Abstract
Human T-cell leukemia virus type 1 (HTLV-1) is associated with leukemia/lymphoma and neurologic disorders. Although the viral transcriptional activator Tax is the critical viral oncoprotein, Rex, which regulates the expression of the viral structural and enzymatic genes, is essential for efficient viral replication. Herein, we investigate the contribution of Rex in HTLV-1 immortalization of primary T cells in vitro and viral survival in an infectious rabbit animal model. A Rex-deficient HTLV-1 (HTLVRex-) was constructed and characterized for viral gene expression, protein production, and immortalization capacity. Cells transiently transfected with the HTLVRex- proviral clone produced low detectable levels of p19 Gag. 729HTLVRex- stable transfectants produced functional Tax, but undetectable levels of Rex or p19 Gag. Coculture of irradiated 729HTLVRex- cells with peripheral blood mononuclear cells (PBMCs) resulted in sustained interleukin-2 (IL-2)-dependent growth of primary T lymphocytes. These cells carried the HTLVRex- genome and expressed tax/rex mRNA but produced no detectable Rex or p19 Gag. Rabbits inoculated with irradiated 729HTLVRex- cells or 729HTLVRex- cells transiently transfected with a Rex cDNA expression plasmid failed to become persistently infected or mount a detectable antibody response to the viral gene products. Together, our results provide the first direct evidence that Rex and its function to modulate viral gene expression and virion production is not required for in vitro immortalization by HTLV-1. However, Rex is critical for efficient infection of cells and persistence in vivo.
Introduction
Human T-cell leukemia virus type 1 (HTLV-1) is a pathogenic retrovirus associated with adult T-cell leukemia (ATL) and a variety of immune-mediated disorders including the chronic neurologic disease HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP).1-4 In addition to the structural and enzymatic genes gag, pol, and env, HTLV encodes the Tax and Rex trans regulatory gene products essential for viral replication and several accessory gene products shown to be important for viral persistence in vivo. Tax acts in trans to activate transcription initiating from the viral long terminal repeat (LTR). In addition, Tax modulates the transcription of various cellular genes involved in growth and differentiation and disrupts cell cycle control and DNA repair processes.5-9 Strong evidence suggests that these pleiotropic effects of Tax on cellular processes are required for the transforming or oncogenic capacity of HTLV.10-14
HTLV Rex is a trans-acting regulatory protein required for efficient cytoplasmic expression of the unspliced and incompletely spliced viral RNA transcripts encoding the viral structural and enzymatic proteins.15,16 Rex specifically acts by binding viral mRNAs containing a cis-acting sequence termed the Rex response element (RxRE), located in the R region of the viral LTR.15,17-21 Mutational analyses of HTLV-1 and HTLV-2 Rex have defined several domains within the protein critical for function.22-30 These include the arginine-rich N-terminal RNA binding domain (RBD) that overlaps with a nuclear localization signal (NLS), a leucine-rich activation domain encompassing the nuclear export signal (NES), and 2 regions flanking the NES that are important for Rex-Rex multimerization. In addition, a novel carboxy terminal domain containing key phosphorylation sites important for function has recently been described for HTLV-2 Rex.27 Ultimately, Rex functions to regulate the cytoplasmic level of genomic RNA and expression of the structural and enzymatic gene products that are critical for production of virus progeny. Therefore, it is hypothesized that Rex plays an important role in the transition from the early to the late stage of HTLV infection and is required for efficient spread of the virus. Moreover, the modulation of Rex function also may determine whether a productively infected cell becomes latent. Although Rex is critical for efficient viral replication, one study has shown that low levels of p24 Gag are produced from T cells transfected with a HTLV-2 Rex-deficient provirus.16
It is well established that Tax is critical for cellular immortalization/transformation by HTLV, but the contribution of Rex outside of its replication function in this process is not clear. In this study, we generated and characterized a Rex-deficient HTLV-1 (HTLVRex-) to determine the contribution of Rex in the immortalization/transformation process and in viral replication, spread, and persistence in inoculated rabbits. Our results indicate that cells transiently transfected with the HTLVRex- proviral clone consistently produced approximately 116-fold less p19 Gag than wild-type HTLV-1 (wtHTLV-1), thus indicating that Rex is not an absolute requirement for structural protein expression. We further showed that 729HTLVRex- stable transfectants produced functional Tax, but undetectable levels of Rex or p19 Gag. Coculture of irradiated 729HTLVRex- cells with peripheral blood mononuclear cells (PBMCs) resulted in sustained interleukin-2 (IL-2)-dependent growth of primary T lymphocytes that contain the HTLVRex- provirus. This suggests that Rex is not required for HTLV-1 cell-associated infection and immortalization of T lymphocytes. Rabbits inoculated with irradiated 729HTLVRex- cells and 729HTLVRex- cells transiently transfected with a Rex cDNA expression plasmid failed to become persistently infected or mount a detectable antibody response to the viral gene products. These data indicate that Rex is dispensable for HTLV-1 cellular immortalization of primary human T lymphocytes in vitro, but HTLV-1 absolutely requires Rex for efficient and persistent infection in vivo.
Materials and methods
Cells
293T and 729 cell lines were maintained in Dulbecco modified Eagle medium (DMEM) and Iscove medium, respectively. Medium was supplemented to contain 10% fetal calf serum (FCS), 2 mM glutamine, penicillin (100 U/mL), and streptomycin (100 μg/mL). PBMCs were isolated from blood of healthy donors by centrifugation over Ficoll paque (Pharmacia, Piscataway, NJ). PBMCs were cultured in RPMI 1640 medium supplemented with 20% FCS, 2 mM glutamine, and antibiotics in the presence or absence of 10 U/mL IL-2 (Boehringer Mannheim, Mannheim, Germany).
Plasmids
Site-directed mutagenesis of the wtHTLV-1 proviral clone Ach31 was used to generate the Rex-deficient HTLV-1 proviral clone (HTLVRex-). The N-terminal coding sequence of Rex 5121GCATGCCCAAG5131 (based on Ach proviral sequence) was mutated to GCATGTCCTAG, so that the codon AAG of the third amino acid lysine was mutated to a stop codon TAG, and the SphI restriction enzyme site GCATGC at the beginning of Rex coding sequence was destroyed to facilitate diagnostic analyses. The Rex-1 expression vector (pcRex), containing the HTLV-1 Ach rex cDNA expressed from the cytomegalovirus (CMV) immediate early gene promoter and LTR-1-Luc (firefly) reporter have been previously described.32,33 CMV-Luc (firefly) and CMV-Renilla were used as transfection effi-ciency controls.
Transfection and p19 Gag ELISA
293T cells (2 × 105) were transfected by calcium phosphate procedure with 5 μg of proviral DNA (wtHTLV-1, HTLVRex-, or vector control) and 1 μg CMV-luciferase. After 72 hours of growth, culture supernatants and cells were harvested. Cell lysates were produced and normalized for luciferase activity, and supernatants were assayed for p19 Gag production using a p19 Gag enzyme-linked immunosorbent assay (ELISA) (ZeptoMetrix, Buffalo, NY). All the experiments were performed in triplicate and normalized for transfection efficiency. For stable transfectants, plasmid DNA containing neor gene was introduced into 729 cells by electroporation as previously described.34 Stable transfectants containing the desired proviral clones were isolated following incubation in 24-well culture plates (5 × 105 cells/well) in medium containing 1 mg/mL geneticin (G418). Following a 4 to 5 week selection period, viable cells were expanded in culture for further analysis.
Metabolic labeling and immunoprecipitation
Permanently transfected 729 cell lines were metabolically labeled with [35S] methionine-cysteine (Trans-35S-label; 100 μCi [3.7 MBq]/mL; ICN Biochemicals, Irvine, CA) in methionine-cysteine-free RPMI 1640 medium supplemented with 10% dialyzed FCS. Cells were lysed in radio-immunoprecipitation assay buffer, and lysates were clarified by centrifugation at 100 000g as described previously.35 Clarified extracts were immunoprecipitated with HTLV-1 patient antiserum containing antibody directed primarily against p24 Gag or polyclonal antibody directed against the Rex carboxy terminus in the presence of protein A-sepharose (Pharmacia). Immunoreactive proteins were electrophoresed on a sodium dodecyl sulfate (SDS)-10% polyacrylamide gel and visualized by autoradiography.
DNA preparation and PCR
High molecular weight genomic DNA from permanently transfected 729 cells and immortalized PBMCs was extracted using DNAzol reagent (Gibco BRL, Carlsbad, CA). Three hundred nanograms of DNA was subjected to 35-cycle polymerase chain reaction (PCR) analysis. The primers for amplifying a 90-bp fragment containing the Rex start codon were RexL (GCCAGTGGAAAGGACCACAG nucleotide [nt] 5011-5030) and 39-I (AAGTGGCGAGAAACTTAC nt 5200-5182). PCR-amplified product was separated on 2% agarose gel and visualized by ethidium bromide staining.
Immortalization assays
Immortalization assays were performed as previously described.35 Briefly, 729 stable transfectants (1 × 106) were gamma-irradiated with 10 000 rad and cocultured with 2 × 106 PBMCs in 24-well culture plates in the absence or presence of 10 U/mL human IL-2 (hIL-2). Viable cells were counted once a week by trypan blue exclusion.
Immortalized PBMCs were phenotyped by fluorescence-activated cell-sorter scanner (FACS) analysis at approximately 9 weeks after coculture. Cells were stained with anti-CD3 antibody-fluorescein isothiocyanate (FITC), anti-CD4 antibody-phycoerythrin (PE), and anti-CD8 antibody-PECy5 (PharMingen, San Diego, CA), and analyzed on a Coulter Epics Elite flow cytometer (Beckman Coulter, Miami, FL).
RNA preparation, RT-PCR, and nested PCR
Total RNA was harvested from immortalized PBMCs using Tri reagent as previously described.16 tax/rex doubly spliced mRNA in immortalized cells was detected by nested PCR. Primer RexL and 671 (GAGCCGATAACGCGTCCATCG nt 7493-7472) were used to perform the coupled reverse transcriptase-polymerase chain reaction (RT-PCR) as previously described.16 Three hundred nanograms of total RNA was subjected to 40-cycle amplification. Following RT-PCR, nested PCR was performed for 25 cycles by using an internal set of primers, LA79-I (CCAGTGGATCCCGTGGAGAC nt 5086-5106) and LA78-I (GTCCAAACCCTGGGAAGTGG nt 7321-7302), which results in amplification of a 117-bp tax/rex-specific fragment.
Rabbit inoculation procedures
Twelve-week-old specific pathogen-free New Zealand White rabbits (Hazelton, Kalamazoo, MI) were inoculated via the lateral ear vein with 1 × 107 gamma-irradiated (5000 rad) 729wtHTLV-1, 729HTLVRex-, 729HTLVRex- transiently transfected with a Rex cDNA, or 729 uninfected control (2 rabbits per group). At weeks 1, 2, 3, 4, 6, and 8 after inoculation, 10 mL of blood was drawn from the central ear artery of each animal. Plasma reactivity to specific viral antigenic determinants was detected using a commercial HTLV-1 Western blot assay (ZeptoMetrix) adapted for rabbit plasma by use of avidin-conjugated goat anti-rabbit IgG (1:3000 dilution) (Sigma, St Louis, MO).36 Plasma showing reactivity to Gag (p24 or p19) and Env (gp21 or gp46) antigens was classified as positive for HTLV-1 seroreactivity. Rabbit lymphocytes were collected and ex vivo-cultured for 2 weeks. p19 Gag production was assessed in the cell supernatant by p19 Gag ELISA. To detect integrated proviruses, high molecular weight DNA was isolated, and 300 ng of DNA was subjected to PCR using primers RexDL (GGCAGCTTCACCACTCCCCCCG nt 4905-4927) and 39-I designed to amplify a 295-bp fragment.
Results
Construction and characterization of the HTLVRex- proviral clone
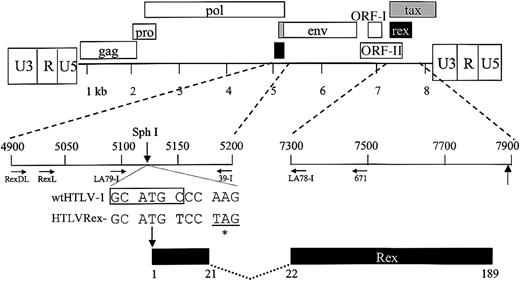
It has been shown that HTLV Rex regulates the expression of the viral structural and enzymatic gene products.15,16 Because Rex modulates the level of specific viral gene products and is critical for efficient viral replication, it has been hypothesized to play an important role in the transition from the early to the late stage of HTLV infection. However, it is unclear whether Rex is absolutely required for virion production and/or if it directly contributes to HTLV-mediated immortalization/transformation of primary human T lymphocytes. In order to determine the potential role of Rex in HTLV-1-mediated cellular immortalization in vitro and viral persistence in vivo, a Rex-deficient proviral clone (HTLVRex-) was generated from the infectious HTLV-1 molecular clone Ach. To construct HTLVRex-, 2 nucleotides surrounding the Rex initiator codon were altered by site-directed mutagenesis. These changes introduced a stop codon at amino acid 3 of the of the rex open reading frame and destroyed a SphI restriction enzyme site that facilitated diagnostic analyses (Figure 1).
Organization of the HTLV-1 genome and expanded Rex coding region. The complete proviral genome is shown schematically. LTRs are depicted with their U3, R, and U5 regions. The location of the gag, pro, pol, env, tax, and rex genes and their corresponding reading frames are indicated along with ORF I and ORF II. Numbers below the genome denote kilobases. The genome containing the 2 rex coding exons has been expanded, and the location of Rex based on the nucleotide sequence of the HTLV-1 proviral clone Ach is presented. The nucleotide sequence around the Rex start site (ATG) for wtHTLV-1 and mutant HTLVRex- proviral clone are indicated. Vertical arrows denote the location of Rex protein start sites (ATG) and the SphI diagnostic restriction enzyme site (CGATGC). Asterisk denotes stop codon (TAG) in HTLVRex-. Location and orientation of oligonucleotide primers used in PCR and RT-PCR are indicated by horizontal arrows. Numbers below the Rex coding region denote amino acid number.
Organization of the HTLV-1 genome and expanded Rex coding region. The complete proviral genome is shown schematically. LTRs are depicted with their U3, R, and U5 regions. The location of the gag, pro, pol, env, tax, and rex genes and their corresponding reading frames are indicated along with ORF I and ORF II. Numbers below the genome denote kilobases. The genome containing the 2 rex coding exons has been expanded, and the location of Rex based on the nucleotide sequence of the HTLV-1 proviral clone Ach is presented. The nucleotide sequence around the Rex start site (ATG) for wtHTLV-1 and mutant HTLVRex- proviral clone are indicated. Vertical arrows denote the location of Rex protein start sites (ATG) and the SphI diagnostic restriction enzyme site (CGATGC). Asterisk denotes stop codon (TAG) in HTLVRex-. Location and orientation of oligonucleotide primers used in PCR and RT-PCR are indicated by horizontal arrows. Numbers below the Rex coding region denote amino acid number.
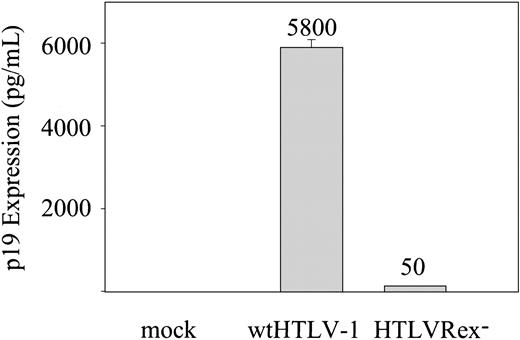
We first assessed the effect that Rex had on p19 Gag production in the supernatant of transfected cells. The concentration of p19 Gag in the culture supernatant is typically used as a measure of virion production. wtHTLV-1 or HTLVRex- proviral clones were transfected into 293T cells and p19 Gag production in the culture supernatant was quantified by ELISA. As expected, cells transfected with wtHTLV-1 clone produced high levels of p19 Gag expression (Figure 2). In contrast, cells transfected with the HTLVRex- clone produced significantly lower but detectable levels of p19 Gag; approximately 116-fold less than wtHTLV-1. These results indicate that Rex is required for efficient p19 Gag production. However, p19 Gag expression is not totally impaired in HTLVRex-, suggesting that virion production and infection could potentially occur, albeit with low efficiency in the absence of Rex.
p19 Gag expression in 293T cells. 2 × 105 293T cells were transfected with 5 μg of wtHTLV-1, HTLVRex-, or expression vector control DNA. At 72 hours after transfection, p19 Gag production was measured in the supernatant by ELISA. The values, which represent p19 Gag levels for 3 independent experiments, are normalized for transfection efficiency. Error bars indicate standard deviations. The data indicate detectable Gag production in the absence of Rex.
p19 Gag expression in 293T cells. 2 × 105 293T cells were transfected with 5 μg of wtHTLV-1, HTLVRex-, or expression vector control DNA. At 72 hours after transfection, p19 Gag production was measured in the supernatant by ELISA. The values, which represent p19 Gag levels for 3 independent experiments, are normalized for transfection efficiency. Error bars indicate standard deviations. The data indicate detectable Gag production in the absence of Rex.
Establishment and characterization of stable provirus-expressing cell lines
To determine the capacity of HTLVRex- proviral clone to synthesize viral proteins, direct viral replication, and induce cellular immortalization/transformation, several permanent 729 B-cell transfectants expressing the wild-type proviral clone and the HTLVRex- proviral clone were isolated and characterized. Each of the stable transfectants contained complete copies of the provirus (data not shown). Diagnostic PCR analyses were performed to determine if the 729HTLVRex- stable cell line DNA contained the provirus with the expected mutations. Figure 3A shows that 729wtHTLV-1, but not 729HTLVRex-, DNA is restricted by SphI, indicating that the mutation introduced at the Rex initiator codon is maintained in the stable transfectant. Nucleotide sequencing of the PCR amplified product further confirmed the expected mutations (data not shown). To monitor the production of Rex protein in these stable transfectants, cells were metabolically labeled with radioactive amino acids and immunoprecipitations were performed with cell lysates. No Rex protein was detected in 729HTLVRex- cells (Figure 3B). To monitor the production of a Rex-dependent protein produced in these stable transfectants, the concentration of p19 Gag in the culture supernatant was quantified by ELISA. In contrast to transient overexpression in 293T cells, 729HTLVRex- cells produced no detectable levels of p19 Gag, whereas 729wtHTLV-1 produced approximately 800 pg/mL of p19 Gag (Table 1). This result is consistent with HTLVRex- p19 Gag production in transiently transfected 293T cells; 116-fold decrease of p19 Gag production in the absence of Rex would be expected to be below the limit of detection of the assay (25 pg/mL). However, this does not rule out low-level virion production or the capacity to transmit the virus by cell-to-cell contact.
729HTLVRex- stable cell line contains the Rex mutation and does not produce detectable Rex. (A) HTLV-1 genome fragment containing the Rex start codon was amplified by PCR from genomic DNA of 729wtHTLV-1 and 729HTLVRex- cells. PCR-amplified product was incubated in the presence (+) or absence (-) of SphI, separated on 2% agarose gel, and visualized by ethidium bromide staining. (B) Rex-1 immunoprecipitation. 729, 729wtHTLV-1, and 729HTLVRex- cells were metabolically labeled with [35S] methionine-cysteine. Cell lysates were immunoprecipitated with polyclonal antibody against the HTLV-1 Rex in the presence of protein A-sepharose. The sizes (in daltons, indicated on the left) were determined by comparison to protein markers.
729HTLVRex- stable cell line contains the Rex mutation and does not produce detectable Rex. (A) HTLV-1 genome fragment containing the Rex start codon was amplified by PCR from genomic DNA of 729wtHTLV-1 and 729HTLVRex- cells. PCR-amplified product was incubated in the presence (+) or absence (-) of SphI, separated on 2% agarose gel, and visualized by ethidium bromide staining. (B) Rex-1 immunoprecipitation. 729, 729wtHTLV-1, and 729HTLVRex- cells were metabolically labeled with [35S] methionine-cysteine. Cell lysates were immunoprecipitated with polyclonal antibody against the HTLV-1 Rex in the presence of protein A-sepharose. The sizes (in daltons, indicated on the left) were determined by comparison to protein markers.
p19 gag production in cell supernatant
Cells . | Gag, pg/mL* . |
|---|---|
| 729wtHTLV-1 | 800 |
| 729HTLVRex— | < 25 |
| 729HTLVRex— + pcRex | 400† |
| 729 | < 25 |
| 729 + pcRex | < 25† |
| PBMC/wtHTLV-1 | 725-1440 |
| PBMC/HTLVRex— | < 25 |
Cells . | Gag, pg/mL* . |
|---|---|
| 729wtHTLV-1 | 800 |
| 729HTLVRex— | < 25 |
| 729HTLVRex— + pcRex | 400† |
| 729 | < 25 |
| 729 + pcRex | < 25† |
| PBMC/wtHTLV-1 | 725-1440 |
| PBMC/HTLVRex— | < 25 |
Culture supernatants of stable transfectants or immortalized PBMCs as indicated were assayed for p19 Gag.
Values less than 25 are below the sensitivity of the assay
5 × 106 729 cells were transfected by electroporation with 5 μg of control vector or the Rex expression vector pcRex (+ pcRex), as described in “Materials and methods.” Culture supernatants were assayed at 96 hours after transfection for Gag protein production by p19 Gag ELISA
We next assessed whether p19 Gag production could be rescued in 729HTLVRex-. Introduction of Rex in trans by transfection of a Rex cDNA expression vector into 729HTLVRex- resulted in significant expression of p19 Gag (Table 1). Taken together, these results indicate that 729HTLVRex- cells contain an intact and viable provirus and, just as importantly, confirms that the mutation in the rex reading frame does not abrogate Tax expression and function, an absolute requirement for efficient viral transcription and cellular immortalization/transformation by HTLV-1.
HTLVRex- immortalizes human PBMCs
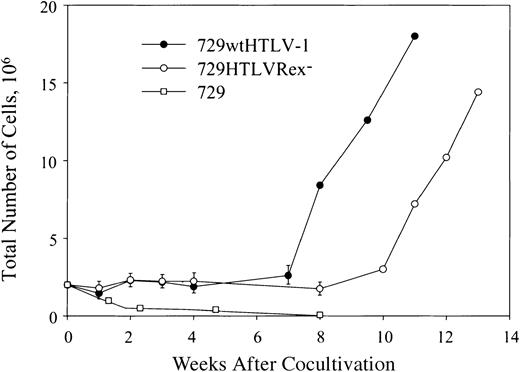
To determined if 729HTLVRex- cells could transmit virus to and immortalize/transform primary human T lymphocytes, lethally irradiated 729wtHTLV-1 and 729HTLVRex- cells were cocultured with human PBMCs. It is important to note that our assay uses freshly isolated unstimulated PBMCs and cell-associated virus transmission designed to closely mimic the in vivo infection. A low concentration of recombinant IL-2 (10 U/mL) was provided in the culture medium at 4 weeks after coculture to maintain and/or amplify cell viability induced by HTLV-1 infection. Cell number and viability were monitored at approximately weekly intervals to follow the immortalization process and the characteristic expansion of T cells from the PBMC mixed cell population. A growth curve of a representative assay indicated a progressive loss of viable cells over time in cocultures containing irradiated uninfected 729 cells and PBMCs (Figure 4). In contrast, the immortalization process was clearly apparent in PBMC/729wtHTLV-1 and PBMC/729HTLVRex- cocultures. The growth curve of immortalized cells by these 2 viruses were similar with the exception that HTLVRex- displayed a 3-week lag in exponential growth as compared to wtHTLV-1 (Figure 4). Flow cytometric analyses of immortalized PBMCs indicated a CD4+/CD8+ T-cell phenotype consistent with immortalization/transformation by HTLV-1 (data not shown).
Growth curve of HTLV T-lymphocyte immortalization assay. Human PBMCs were isolated by Ficoll/Paque and cocultivated with irradiated (10 000 rad) 729 stable cell lines (729wtHTLV-1, 729HTLVRex-) or 729 uninfected control cells as indicated. PBMCs (2 × 106) were cocultured with irradiated donor cells (1 × 106) in 24-well plates. Cells were fed once per week with RPMI 1640 supplemented to contain 20% FCS. Cell viability was determined by trypan blue exclusion staining at 0, 1, 2, 3, 4, 6, and 8 weeks after cocultivation. After 4 weeks, 10 U/mL IL-2 was provided in the culture medium. The mean and standard deviation of each time point were determined from 3 independent samples.
Growth curve of HTLV T-lymphocyte immortalization assay. Human PBMCs were isolated by Ficoll/Paque and cocultivated with irradiated (10 000 rad) 729 stable cell lines (729wtHTLV-1, 729HTLVRex-) or 729 uninfected control cells as indicated. PBMCs (2 × 106) were cocultured with irradiated donor cells (1 × 106) in 24-well plates. Cells were fed once per week with RPMI 1640 supplemented to contain 20% FCS. Cell viability was determined by trypan blue exclusion staining at 0, 1, 2, 3, 4, 6, and 8 weeks after cocultivation. After 4 weeks, 10 U/mL IL-2 was provided in the culture medium. The mean and standard deviation of each time point were determined from 3 independent samples.
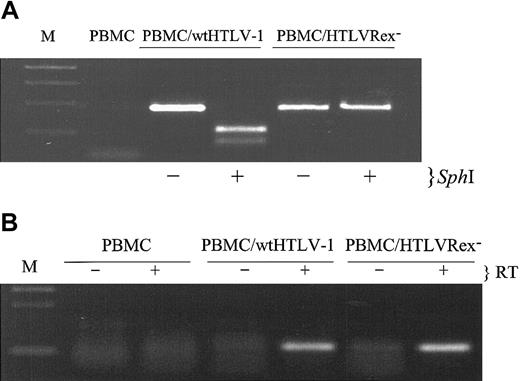
We next assessed the presence of HTLV sequences and viral gene expression in T cells immortalized by wtHTLV-1 and HTLVRex-. Diagnostic DNA PCR analyses were performed to determine if immortalized cells contained the expected viral sequences. Figure 5A shows that high molecular weight DNA from cells immortalized by both wtHTLV-1 and HTLVRex- contain specific HTLV-1 DNA sequences. wtHTLV-1 DNA, but not HTLVRex- DNA was restricted by SphI, indicating that the mutation introduced at the Rex initiator codon was present in the immortalized cells. Nucleotide sequencing of the PCR amplified product further confirmed the expected mutations (data not shown). Consistent with a defective Rex phenotype, PBMCs immortalized by HTLVRex- produced no detectable p19 Gag as compared to significant expression in wtHTLV-1-immortalized cells (Table 1).
T lymphocytes immortalized by HTLVRex- contain the expected Rex- mutation and express tax/rex mRNA. (A) HTLV-1 genome fragment containing the Rex start codon was amplified by PCR from high molecular weight DNA of wtHTLV-1 and HTLVRex- immortalized cells. PCR-amplified product was incubated in the presence or absence of SphI, separated on 2% agarose gel, and visualized by ethidium bromide staining. (B) Detection of tax/rex mRNA in the wtHTLV-1 and HTLVRex- immortalized PBMCs. Total RNA was prepared from fresh PBMCs, wtHTLV-1, and HTLVRex- immortalized PBMCs. Approximately 0.3 μg RNA was subjected to a coupled 40-cycle RT-PCR in the presence (+) or absence (-) of reverse transcriptase (RT). Following RT-PCR, 25-cycle nested PCR was performed. PCR product was separated on 2% agarose gel and visualized by ethidium bromide staining.
T lymphocytes immortalized by HTLVRex- contain the expected Rex- mutation and express tax/rex mRNA. (A) HTLV-1 genome fragment containing the Rex start codon was amplified by PCR from high molecular weight DNA of wtHTLV-1 and HTLVRex- immortalized cells. PCR-amplified product was incubated in the presence or absence of SphI, separated on 2% agarose gel, and visualized by ethidium bromide staining. (B) Detection of tax/rex mRNA in the wtHTLV-1 and HTLVRex- immortalized PBMCs. Total RNA was prepared from fresh PBMCs, wtHTLV-1, and HTLVRex- immortalized PBMCs. Approximately 0.3 μg RNA was subjected to a coupled 40-cycle RT-PCR in the presence (+) or absence (-) of reverse transcriptase (RT). Following RT-PCR, 25-cycle nested PCR was performed. PCR product was separated on 2% agarose gel and visualized by ethidium bromide staining.
Previous studies have shown that Tax is critical for efficient HTLV-1 gene expression and ultimately cellular immortalization/transformation of primary T lymphocytes.11,37 However, the expression of Tax is extremely low early on in the transformation process and requires a sensitive approach, such as nested RT-PCR, to consistently detect tax/rex mRNA expression. tax/rex mRNA expression was detected in both HTLVRex- and wtHTLV-1-immortalized PBMCs, consistent with expression of a functional Tax protein (Figure 5B). Taken together these results provide direct evidence that Rex is not required for HTLV-1-mediated in vitro cellular immortalization. The overall implication is that even cells harboring proviruses that do not produce detectable progeny virions based on current measurement assessments are capable of viral transmission.
HTLVRex- fails to infect rabbits
The above experiments demonstrated that Rex or Rex-dependent viral replication is not required for HTLV-1-mediated cellular immortalization in vitro. However, the in vitro system does not reflect the pressures of immune surveillance and viral target cell microenvironment in the infected host. We determined if the abrogation of Rex affected the ability of HTLV-1 to infect and replicate in vivo. Rabbits were inoculated with lethally irradiated 729, 729wtHTLV-1, 729HTLVRex-, or 729HTLVRex- + Rex (729HTLVRex- cells transiently transfected with a pcRex cDNA expression vector). Rabbit blood was drawn at weeks 1, 2, 3, 4, 6, and 8 after inoculation. Rabbit PBMCs were isolated from blood to determine viral loads and viral DNA integration and inoculated rabbit plasma was assessed for anti-HTLV-1 antibody response.
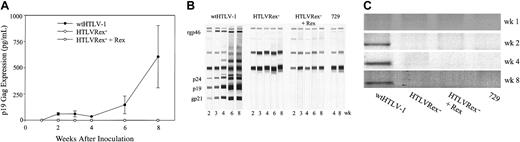
Rabbit PBMCs were ex vivo-cultured for 2 weeks, and p19 Gag expression in the supernatants was quantified by ELISA. p19 Gag was first detected from PBMCs of wtHTLV-1-inoculated rabbits at 2 weeks after inoculation, and the expression increased along the time course till week 8, when the experiment was terminated (Figure 6A). The PBMCs from the rabbits inoculated with HTLVRex- and HTLVRex- + Rex showed no detectable expression of p19 Gag. These results are consistent with our in vitro immortalization of T lymphocytes in which only cells immortalized by the wtHTLV-1 produced detectable p19 Gag.
Assessment of HTLV-1 infection in rabbits. Rabbits were inoculated with 1 × 107 irradiated 729wtHTLV-1, 729HTLVRex-, or 729HTLVRex- + Rex (Table 1). At weeks 1, 2, 3, 4, 6, 8 after inoculation, rabbit PBMCs and sera were isolated from blood. (A) p19 Gag expression in the supernatant of ex vivo-cultured rabbit PBMCs. PBMCs were cultured ex vivo for 2 weeks, and p19 Gag in the culture supernatant was detected by ELISA. Results are the average of 2 rabbits for each cell line, indicating that only PBMCs from 729wtHTLV-1-inoculated rabbits produce detectable p19 Gag. Error bars indicate standard deviations. (B) HTLV-1-specific serologic response. Sera from inoculated rabbits were tested for reactivity to specific HTLV-1 proteins by Western blot. A representative rabbit from each group as indicated is shown with reactive viral proteins labeled on the left. Results indicate that only 729wtHTLV-1-inoculated rabbits are seropositive. (C) Detection of HTLV-1-specific sequences in rabbits. Genomic DNA was isolated from rabbit PBMCs and subjected to PCR using HTLV-1-specific oligonucleotide primers. A representative result from one rabbit in each group is shown. Viral DNA integration was only detected in wtHTLV-1-inoculated rabbits.
Assessment of HTLV-1 infection in rabbits. Rabbits were inoculated with 1 × 107 irradiated 729wtHTLV-1, 729HTLVRex-, or 729HTLVRex- + Rex (Table 1). At weeks 1, 2, 3, 4, 6, 8 after inoculation, rabbit PBMCs and sera were isolated from blood. (A) p19 Gag expression in the supernatant of ex vivo-cultured rabbit PBMCs. PBMCs were cultured ex vivo for 2 weeks, and p19 Gag in the culture supernatant was detected by ELISA. Results are the average of 2 rabbits for each cell line, indicating that only PBMCs from 729wtHTLV-1-inoculated rabbits produce detectable p19 Gag. Error bars indicate standard deviations. (B) HTLV-1-specific serologic response. Sera from inoculated rabbits were tested for reactivity to specific HTLV-1 proteins by Western blot. A representative rabbit from each group as indicated is shown with reactive viral proteins labeled on the left. Results indicate that only 729wtHTLV-1-inoculated rabbits are seropositive. (C) Detection of HTLV-1-specific sequences in rabbits. Genomic DNA was isolated from rabbit PBMCs and subjected to PCR using HTLV-1-specific oligonucleotide primers. A representative result from one rabbit in each group is shown. Viral DNA integration was only detected in wtHTLV-1-inoculated rabbits.
To determine serologic response of rabbits to inoculated lines, we measured anti-HTLV-1 antibody response in rabbits by Western blot analysis. A representative seroconversion pattern from each of the inoculated groups is shown in Figure 6B. Seroconversion was detected in the 729wtHTLV-1-inoculated rabbits starting at week 2, and antibody titers rose over the time course of the experiment. We were unable to detect any antibody response in rabbits inoculated with 729HTLVRex- or 729HTLVRex- + Rex.
To further determine the infection status of the inoculated rabbits, amplification of HTLV-1-specific sequences by PCR from rabbit PBMCs was performed. We detected viral DNA integration in 729wtHTLV-1-inoculated rabbits starting from week 2 after inoculation, and the viral DNA consistently persisted until the end of the experiment. However, we were unable to amplify viral DNA sequences in 729HTLVRex- or 729HTLVRex- + Rex inoculated rabbits at any time point (Figure 6C). Taken together our results demonstrated that Rex-dependent viral replication and viral spread is required for establishing and maintaining infection of rabbits.
Discussion
HTLV-1 Rex acts posttranscriptionally to facilitate accumulation of full-length gag/pol and singly spliced env viral RNA in the cytoplasm of HTLV-1-infected cells, and its function has been shown to be critical for efficient replication.15,16,22,38 In this study, we confirmed that the abrogation of Rex function impairs viral replication. However, this loss of Rex did not abolish the ability of HTLV-1 to infect and immortalize PBMCs in vitro. Furthermore, rabbits inoculated with irradiated 729HTLVRex- cells or 729HTLVRex- cells transfected with a Rex expression vector failed to become persistently infected or mount a detectable antibody response to the viral gene products. Overall, our results provide the first direct evidence that HTLV-1-mediated cellular immortalization does not require Rex-dependent viral replication or other possible Rex contributions but indicates that Rex-dependent replication and modulation of viral gene expression is critical for infection, spread, and persistence in vivo.
We used transient transfection of wild-type and Rex-defective full-length proviral clones followed by quantitation of p19 Gag in the culture supernatant to determine the effect of Rex on virion production. We found that in the absence of functional Rex, the viral p19 Gag expression level in the supernatant was significantly reduced, approximately 116-fold. However, low but detectable levels of p19 Gag were still expressed (50 pg/mL). It is likely that small amounts of incompletely spliced mRNA can “leak” into the cytoplasm in the absence of Rex, potentially resulting in low levels of virion production. One study consistent with this hypothesis showed that human T cells transfected with a HTLV-2 Rex-defective provirus expressed reduced levels of genomic and env mRNA in the cytoplasm and significantly reduced levels of p24 Gag.16 Characterization of permanently transfected 729 cell lines revealed that cells harboring HTLVRex- provirus produce no detectable Gag protein, whereas 729wtHTLV-1 produce 700 to 800 pg/mL of p19 Gag. Since our data indicate that Gag production is modulated approximately 116-fold by Rex, levels of Gag from 729HTLVRex- would be expected to be below the limit of detection of 25 pg/mL. This does not rule out the possibility of production of low levels of infectious virions. It has been established that cell-free infection by HTLV is very inefficient, and infection of the natural target cell usually occurs by cell-associated transmission. Direct cell-to-cell contact in our coculture assay could ensure that even a minute amount of virion production would result in the infection of target PBMCs.
In our coculture immortalization/transformation assay, we found that both wtHTLV-1 and HTLVRex- had the capacity to immortalized PBMCs. A 3-week lag in cell proliferation in PBMC/HTLVRex- was observed in comparison to PBMCs immortalized by wtHTLV-1. This delay likely reflects the low virus production from 729HTLVRex- and reduced number of PBMCs infected. Coculture of PBMCs from different donors and 2 different 729HTLVRex- cell lines gave similar results, thus eliminating the possible explanation of individual donor cell or producer cell line variation (data not shown). Furthermore, we showed that PBMCs immortalized by HTLVRex- contain the expected rex mutation and produced no detectable p19 Gag, consistent with the Rex minus phenotype.
Previous studies have indicated that Tax is critical for HTLV-mediated cellular immortalization/transformation.10,11,37 Our data are in agreement with these reports in that PBMCs immortalized by HTLVRex- or wtHTLV-1 expressed low but detectable levels of tax/rex mRNA. This remains consistent with the adult T-cell leukemia in which tumor cells maintain an intact tax gene. It has also been hypothesized that the modulation of viral RNA expression by Rex (primarily controlling Tax expression) may contribute to the immortalization/transformation process in vitro. Our data indicate that the loss of Rex has no significant effect on the ability of the virus to ultimately immortalize primary human T cells in vitro. We do however speculate that the 3-week lag in cell proliferation may be the result of the disruption of Rex regulation of viral gene expression. One possibility consistent with this hypothesis is that this modulation of gene expression affects the concentration of viral proteins in the infected cell, particularly Tax, altering lymphocyte proliferation or the induction of apoptosis.
Our reproducible rabbit animal model has been used to successfully study HTLV infection, transmission, and persistence.36,39,40 Here we used the model to study the effect of Rex abrogation on HTLV infection and persistence in vivo. As expected, viral persistence and antiviral immune response was detected in wtHTLV-1-infected rabbits. Antibodies against all major viral antigens were detected in HTLV-1-infected rabbits beginning at 2 weeks after inoculation. We found that HTLVRex- failed to infect rabbits or induce an immune response, indicating that Rex function is required for the establishment of infection in vivo. Interestingly, ORF I and ORF II of the pX region of HTLV have been shown to have no effect on HTLV virion production, infectivity, and immortalization in vitro, but have a significant effect on HTLV viral loads and persistence in vivo.36,40,41 Therefore, the function of rex, ORF I, and ORF II gene products are most critical in vivo, in which efficient replication, viral spread to sufficient number of activated target cells, and modulation of gene expression are required for virus survival and persistence.
In summary, our results provide the first direct evidence that Rex is dispensable for HTLV-1 immortalization of primary human T lymphocytes in vitro. This work also provides strong evidence that cells harboring proviruses that produce no detectable virions are still capable of cell-associated transmission in vitro. Clearly, Rex is required for efficient virus production and infection in the rabbit model. Further studies using this animal model may provide insight as to whether Rex may also be an important modulator of viral gene expression controlling concentrations of specific gene products, viral latency, and infected cell survival in the host.
Prepublished online as Blood First Edition Paper, August 7, 2003; DOI 10.1182/blood-2003-05-1490.
Supported by grants from the National Institutes of Health (CA93584 and CA100730).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked ”advertisement” in accordance with 18 U.S.C. section 1734.
We thank M. Anderson and I. Younis for their assistance with the rabbit experiments. We also thank M. Anderson, I. Younis, L. Khair, and L. Xie for critical comments on the manuscript.

![Figure 3. 729HTLVRex- stable cell line contains the Rex mutation and does not produce detectable Rex. (A) HTLV-1 genome fragment containing the Rex start codon was amplified by PCR from genomic DNA of 729wtHTLV-1 and 729HTLVRex- cells. PCR-amplified product was incubated in the presence (+) or absence (-) of SphI, separated on 2% agarose gel, and visualized by ethidium bromide staining. (B) Rex-1 immunoprecipitation. 729, 729wtHTLV-1, and 729HTLVRex- cells were metabolically labeled with [35S] methionine-cysteine. Cell lysates were immunoprecipitated with polyclonal antibody against the HTLV-1 Rex in the presence of protein A-sepharose. The sizes (in daltons, indicated on the left) were determined by comparison to protein markers.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/12/10.1182_blood-2003-05-1490/6/m_h82335288003.jpeg?Expires=1767709916&Signature=gZhlJYclB9f8oef35sJq2lHAp8P5em~SW~xsGxv3m43b8QIeMATkLf4Rw0jmFvdFp-DNdxIildXy9g529VMmgtPhdFaEogB1EWiBX2yy-rNrirEKLwRSr1SFjFryBk8SVEfjnpBHOTObt8Au2mHZX1ttKlUT9PXeS~eD6a9-B4cAndwOTp3YwWPwjfDWp-j-p2FpcZZk~KO8s0byQQShKtd-mhKqkFYf8s2cCvdA7pbr7taf2NzqvXRdBsXPXtbOhUyCIic-scJwBbZu26V1u9gZGLJ0Ez1hJlOkgm96BW8KygyRvzr0ixRwcbi0WH1~BgI2LIdlbdjfzo6IoeAXgQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal