine the effects on BMCs.
Allogeneic ALAK cells mediate more potent purging effects against C1498 leukemia than H2-matched ALAK cells
We have previously shown that ex vivo purging of C1498 leukemia with syngeneic NK cells before lethally irradiated recipients underwent BMT resulted in the rescue and long-term survival of some animals.53 Because allogeneic NK cells mediated more cytotoxicity against various tumor cells, including C1498 leukemia, than syngeneic or H2-matched NK cells in vitro, we examined the efficacy of ex vivo purging of C1498 using allogeneic NK cells. BMCs from B6 mice were mixed with C1498 and cultured with H2-matched B10 or allogeneic B10.D2 ALAK cells at an NK/tumor/BMC ratio of 100:1:100 for 24 hours (Figure 2B). A fraction of the cocultured cells was then replated in 96-well microtiter plates to assess the effect of ALAK cells on tumor growth by [3H]-thymidine uptake in vitro, and the remaining cells were injected into irradiated B6 mice at 3 × 104 C1498 (dose based on the cell number at the initiation of coculture) per mouse. The incorporation of [3H]thymidine by proliferating cells showed that C1498 cells cultured with H2-matched B10 ALAK cells began to grow by day 6 in culture and ultimately reached the maximum proliferation rate as the tumor-only control (data not shown) by 10 days after culture (Figure 3A). In contrast, the growth of C1498 cells cultured with allogeneic B10.D2 ALAK cells could not be observed even up to day 10 in culture, demonstrating that allogeneic ALAK cells killed the tumor cells more effectively than H2-matched ALAK cells during purging. Furthermore, when irradiated B6 mice received transplanted BMCs and the C1498 mixture cultured with allogeneic B10.D2 ALAK cells, more than 95% of the animals were protected, which resulted in tumor-free long-term survival (Figure 3B). On the other hand, only 30% to 40% of the animals that underwent transplantation with the BMCs and C1498 mixture cultured with H2-matched B10 ALAK cells did not die (Figure 3B), correlating with tumor growth in vitro. Although there was a small percentage of NK/T cells in the ALAK cell preparations, the purging effect was mediated by NK cells because ALAK cells generated from CD1d knockout mice, which lack NK/T cells,56 could effectively purge C1498 and rescue all mice (data not shown). These results demonstrate that NK cells allogeneic to the tumor mount potent antitumor effects during ex vivo purging, resulting in effective eradication of the tumor to the level at which minimal fatality is observed even in irradiated animals.
Purging of C1498 leukemia with allogeneic B10.D2 ALAK cells ex vivo results in increased inhibition of tumor growth and survival of mice. BMCs (3.6 × 107) from B6 mice and C1498 leukemia cells (3.6 × 105) were cultured with or without B10 or B10.D2 ALAK cells (3.6 × 107) in 10% IMDM containing 5000 IU/mL rhIL-2 in T-25 flasks for 24 hours. (A) A fraction of the cocultured cells was plated back in 96-well, flat-bottomed microtiter plates at 2 × 103 C1498 cells per well based on the cell numbers at the initiation of the coculture in triplicate to assess proliferation using [3H]-thymidine, as described in “Materials and methods.” The [3H]-thymidine incorporation of BMC + C1498 control was 2.1 × 104 cpm at day 5. Results from 1 of 2 representative independent experiments are shown, and the statistical difference between cell growths at various days was determined using the unpaired t test with Welch correction. (B) Cocultured cells were injected into irradiated (8 Gy) B6 recipient mice (10 mice per group per experiment) at 3 × 104 C1498 cells per mouse (intravenously) based on the cell number at the initiation of coculture, and mice were monitored for survival. Pooled data from 2 independent experiments are shown, and the statistical difference between the groups was determined using the log-rank test.
Purging of C1498 leukemia with allogeneic B10.D2 ALAK cells ex vivo results in increased inhibition of tumor growth and survival of mice. BMCs (3.6 × 107) from B6 mice and C1498 leukemia cells (3.6 × 105) were cultured with or without B10 or B10.D2 ALAK cells (3.6 × 107) in 10% IMDM containing 5000 IU/mL rhIL-2 in T-25 flasks for 24 hours. (A) A fraction of the cocultured cells was plated back in 96-well, flat-bottomed microtiter plates at 2 × 103 C1498 cells per well based on the cell numbers at the initiation of the coculture in triplicate to assess proliferation using [3H]-thymidine, as described in “Materials and methods.” The [3H]-thymidine incorporation of BMC + C1498 control was 2.1 × 104 cpm at day 5. Results from 1 of 2 representative independent experiments are shown, and the statistical difference between cell growths at various days was determined using the unpaired t test with Welch correction. (B) Cocultured cells were injected into irradiated (8 Gy) B6 recipient mice (10 mice per group per experiment) at 3 × 104 C1498 cells per mouse (intravenously) based on the cell number at the initiation of coculture, and mice were monitored for survival. Pooled data from 2 independent experiments are shown, and the statistical difference between the groups was determined using the log-rank test.
Allogeneic ALAK cells do not have adverse effects on normal BMCs in 24-hour coculture and do not suppress hematopoietic reconstitution after congenic BMT
Because allogeneic NK cells exhibited such strong antitumor effects against C1498 leukemia contaminating the bone marrow, it was necessary to examine whether NK cells also allogeneic to BMCs exert any alloreactivity to BMCs during purging. Therefore, BMCs from B6-Ly5.2 congenic mice expressing CD45.1, a different isoform of CD45 compared with conventional B6 mice, which express CD45.2, were cultured with B10 or B10.D2 ALAK cells at a BMC/ALAK ratio of 1:1 for 24 hours (Figure 2C). The growth of granulocyte/monocyte progenitors (granulocyte/monocyte colony-forming units [CFU-GMs]) in the BMCs was then determined in vitro by culturing the cells in semisolid media containing recombinant murine (rm) IL-3 and rmGM-CSF for 7 days. Figure 4A shows that allogeneic ALAK cells mediated minimal suppressive effects on granulocyte/monocyte progenitors in 24 hours, as evidenced by a lack of a significant inhibition of growth of CFU-GM. To investigate whether the ability of BMCs exposed to allogeneic NK cells to repopulate was compromised, irradiated B6 recipients received transplanted B6-Ly5.2 BMCs cultured with B10 or B10.D2 ALAK cells for 24 hours. At various days after BMT, different parameters of hematopoietic reconstitution were analyzed (Figure 2C). Results from colony assays using splenocytes and BMCs harvested from recipient mice showed comparable growth of CFU-GM in spleens and bone marrow among the groups of mice that received transplanted BMCs cultured alone or with H2-matched or allogeneic ALAK cells (Figure 4B). These results indicate that allogeneic ALAK cells mediated little or no suppression on myeloid reconstitution in a 24-hour purging. In addition, analysis of the levels of different cellular components in peripheral blood collected at various days after BMT demonstrated that the ability of BMCs for lymphoid or erythroid reconstitution after BMT was not inhibited by allogeneic ALAK cells (Figure 5). When the levels of donor cell engraftment in spleen were examined at day 60 after BMT, comparable numbers of donor-derived cells were observed in all mice regardless of the purging condition in which BMCs were treated before transplantation (Table 1). These results demonstrate that allogeneic NK cells do not mediate deleterious alloreactivity or suppress normal BMCs under the same culture conditions in which the more powerful antitumor effects are observed. In contrast, in BMCs cultured with allogeneic ALAK cells for 48 hours, CFU-GM growth from the cocultures (P < .05), myeloid reconstitution (P < .01), and donor chimerism (P < .01) were more severely inhibited than those cultured with H2-matched ALAK cells (Figure 6), indicating that purging conditions can influence the extent of adverse effects on hematopoietic cells mediated by allogeneic ALAK cells.
Allogeneic ALAK cells have minimal effects on myeloid reconstitution after BMT. B6-Ly5.2 BMCs (5 × 107) and ALAK cells (5 × 107) from B10 or B10.D2 mice were cultured for 24 hours, as described in “Materials and methods.” (A) A fraction of cocultured cells was cultured in semisolid colony assay media in triplicate for another 7 days, as described in “Materials and methods.” Pooled data from 2 independent experiments are shown. (B) B6 mice were irradiated at 8.5 Gy and underwent transplantation with the cocultured cells at 3 × 106 BMCs per mouse based on the cell numbers at the initiation of coculture, as described in “Materials and methods.” At various days after BMT, spleen and femurs were harvested (3 mice per group per time point), splenocytes (2.5 or 5 × 105) and BMCs (5 × 104) were cultured in semisolid colony assay media in triplicate. Colonies were enumerated on a stereoscope at day 7, as described in “Materials and methods.” Representative data and SEMs from 2 independent experiments are shown.
Allogeneic ALAK cells have minimal effects on myeloid reconstitution after BMT. B6-Ly5.2 BMCs (5 × 107) and ALAK cells (5 × 107) from B10 or B10.D2 mice were cultured for 24 hours, as described in “Materials and methods.” (A) A fraction of cocultured cells was cultured in semisolid colony assay media in triplicate for another 7 days, as described in “Materials and methods.” Pooled data from 2 independent experiments are shown. (B) B6 mice were irradiated at 8.5 Gy and underwent transplantation with the cocultured cells at 3 × 106 BMCs per mouse based on the cell numbers at the initiation of coculture, as described in “Materials and methods.” At various days after BMT, spleen and femurs were harvested (3 mice per group per time point), splenocytes (2.5 or 5 × 105) and BMCs (5 × 104) were cultured in semisolid colony assay media in triplicate. Colonies were enumerated on a stereoscope at day 7, as described in “Materials and methods.” Representative data and SEMs from 2 independent experiments are shown.
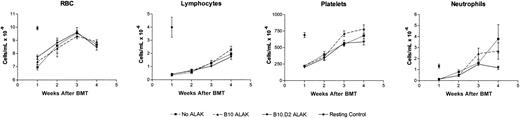
Allogeneic ALAK cells do not exert suppressive effects on lymphoid and erythroid reconstitution after BMT. B6-Ly5.2 BMCs (5 × 107) and ALAK cells (5 × 107) from B10 or B10.D2 mice were cultured for 24 hours, after which the cells were transplanted into irradiated B6 recipients (8.5 Gy) at 3 × 106 BMCs per mouse (intravenously) based on the cell numbers at the initiation of coculture, as described in “Materials and methods.” At various days after BMT, peripheral blood samples were collected from 3 mice per group, and CBCs were measured, as described in “Materials and methods.” Pooled data and SEMs from 2 independent experiments are shown. RBC indicates red blood cell.
Allogeneic ALAK cells do not exert suppressive effects on lymphoid and erythroid reconstitution after BMT. B6-Ly5.2 BMCs (5 × 107) and ALAK cells (5 × 107) from B10 or B10.D2 mice were cultured for 24 hours, after which the cells were transplanted into irradiated B6 recipients (8.5 Gy) at 3 × 106 BMCs per mouse (intravenously) based on the cell numbers at the initiation of coculture, as described in “Materials and methods.” At various days after BMT, peripheral blood samples were collected from 3 mice per group, and CBCs were measured, as described in “Materials and methods.” Pooled data and SEMs from 2 independent experiments are shown. RBC indicates red blood cell.
Effects of allogeneic ALAK cells on reconstitution of B6 recipients with donor-derived cells in spleen
Treatment* . | ALAK/BMC ratio . | No. mice . | Cellularity, × 106 . | Donor chimerism, %† . |
|---|---|---|---|---|
| No ALAK | 0 | 5 | 80.6 ± 16.7 | 79.5 ± 5.2 |
| B10 ALAK | 1:1 | 5 | 82.0 ± 15.1 | 80.9 ± 3.5 |
| B10.D2 ALAK | 1:1 | 6 | 86.2 ± 11.9 | 77.3 ± 5.6 |
Treatment* . | ALAK/BMC ratio . | No. mice . | Cellularity, × 106 . | Donor chimerism, %† . |
|---|---|---|---|---|
| No ALAK | 0 | 5 | 80.6 ± 16.7 | 79.5 ± 5.2 |
| B10 ALAK | 1:1 | 5 | 82.0 ± 15.1 | 80.9 ± 3.5 |
| B10.D2 ALAK | 1:1 | 6 | 86.2 ± 11.9 | 77.3 ± 5.6 |
Values represent mean ± SD.
BMCs from B6-Ly5.2 donors were cultured alone or in the presence of H2-matched or allogeneic ALAK cells for 24 hours before injection into irradiated (8.5 Gy) B6 recipients.
At day 60 after BMT, splenocytes from mice were double stained with FITC-antimouse CD45.2 (Ly5.1, host) and PE-anti-CD45.1 (Ly5.2, donor) mAb and were analyzed on FACScan. Percentages of CD45.1+ cells were obtained by gating on live cells.
Allogeneic ALAK cells can exert suppressive effects on myeloid reconstitution and donor chimerism after BMT. B6-Ly5.2 BMC (5 × 107) and ALAK cells (5 × 107) from B10 or B10.D2 mice were cultured for 48 hours, as described in “Materials and methods.” (A) A fraction of cocultured cells was cultured in semisolid colony assay media in triplicate for another 7 days, as described in “Materials and methods.” (B) B6 mice were irradiated at 8.5 Gy and underwent transplantation with the cocultured cells at 3 × 106 BMCs per mouse based on the cell numbers at the initiation of coculture, and the level of myeloid reconstitution was determined, as described in “Materials and methods.” (C) At day 29 after BMT, splenocytes were stained for CD45.1 expression, as described in “Materials and methods” to determine the level of donor chimerism. Statistical differences between treatment groups were determined by the unpaired t test with Welch correction. *Significant difference compared with the BMC-only control group. **Significant difference compared with H2-matched ALAK cells.
Allogeneic ALAK cells can exert suppressive effects on myeloid reconstitution and donor chimerism after BMT. B6-Ly5.2 BMC (5 × 107) and ALAK cells (5 × 107) from B10 or B10.D2 mice were cultured for 48 hours, as described in “Materials and methods.” (A) A fraction of cocultured cells was cultured in semisolid colony assay media in triplicate for another 7 days, as described in “Materials and methods.” (B) B6 mice were irradiated at 8.5 Gy and underwent transplantation with the cocultured cells at 3 × 106 BMCs per mouse based on the cell numbers at the initiation of coculture, and the level of myeloid reconstitution was determined, as described in “Materials and methods.” (C) At day 29 after BMT, splenocytes were stained for CD45.1 expression, as described in “Materials and methods” to determine the level of donor chimerism. Statistical differences between treatment groups were determined by the unpaired t test with Welch correction. *Significant difference compared with the BMC-only control group. **Significant difference compared with H2-matched ALAK cells.
Blockade of inhibitory receptors on allogeneic NK cells during ex vivo purging augments antitumor effects
Although allogeneic NK cells mediate significant antitumor effects against C1498 without any significant alloreactivity to normal BMCs under this ex vivo purging condition, tumor cells that are more aggressive and resistant to NK cells or are of large tumor burden may require altered or prolonged purging conditions. We have previously demonstrated that blocking inhibitory receptors on syngeneic NK cells increases antitumor effects without significantly inhibiting BMCs.53 Thus, we first examined whether the blockade of inhibitory receptors on allogeneic NK cells would result in increased antitumor effects in vitro. B10 and B10.D2 ALAK cells express comparable levels of Ly49C/I and Ly49G2 inhibitory receptors9 through which inhibitory signals are induced on interacting with H2Db and H2Kb or H2Dd, respectively.57 B10 and B10.D2 ALAK cells were pretreated with F(ab′)2 fragments of normal mouse IgG, anti-Ly49C/I (5E6), or anti-Ly49G2 (4D11) antibodies for 2 hours before coculture with C1498 (E/T ratio, 10:1) or P815 (H2d; E/T ratio, 50:1) tumor cells for 20 to 24 hours (Figure 2A). The cocultured cells were then transferred to 96-well microtiter plates and incubated for various numbers of days to determine the level of cell proliferation. Results showed that blocking Ly49C/I on B10 ALAK cells using 5E6 F(ab′)2 decreased C1498 growth, whereas F(ab′)2 fragments of normal mouse IgG and 4D11 control antibodies had no effect on tumor growth as observed earlier (Figure 7A; P < .005). Similarly, blocking Ly49C/I on B10.D2 ALAK cells, allogeneic to C1498, significantly inhibited tumor growth, and this inhibition was more effective than that observed with inhibitory receptor blockade on H2-matched ALAK cells (Figure 7A; P = .0026). Similar results were obtained using P815 cells (H2d) as targets in that blocking the Ly49G2 inhibitory receptor specific for H2Dd using 4D11 F(ab′)2 increased the antitumor effects mediated by H2-matched B10.D2 and allogeneic B10 ALAK cells compared with ALAK cells treated with normal mouse IgG or 5E6 F(ab′)2 control antibodies (P < .05). The antitumor effects mediated by allogeneic B10 ALAK cells on the blockade of Ly49G2 inhibitory receptor were significantly more efficient than those mediated by H2-matched B10.D2 ALAK cells with inhibitory receptor blockade (Figure 7B; P = .0031). These results demonstrate that the subset of allogeneic NK cells expressing inhibitory receptors specific for MHC class I on tumor targets is inactivated and that blocking the interaction between the inhibitory receptor and its ligand using F(ab′)2 fragments of the specific antibody augments allogeneic NK-cell-mediated antitumor activity.
Blocking inhibitory receptors on allogeneic ALAK cells augments the inhibition of tumor growth in vitro. B10 or B10.D2 ALAK cells were preincubated with F(ab′)2 fragments of normal mouse IgG, anti-Ly49C/I (5E6), or anti-Ly49G2 (4D11) antibodies for 2 hours. C1498 and P815 tumor cells were cultured with pretreated ALAK cells for 20 to 24 hours, after which cocultured cells were replated in 96-well, flat-bottomed microtiter plates at various numbers of cells per well in triplicate, as described in “Materials and methods.” The level of cell proliferation was determined using [3H]-thymidine at 1 μCi/well (0.037 MBq), as described in “Materials and methods.” C1498 (A) and P815 (B) cells were plated at 3000 or 30 cells per well, respectively, based on the cell numbers at the initiation of coculture, and cell proliferation was assessed at day 3 in culture. [3H]-thymidine incorporation of C1498- and P815-only control was 1.2 × 105 cpm and 7.2 × 104 cpm, respectively. Representative data from 3 independent experiments are shown, and statistical differences between various antibody treatments were determined by the unpaired t test with Welch correction. *Significant difference compared with control antibodies. **Significant difference compared with H2-matched ALAK cells. Error bars indicate SEM.
Blocking inhibitory receptors on allogeneic ALAK cells augments the inhibition of tumor growth in vitro. B10 or B10.D2 ALAK cells were preincubated with F(ab′)2 fragments of normal mouse IgG, anti-Ly49C/I (5E6), or anti-Ly49G2 (4D11) antibodies for 2 hours. C1498 and P815 tumor cells were cultured with pretreated ALAK cells for 20 to 24 hours, after which cocultured cells were replated in 96-well, flat-bottomed microtiter plates at various numbers of cells per well in triplicate, as described in “Materials and methods.” The level of cell proliferation was determined using [3H]-thymidine at 1 μCi/well (0.037 MBq), as described in “Materials and methods.” C1498 (A) and P815 (B) cells were plated at 3000 or 30 cells per well, respectively, based on the cell numbers at the initiation of coculture, and cell proliferation was assessed at day 3 in culture. [3H]-thymidine incorporation of C1498- and P815-only control was 1.2 × 105 cpm and 7.2 × 104 cpm, respectively. Representative data from 3 independent experiments are shown, and statistical differences between various antibody treatments were determined by the unpaired t test with Welch correction. *Significant difference compared with control antibodies. **Significant difference compared with H2-matched ALAK cells. Error bars indicate SEM.
To determine whether blocking inhibitory receptors on allogeneic NK cells during ex vivo purging has any effect on the survival of animals, B10.D2 ALAK cells were pretreated with normal mouse IgG, 5E6, or 4D11 F(ab′)2 and then were cocultured with C1498-contaminated B6 BMCs at an NK/C1498/BMC ratio of 10:1:100 or 25:1:100 for 24 hours (Figure 2B). The cocultured cells were transferred to irradiated B6 mice, and the animals were monitored for survival from the tumor. Figure 8A shows that at an E/T ratio of 10:1, B10.D2 ALAK cells pretreated with normal mouse IgG or 4D11 F(ab′)2 could not protect mice from the tumor. However, when the number of B10.D2 ALAK cells was increased to an E/T ratio of 25:1, the survival rate was increased and approximately 20% of mice could be rescued with long-term survival compared with control mice receiving transplanted C1498-contaminated BMCs (Figure 8B). More important, ex vivo purging of C1498-contaminated BMCs with B10.D2 ALAK cells in the presence of 5E6 F(ab′)2 significantly increased the proportion of mice with long-term survival to 25% at an E/T ratio of 10:1 and to 45% at an E/T ratio of 25:1 compared with purging in the presence of control antibodies, normal mouse IgG, or 4D11 F(ab′)2. Moreover, when the levels of hematopoietic reconstitution and donor chimerism were analyzed in the mice receiving BMCs exposed to allogeneic NK cells with inhibitory receptor blockade (NK/BMC ratio, 25:100), no significant suppression was observed at various days after BMT (data not shown). These results demonstrate that inhibitory receptor blockade of allogeneic NK cells augments antitumor effects without increasing alloreactivity against hematopoietic cells; thus, allogeneic NK cells with or without inhibitory receptor blockade may be a powerful tool to increase the efficacy of ex vivo purging of tumor before BMT.
Blocking inhibitory receptors on allogeneic ALAK cells during ex vivo purging of C1498 increases the proportion of mice with long-term survival. B10.D2 ALAK cells were preincubated with normal mouse IgG, 5E6, or 4D11 F(ab′)2 fragments for 2 hours and further incubated with BMCs and C1498 leukemia cells at ALAK-to-tumor (E/T) ratios of 10:1 (A) or 25:1 (B) for 20 to 24 hours, as described in “Materials and methods.” B6 recipients (8-10 mice per group per experiment) were irradiated (8 Gy) and underwent transplantation with cocultured cells at 3 × 104 C1498 per mouse (intravenously) based on the cell numbers at the initiation of the cocultures. Pooled data from 2 independent experiments are shown, and the statistical difference between the groups was determined by log-rank test.
Blocking inhibitory receptors on allogeneic ALAK cells during ex vivo purging of C1498 increases the proportion of mice with long-term survival. B10.D2 ALAK cells were preincubated with normal mouse IgG, 5E6, or 4D11 F(ab′)2 fragments for 2 hours and further incubated with BMCs and C1498 leukemia cells at ALAK-to-tumor (E/T) ratios of 10:1 (A) or 25:1 (B) for 20 to 24 hours, as described in “Materials and methods.” B6 recipients (8-10 mice per group per experiment) were irradiated (8 Gy) and underwent transplantation with cocultured cells at 3 × 104 C1498 per mouse (intravenously) based on the cell numbers at the initiation of the cocultures. Pooled data from 2 independent experiments are shown, and the statistical difference between the groups was determined by log-rank test.
Discussion
A high rate of relapse of the original tumor resulting from a small fraction of tumor cells contaminating the bone marrow graft is one of the factors limiting the efficacy of autologous BMT as a therapy for cancer.46,47 Because of the ability of NK cells to kill some tumor cells without prior sensitization, a few studies explored the possibility of using activated autologous LAK cells, which include NK cells, as an ex vivo purging agent and have shown that autologous LAK cells can be used as a purging agent to reduce the tumor burden to improve the efficacy of autologous BMT.58,59 In addition, we have reported that in mice, blocking inhibitory receptors on syngeneic NK cells specific for self-MHC class I augments NK-cell-mediated antitumor effects during ex vivo purging without autoreactivity and results in tumor-free, long-term survival under carefully optimized conditions.53 In this study, we have examined the antitumor activity mediated by NK cells allogeneic to the tumor cells and show that allogeneic NK cells mediate superior antitumor effects compared with syngeneic NK cells during ex vivo purging of the tumor.
Results from a 4-hour cytotoxicity assay and a tumor-colony assay using various tumor cell lines show that NK cells allogeneic to the tumor target mediate superior killing compared with syngeneic or H2-matched NK cells. Differences in the length of the coculture with NK cells and the means to quantitate superior killing by allogeneic NK cells suggest that various tumor cells may be sensitive to different pathways of cytotoxicity. C1498 and RENCA cells, which show significant differences in sensitivity to syngeneic compared with allogeneic NK-cell-mediated cytotoxicity in 4 hours, may be more sensitive to perforin- or granzyme-mediated killing.60,61 On the other hand, cytotoxicity against P815 cells, which require a minimum coculture period of 24 hours and an E/T ratio of 50:1 or 100:1 for any level of killing to be observed, may be mediated by other means such as TRAIL and FasL,62-66 which may require longer times for induction. Regardless, the superior antitumor effects mediated by allogeneic NK cells against various tumor cell lines suggest that allogeneic recognition by NK cells can affect different cytotoxic pathways.
We also demonstrate that allogeneic ALAK cells mediate more powerful antitumor effects against C1498 leukemia during ex vivo purging, such that 95% of the animals receiving transplanted BMCs and the C1498 mixture purged with allogeneic ALAK cells remained tumor free compared with 40% of the animals receiving the tumor-contaminated bone marrow graft purged with H2-matched ALAK cells (Figure 3). The high percentage of long-term survivors after BMT of the purged graft into irradiated mice demonstrated successful eradication of the tumor and thus the potency of allogeneic ALAK cells as a purging agent. NK cells from B10 and B10.D2 mice expressed comparable levels of Ly49C/I inhibitory receptors (data not shown); hence, the proportion of B10 and B10.D2 NK cells inactivated by H2Kb and H2Db expressed on C1498 should be equivalent. This suggests that Ly49C/I- NK cells from B10.D2 mice may mount more potent antitumor effects because the tumor cells lack the expression of MHC class I that is self to the NK cells. On the other hand, the cytotoxicity assay against RENCA (H2d) cells shows increased killing by B6 compared with BALB/c ALAK cells (Figure 1), perhaps resulting from the expression of the Ly49D-activating receptor specific for H2Dd and the lack thereof by B6 and BALB/c ALAK cells, respectively.9,67 Interestingly, in spite of comparable levels of Ly49C/I and Ly49G2 expression by B10 and B10.D2 ALAK cells, blocking these inhibitory receptors augments the antitumor effects against C1498 and P815 mediated by allogeneic ALAK cells more significantly than those by H2-matched ALAK cells (Figure 7). Our preliminary data from the cytotoxicity assay against C1498 using the Ly49C/I+ NK-cell subset (enriched to more than 85%) indicated that the cytotoxicity mediated by B10.D2 Ly49C/I+ NK cells is greater than that mediated by the subset from B10 mice (data not shown). This subset of NK cells in B10 and B10.D2 mice expresses equivalent levels of the known activating receptors, Ly49D, Ly49H, and NKG2D (data not shown); the ligand for each receptor is H2Dd,9,67 MCMV peptide,68 and Rae-1 and H60,36,39 respectively, all of which are irrelevant in the model used in this study. Thus, an alternative mechanism by which allogeneic NK cells mediate more potent antitumor effects may be the expression of an activating receptor(s) yet to be defined. Such receptors may recognize specific H2 determinants or tumor-specific antigens. The mechanism involved in tumor cell recognition and antitumor effects mediated by allogeneic NK cells is under investigation.
Allogeneic NK cells, though they mounted potent antitumor effects against C1498, did not affect the ability of BMCs to reconstitute irradiated recipient mice during 24-hour coculture (Figures 4 and 5). However, if the coculture period was increased to 48 hours, severe suppression of myeloid reconstitution and donor chimerism were observed (Figure 6), indicating that allogeneic NK cells mediate alloreactivity against BMCs given a longer period of coculture. The different outcomes of effects on BMCs, depending on the length of coculture with allogeneic NK cells, suggests that the mechanism of alloreactivity toward BMCs mediated by NK cells may be different from that of antitumor effects. Recognition of BMCs by allogeneic NK cells may induce indirect effects, such as higher levels of cytokine production, that suppress hematopoietic cells compared with those induced by syngeneic NK cells. The fact that allogeneic NK cells can deleteriously affect BMCs during ex vivo purging under certain conditions, leading to severe inhibition of hematopoietic reconstitution, underscores the importance of the delicate balance between promoting antitumor effects and minimizing alloreactivity against BMCs.
It has been demonstrated that blocking inhibitory receptors on syngeneic NK cells enhances antitumor effects during ex vivo purging.53 The lack of difference between the inhibition of tumor growth induced by H2-matched and allogeneic NK cells treated with control F(ab′)2 at low E/T ratios (Figure 7) may represent conditions, such as a higher tumor burden or aggressive tumor resistant to killing, in which higher numbers of allogeneic NK cells may be required for appreciable antitumor effects. However, purging with higher numbers of allogeneic NK cells may also result in the suppression of hematopoietic reconstitution after BMT using purged bone marrow graft. In this study, we demonstrate that the inhibitory receptor blockade of allogeneic NK cells significantly augments antitumor effects at low E/T ratios compared with that of H2-matched NK cells, as evidenced by a marked increase in the inhibition of tumor growth after the exposure of tumor cells to allogeneic NK cells with the inhibitory receptor blockade (Figure 7). Increased antitumor effects caused by the inhibitory receptor blockade of allogeneic NK cells is also demonstrated in vivo by the increase in the percentage of long-term survivors among mice that received the transplanted BMCs and C1498 mixture after ex vivo purging using fewer allogeneic NK cells with inhibitory receptor blockade. With the blockade of inhibitory receptors, using as few as one tenth allogeneic NK cells as syngeneic NK cells in ex vivo purging could protect mice with long-term survival (Figure 8),53 indicating that the inhibitory receptor blockade of allogeneic NK cells can be a powerful tool to augment antitumor effects during ex vivo purging without increasing the number of allogeneic NK cells.
Taken together, data presented in this report demonstrate that allogeneic NK cells mediate more potent antitumor effects than syngeneic or H2-matched NK cells and that allogeneic NK cells can be an effective means to purge tumor cells contaminating the bone marrow without mediating suppressive alloreactivity against BMCs. In addition, the data demonstrate that blocking inhibitory receptors on allogeneic NK cells augments antitumor effects during ex vivo purging. Therefore, using allogeneic NK cells with or without inhibitory receptor blockade may be a valuable tool for increasing the efficacy of autologous BMT as a means of cancer therapy.
Prepublished online as Blood First Edition Paper, July 31, 2003; DOI 10.1182/blood-2003-04-1367.
Supported in part by American Cancer Society grants RSG-02-169-01 and R01 CA72669.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Tracy Ulderich for generating NK and ALAK cells, Carol Smith and Steve Stull for their technical assistance with animal studies, and DeMing Zhou for generating F(ab′)2 fragments. We thank Nicole Maddox for secretarial assistance.


![Figure 3. Purging of C1498 leukemia with allogeneic B10.D2 ALAK cells ex vivo results in increased inhibition of tumor growth and survival of mice. BMCs (3.6 × 107) from B6 mice and C1498 leukemia cells (3.6 × 105) were cultured with or without B10 or B10.D2 ALAK cells (3.6 × 107) in 10% IMDM containing 5000 IU/mL rhIL-2 in T-25 flasks for 24 hours. (A) A fraction of the cocultured cells was plated back in 96-well, flat-bottomed microtiter plates at 2 × 103 C1498 cells per well based on the cell numbers at the initiation of the coculture in triplicate to assess proliferation using [3H]-thymidine, as described in “Materials and methods.” The [3H]-thymidine incorporation of BMC + C1498 control was 2.1 × 104 cpm at day 5. Results from 1 of 2 representative independent experiments are shown, and the statistical difference between cell growths at various days was determined using the unpaired t test with Welch correction. (B) Cocultured cells were injected into irradiated (8 Gy) B6 recipient mice (10 mice per group per experiment) at 3 × 104 C1498 cells per mouse (intravenously) based on the cell number at the initiation of coculture, and mice were monitored for survival. Pooled data from 2 independent experiments are shown, and the statistical difference between the groups was determined using the log-rank test.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/12/10.1182_blood-2003-04-1367/6/m_h82335296003.jpeg?Expires=1767779112&Signature=BFcqlnwb6~S9-GeCErzBwAJ4LSsaZ0dj63-obquN5oLBOtQL44FmyzdamqazOipNySIfm7d-apj-9LF2OM5Np9M3KDruno~sVi4iUhCL7xj~JDkdryX8xC07zyCUQRaBPUppDkrSebBCZ3jmTp3eRWWK6UrbURrFtd-6Un2fH7ley-mwY-j7GygtrbTVVlg2OSowbDbp8FV0ynlm5r-GE7ILbCCMQNNUe3FFiI-EawTlqRWGEhLMlZK6xi1gCIchANv1RoGNn9JCj-w8e2H-IqFGga3xOxZJs4jjDrI0IHFolyk0pjK7NfNJIEmLj1VAEXtBg14nv5DcNCN2WNWl4A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Figure 7. Blocking inhibitory receptors on allogeneic ALAK cells augments the inhibition of tumor growth in vitro. B10 or B10.D2 ALAK cells were preincubated with F(ab′)2 fragments of normal mouse IgG, anti-Ly49C/I (5E6), or anti-Ly49G2 (4D11) antibodies for 2 hours. C1498 and P815 tumor cells were cultured with pretreated ALAK cells for 20 to 24 hours, after which cocultured cells were replated in 96-well, flat-bottomed microtiter plates at various numbers of cells per well in triplicate, as described in “Materials and methods.” The level of cell proliferation was determined using [3H]-thymidine at 1 μCi/well (0.037 MBq), as described in “Materials and methods.” C1498 (A) and P815 (B) cells were plated at 3000 or 30 cells per well, respectively, based on the cell numbers at the initiation of coculture, and cell proliferation was assessed at day 3 in culture. [3H]-thymidine incorporation of C1498- and P815-only control was 1.2 × 105 cpm and 7.2 × 104 cpm, respectively. Representative data from 3 independent experiments are shown, and statistical differences between various antibody treatments were determined by the unpaired t test with Welch correction. *Significant difference compared with control antibodies. **Significant difference compared with H2-matched ALAK cells. Error bars indicate SEM.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/12/10.1182_blood-2003-04-1367/6/m_h82335296007.jpeg?Expires=1767779112&Signature=sq3db6SZj1QJDQlNk~VnYRtUO6HHF5W0ofoJeL3NFQA~G-LsJ3cl-LKeWerBu4CcqatfNX4tw7O~j0RQGQY57zU0YgT8HqO148fJxz1xSd9Yr-DnxF~k8kLTIEodfwd5Oy2TVqHiFmqNMYrdl-kEr4tW~601-E9r9Iv8xMkGwQwnYoMRA35UXLaNkzLuG-jpfFTEuXAwrskdI2pyFqhVyR38xI0NPF4FtqWzTYzb4Wl8ak~rYZOig91iNEJKI~uWwOuwcbTVLN4npaemYaTW6NCEQDy1o9FyVjDqhQ1Zi8ast0~os-ffI4aO8Kzx8KDKaQYUYpi0gi0SjxG-O8d~Ig__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal