Abstract
Replication of the pathogenic human parvovirus B19 is restricted to erythroid progenitor cells. Although blood group P antigen has been reported to be the cell surface receptor for parvovirus B19, a number of nonerythroid cells, which express P antigen, are not permissive for parvovirus B19 infection. We have documented that P antigen is necessary for parvovirus B19 binding but not sufficient for virus entry into cells. To test whether parvovirus B19 utilizes a cell surface coreceptor for entry, we used human erythroleukemia cells (K562), which allow parvovirus B19 binding but not entry. We report here that upon treatment with phorbol esters, K562 cells become adherent and permissive for parvovirus B19 entry, which is mediated by α5β1 integrins, but only in their high-affinity conformation. Mature human red blood cells (RBCs), which express high levels of P antigen, but not α5β1 integrins, bind parvovirus B19 but do not allow viral entry. In contrast, primary human erythroid progenitor cells express high levels of both P antigen and α5β1 integrins and allow β1 integrin–mediated entry of parvovirus B19. Thus, in a natural course of infection, RBCs are likely exploited for a highly efficient systemic dissemination of parvovirus B19.
Introduction
Two parvoviruses of human origin, the nonpathogenic adeno-associated virus 2 (AAV) and the pathogenic parvovirus B19, have been studied extensively.1,2 Recombinant AAV vectors have gained attention as a potentially useful alternative to the more commonly used recombinant retroviral and adenoviral vectors in human gene therapy.3 AAV possesses a broad host range that transcends the species barrier because it utilizes the ubiquitously expressed cell surface heparan sulfate proteoglycan as a primary receptor for viral binding.4 We and others have reported the requirement of fibroblast growth factor receptor 1 (FGFR1) and αVβ5 integrin as coreceptors for viral entry.5,6 Parvovirus B19, on the other hand, has been shown to have an extremely limited tissue-tropism, and the virus replication is restricted to human erythroid progenitor cells,7 presumably because (1) the blood group P antigen (synonym “globoside”; “globotetraosylceramide”) is used as primary cellular receptor for parvovirus B19,8 (2) putative intracellular factors, largely restricted to human erythroid cells, are required for optimal transcriptional activation of the B19 promoter at map unit 6 (B19p6) and viral replication,9,10 and (3) B19 capsid protein expression in nonpermissive cells is impaired due to a block in full-length transcription of the viral genome, atypical mRNA splicing, and impaired ribosome loading of structural gene transcripts.11-13 However, P antigen expression is not restricted to erythroid cells, and a number of P antigen–positive nonerythroid cells are nonpermissive for a successful infection by parvovirus B19.2 In addition, mature red blood cells (RBCs), which are known to express high levels of P antigen, are unlikely to be ideal targets for B19 infection and replication because they lack nuclei. Similarly, B19p6 promoter-driven expression analyses were carried out with plasmid DNA transfections,11-13 which do not accurately reflect a natural infection by parvovirus B19.
We have reported the generation of recombinant AAV-B19 hybrid virus, in which either the entire wild-type (wt) parvovirus B19 genome was encapsidated in AAV capsids14 or the AAV promoter at map unit 5 (AAVp5) was replaced by the B19p6 promoter in the wt AAV genome encapsidated in AAV capsids.15 In both instances, the hybrid virus retained its erythroid specificity. Thus, P antigen alone is insufficient to impart erythroid specificity to parvovirus B19.
More recently, we have generated recombinant parvovirus B19 vectors in which a recombinant AAV genome is encapsidated into parvovirus B19 capsids.16 Using these vectors, we established that P antigen is necessary for virus binding but not sufficient for virus entry into human cells because parvovirus B19 could bind to, but not enter, a number of P antigen–expressing human cell lines.17 During these studies, we observed that cells that grew as adherent monolayers were permissive for parvovirus B19 infection, whereas cells that grew as suspension cultures could not be infected, although both expressed P antigen and bound the virus, and we proposed that parvovirus B19 also requires the presence of a putative cell surface coreceptor for successful infection.17
To investigate whether the adhesive phenotype of a cell was related to its permissibility for parvovirus B19 internalization, we used a human erythroleukemia cell line, K562, which grows in suspension but can undergo a phenotype shift to adherent growth either spontaneously or upon treatment with phorbol ester, phorbol 12-myristate 13-acetate (PMA).18 We reported previously that K562 cells express P antigen and bind but do not internalize parvovirus B19 when grown in suspension.17 Here, we document that spontaneously adherent and PMA-treated K562 cells internalize parvovirus B19 and that activated α5β1 integrins expressed on K562 cells serve as a coreceptor for parvovirus B19.
Materials and methods
Cells, viruses, and antibodies
K562 cells were purchased from American Type Culture Collection (ATCC; Manassas, VA) and grown in Iscove modified Dulbecco medium (IMDM) supplemented with 10% newborn calf serum and 1% penicillin/streptomycin (Sigma, St Louis, MO). For suspension growth, K562 cells were subcultured every 48 hours. Normal donor red blood cells (RBCs) were purchased from the Central Indiana Regional Blood Center, Indianapolis, and used on the day of purchase. Human bone marrow–derived highly purified CD71+ primary erythroid progenitor cells were purchased from AllCells (Berkeley, CA). Recombinant parvovirus B19-Luc vector stocks were generated and purified by CsCl equilibrium density gradient centrifugation as previously described.16,17 Wild-type parvovirus B19-containing sera were a kind gift from Kent Dupuis, Cerus, Concord, CA. Activating and inhibitory monoclonal antibodies against α and β integrins were purchased from Chemicon (Temecula, CA).
Induction of the adherence phenotype
To induce spontaneous adherence, K562 cells were subcultured every 5 to 7 days; however, medium changes were performed every 48 hours without disturbing the settled cells. To induce an adhesive phenotype in K562 cells pharmacologically, suspension K562 cells were treated with 15 nM or 32 nM phorbol 12-myristate 13-acetate (PMA) for 48 hours, as described previously.18
Confocal microscopy
Approximately 2 × 105 cells (suspension, adherent, PMA-treated, or RBCs) were washed twice with IMDM supplemented with 1 mM Mg2+ and 1 mM Mn2+, preincubated (K562 cells) with 5 μg/mL of activating (N29, 21C8) and inhibitory (P4C10) β1 integrin antibodies for 30 minutes at room temperature, and either mock infected or infected with 500 particles per cell (ppc) of cyanine 3 (Cy3)–labeled parvovirus B19 (Molecular Probes, Eugene, OR) for 30 minutes at 37°C. After infection cells were either directly transferred to glass coverslips coated with TAK adhesive protein (Sigma) (K562 cells) or either mock treated or treated with 0.05% trypsin plus 0.02% EDTA (ethylenediaminetetraacetic acid) at 37°C for 5 minutes (RBCs) and then transferred to the coverslips. The coverslips were spun briefly (500 rpm maximum speed) to attach the cells, washed with phosphate-buffered saline/1% bovine serum albumin (PBS/1% BSA), and fixed in 0.5% glutaraldehyde in PBS on ice for 30 minutes. After another washing step, nuclei (K562 cells) were stained using SYTO 16 green fluorescent nucleic acid stain (Molecular Probes). The coverslips were mounted in 50:50 glycerol/PBS and analyzed by confocal microscopy using a Zeiss confocal microscope (Zeiss, Thornwood, NY). A mean of 12 to 20 slices per visible field were acquired using a multitrack mode to record red and green fluorescence separately, and overlay images were subsequently generated using the LSM5 software (Zeiss). For RBCs, image acquisition was achieved with red fluorescence and transmission light to visualize cell contour/morphology.
Recombinant parvovirus B19 vector transduction assays
Approximately equal numbers of untreated or PMA-treated K562 cells (6 × 105) were washed twice with IMDM supplemented with 1 mM Mg2+ and 1 mM Mn2+ and either mock infected or infected for 1 hour at 37°C with 500 ppc recombinant parvovirus B19-Luc vectors. In some experiments, cells were either mock treated or preincubated with 10 μg/mL of activating (N29, 21C8) and inhibitory (P4C10, JB1a) β1 integrin antibodies for 30 minutes at room temperature or 500 μg/mL GRGDS peptides for 20 minutes at room temperature. For integrin cross-linking, K562 cells were first incubated with 10 μg/mL of β1 integrin inhibitory antibodies (JB1a) or 500 μg/mL GRGDS peptides for 20 minutes at room temperature followed by that with 5 μg/mL of goat antimouse immunoglobulin G (IgG) antibodies for an additional 20 minutes at room temperature. Cells were then infected with the recombinant parvovirus B19-Luc vector for 1 hour at 37°C in a CO2 incubator. Twenty-four hours after infection, firefly luciferase activity was assayed in cell lysates using a Luc reporter system (Promega, Madison, WI).
Entry of wild-type parvovirus B19 into K562 or primary human erythroid progenitor cells
Untreated, PMA-treated K562 cells (2 × 106), or primary human erythroid progenitor cells (1 × 106) were washed twice with IMDM supplemented with 1 mM Mg2+ and 1 mM Mn2+, preincubated first with 20 μg/mL normal mouse IgG for 10 minutes at room temperature to block Fc receptors, and subsequently with 25 μg/mL or 50 μg/mL activating (N29) or inhibitory (P4C10) β1 integrin antibodies for 30 minutes at room temperature. A total of 10 μL (for infection of K562 cells) or 5 μL (for infection of CD71+ cells) of serum containing 109 and 5 × 108 wild-type parvovirus B19 particles, respectively, were either untreated or preincubated with 10 μg and 5 μg antiparvovirus B19 VP2 monoclonal antibodies for 1 hour on ice. Cells were infected for 1 hour at 37°C, washed, and uninternalized viral particles were removed by trypsinization (0.05% trypsin plus 0.02% EDTA at 37°C for 5 minutes) as described previously.17 Low Mr DNA was isolated and analyzed on Southern blots using a parvovirus B19-specific DNA probe.
Fluorescence-activated cell sorting
Normal donor RBCs or CD71+ primary human erythroid progenitor cells were washed twice with IMDM and once with PBS/1% BSA and incubated with 5 μg/mL of primary anti–P antigen and anti-α5 and anti-β1 integrin antibodies for 30 minutes on ice. Subsequently, the cells were washed twice with PBS/1% BSA and incubated with 5 μg/mL fluorescein isothiocyanate (FITC)– or phycoerythrin (PE)–labeled secondary antibodies for 20 minutes on ice. Control cells were incubated with secondary antibody only. Cell surface expression of P antigen and α5 and β1 integrins was analyzed by flow cytometry (Becton Dickinson, San Jose, CA).
Results
Cell adherence renders susceptibility to parvovirus B19 entry
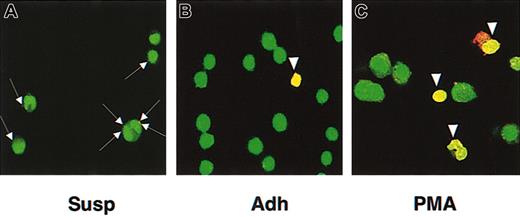
To directly investigate whether cell adhesion renders cells susceptible to parvovirus B19 infection, we used the human erythroleukemia cell line K562 as a model system because these cells grow in suspension and can undergo a phenotype shift to adherent growth either spontaneously or upon prolonged treatment with phorbol esters.18 We reported previously that these cells express P antigen and bind parvovirus B19 but cannot be transduced by recombinant parvovirus B19 vectors containing the firefly luciferase transgene (B19-Luc) when grown as suspension cultures.17 In the present studies, we observed that spontaneously adherent and PMA–treated K562 cells became permissive for parvovirus B19 infection. Using Cy3-labeled recombinant parvovirus B19 particles and confocal microscopy, we documented binding but not internalization of Cy3-labeled parvovirus B19 particles (red) in K562 cells grown in suspension (Susp) cultures supplemented with divalent ions (Mn2+ and Mg2+) (Figure 1A). Upon spontaneous adherence (Adh) or PMA treatment (PMA), however, internalization and trafficking of parvovirus B19 particles to the nucleus (green) could be detected within the first 30 minutes of infection (Figure 1B-C) as indicated by the yellow fluorescence of the nuclei. Importantly, in the absence of divalent ions, no internalization of parvovirus B19 particles could be observed (data not shown). Because PMA treatment did not affect the level of P antigen expression on these cells, as determined by flow cytometry (data not shown), we hypothesized that a cell surface factor(s) involved in cell adherence provided coreceptor activity for parvovirus B19 entry into human K562 cells.
Cell adherence renders K562 cells permissive for parvovirus B19 entry and nuclear localization. K562 cells grown in suspension (Susp) could bind parvovirus B19 (A; arrows) in the presence of divalent ions (1 mM Mn2+, 1 mM Mg2+) but did not allow viral entry. Upon spontaneous adherence (Adh), virus entry and nuclear localization were seen in about 7% of K562 cells (B; arrowhead), the extent of which is increased to about 29% upon PMA treatment (C; arrowheads). K562 cells were infected with Cy3-labeled recombinant parvovirus B19 for 30 minutes, fixed, and nuclei stained with SYTO 16 green fluorescent nucleic acid stain. Confocal images were acquired using a Zeiss LSM510 confocal microscope. Original magnification, × 630 for all panels.
Cell adherence renders K562 cells permissive for parvovirus B19 entry and nuclear localization. K562 cells grown in suspension (Susp) could bind parvovirus B19 (A; arrows) in the presence of divalent ions (1 mM Mn2+, 1 mM Mg2+) but did not allow viral entry. Upon spontaneous adherence (Adh), virus entry and nuclear localization were seen in about 7% of K562 cells (B; arrowhead), the extent of which is increased to about 29% upon PMA treatment (C; arrowheads). K562 cells were infected with Cy3-labeled recombinant parvovirus B19 for 30 minutes, fixed, and nuclei stained with SYTO 16 green fluorescent nucleic acid stain. Confocal images were acquired using a Zeiss LSM510 confocal microscope. Original magnification, × 630 for all panels.
Activated cell surface β1 integrins (α5β1) mediate recombinant parvovirus B19 entry independent of fibronectin binding
K562 cells express a very limited number of integrins, predominantly α5β1 integrins.19 No difference was found in the total levels of α5β1 integrin expression or in the expression of specific activation-dependent epitopes on suspension, spontaneously adherent, and PMA-treated K562 cells by flow cytometry (data not shown). To test whether α5β1 integrins were involved in the adhesion-dependent permissiveness of K562 cells for parvovirus B19 internalization, we incubated PMA-treated K562 cells in the presence of divalent ions and either β1 integrin function-activating (or high-affinity conformation-stabilizing; N29, 21C8) or β1 integrin function-blocking (or high-affinity conformation-destabilizing; P4C10, JB1a) antibodies prior to infection with Cy3-labeled or parvovirus B19-Luc vectors. Confocal microscopy revealed a reduction in nuclear localization of Cy3-labeled parvovirus B19 particles by β1 integrin function-blocking antibodies (P4C10) 30 minutes after infection (Figure 2Ai-Aii). In addition, transduction of PMA-treated K562 cells with recombinant parvovirus B19-Luc vectors was inhibited by 55.17% ± 0.22% and 65.22% ± 1.49% by preincubation of the cells with function-blocking monoclonal antibodies against α5 and β1 integrins, respectively (Figure 2B). Interestingly, stabilization of the high-affinity conformation of surface-expressed β1 integrins by monoclonal antibodies (N29, 21C8) resulted in an increase in parvovirus B19 entry and nuclear localization (Figure 2Aiii-Aiv) as well as transduction (Figure 2B) in PMA-treated K562 cells. Quantification of the confocal data revealed that 28.54% ± 4.68% of PMA-treated K562 cells showed nuclear entry of parvovirus B19 in the absence of antibodies, compared with 58.06% ± 1.09% and 57.86% ± 0.6%, respectively, in the presence of N29 and 21C8 high-affinity β1 integrin–stabilizing antibodies. In addition, activation of β1 integrins by cross-linking of β1 integrin function-blocking antibodies with secondary goat antimouse IgG antibodies (JB1a+sec. Ab)20 also resulted in an approximately 3-fold increase in transduction (Figure 2B). Because internalization of fibronectin by α5β1 integrin is well documented, one possible mechanism of parvovirus B19 entry into human cells could be the internalization of fibronectin-opsonized viral particles.19 We examined, therefore, whether fibronectin binding to cell surface–expressed α5β1 integrins (which is mediated by the Arg-Gly-Asp [RGD]) containing tenth type III repeat of the central cell binding domain of fibronectin and inhibited by RGD peptides21 was necessary for parvovirus B19 infection. RGD peptides, however, in the absence (RGD) or presence of secondary antimouse IgG antibodies (RGD+sec. Ab) did not affect parvovirus B19 infection (Figure 2B). These results suggested that α5β1 integrins expressed on PMA-treated K562 cells and activated in the presence of divalent ions promoted the internalization of parvovirus B19 particles and that stabilization or destabilization of the high-affinity conformation of α5β1 integrins using monoclonal antibodies modulated this coreceptor activity. In addition, these results indicated that fibronectin opsonization of parvovirus B19 particles was not involved in α5β1 integrin–mediated virus internalization and that the virus did not directly interact with the RGD ligand binding site on α5β1 integrins. Furthermore, α5β1 integrin coreceptor function could be enhanced either by increasing β1 integrin affinity or avidity.
Activated cell surface β1 integrins (α5β1) mediate recombinant parvovirus B19 entry independent of fibronectin binding. (A) Parvovirus B19 entry and nuclear localization in PMA-treated K562 cells were inhibited by inhibitory (P4C10) and increased by high-affinity conformation-stabilizing (N29, 21C8) β1 integrin antibodies. K562 cells were infected with Cy3-labeled recombinant parvovirus B19 for 30 minutes, fixed, and nuclei stained with SYTO 16 green fluorescent nucleic acid stain. Original magnification, × 630 for all panels. (B) Transduction of PMA-treated K562 cells with recombinant parvovirus B19-Luc vectors was inhibited by inhibitory α5 and β1 integrin antibodies (α5, JB1a) but was independent of disruption of β1 integrin interaction with fibronectin by RGD peptides (RGD). β1 integrin activation induced either by stabilization of high-affinity β1 integrins (N29) or by cross-linking of antibody-ligated β1 integrins with goat antimouse secondary antibodies (JB1a+sec. Ab) resulted in an increase in transduction, whereas ligand binding without cross-linking (RGD+sec. Ab) had no effect. Data are representative of 3 independent experiments; error bars represent standard deviations (SDs). Firefly luciferase activity was detected in cell extracts 24 hours after infection as described in “Materials and methods.” *P < .05; **P < .001.
Activated cell surface β1 integrins (α5β1) mediate recombinant parvovirus B19 entry independent of fibronectin binding. (A) Parvovirus B19 entry and nuclear localization in PMA-treated K562 cells were inhibited by inhibitory (P4C10) and increased by high-affinity conformation-stabilizing (N29, 21C8) β1 integrin antibodies. K562 cells were infected with Cy3-labeled recombinant parvovirus B19 for 30 minutes, fixed, and nuclei stained with SYTO 16 green fluorescent nucleic acid stain. Original magnification, × 630 for all panels. (B) Transduction of PMA-treated K562 cells with recombinant parvovirus B19-Luc vectors was inhibited by inhibitory α5 and β1 integrin antibodies (α5, JB1a) but was independent of disruption of β1 integrin interaction with fibronectin by RGD peptides (RGD). β1 integrin activation induced either by stabilization of high-affinity β1 integrins (N29) or by cross-linking of antibody-ligated β1 integrins with goat antimouse secondary antibodies (JB1a+sec. Ab) resulted in an increase in transduction, whereas ligand binding without cross-linking (RGD+sec. Ab) had no effect. Data are representative of 3 independent experiments; error bars represent standard deviations (SDs). Firefly luciferase activity was detected in cell extracts 24 hours after infection as described in “Materials and methods.” *P < .05; **P < .001.
Activated cell surface β1 integrins also mediate wild-type parvovirus B19 entry
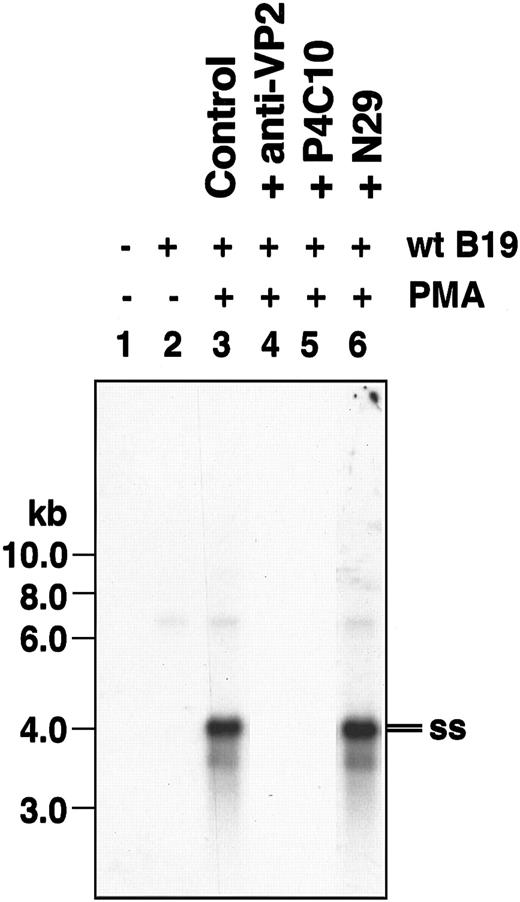
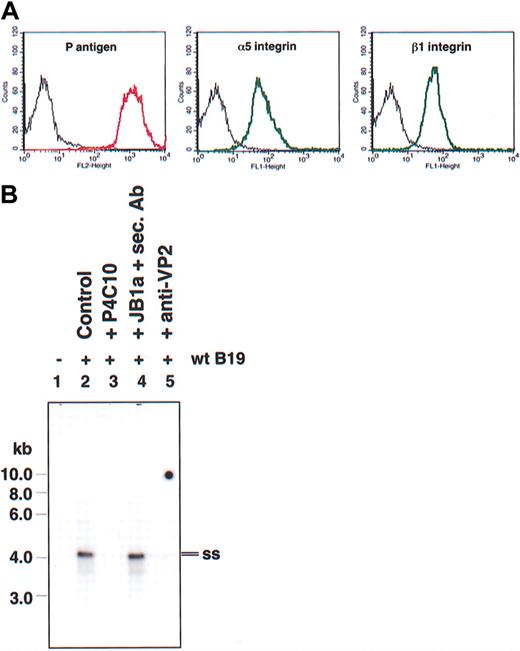
Because the results reported thus far were obtained using recombinant parvovirus B19 vectors, we next investigated the role of β1 integrins in wild-type (wt) parvovirus B19 infection. Undifferentiated and PMA-differentiated K562 cells were infected with 500 ppc of wt parvovirus B19 for 1 hour and uninternalized viral particles were removed from the cell surface by trypsinization as described previously.17 Internalized viral genomes were subsequently detected by Southern hybridization. As can be seen in Figure 3, viral entry did not occur in undifferentiated K562 cells (Figure 3, lane 2). Upon PMA treatment, however, single-stranded (ss) wt parvovirus B19 genomes were readily detected (Figure 3, lane 3). Pretreatment of wt parvovirus B19 with parvovirus B19 anti-VP2 antibodies (Figure 3, lane 4) or pretreatment of cells with inhibitory β1 integrin antibody (P4C10) (Figure 3, lane 5) abolished viral internalization. Interestingly, preincubation of PMA-treated K562 cells with β1 integrin–activating antibody (N29) increased the extent of wt parvovirus B19 internalization (Figure 3, lane 6). Despite successful entry, wt parvovirus B19 failed to undergo a productive replication (data not shown), suggesting the lack of erythroid cell–specific transcriptional activators in PMA-differentiated K562 cells. These results, nonetheless, corroborate our observation that activated β1 integrins play a crucial role in parvovirus B19 entry into human K562 cells.
β1 integrin also serves as a coreceptor for entry of wild-type human parvovirus B19 in PMA-treated K562 cells. Southern blot analysis of low Mr DNA isolated from undifferentiated (lanes 1-2) and PMA-differentiated (lanes 3-6) K562 revealed the presence of internalized single-stranded (ss) wt parvovirus B19 genomes only in PMA-treated cells (lane 3). Both parvovirus B19 anti-VP2 antibodies (lane 4) and inhibitory β1 integrin antibodies (lane 5) abolished it, whereas β1 integrin-activating antibody increased viral entry (lane 6). Southern blot analyses were performed as described in “Materials and methods.”
β1 integrin also serves as a coreceptor for entry of wild-type human parvovirus B19 in PMA-treated K562 cells. Southern blot analysis of low Mr DNA isolated from undifferentiated (lanes 1-2) and PMA-differentiated (lanes 3-6) K562 revealed the presence of internalized single-stranded (ss) wt parvovirus B19 genomes only in PMA-treated cells (lane 3). Both parvovirus B19 anti-VP2 antibodies (lane 4) and inhibitory β1 integrin antibodies (lane 5) abolished it, whereas β1 integrin-activating antibody increased viral entry (lane 6). Southern blot analyses were performed as described in “Materials and methods.”
Postreceptor events determine β1 integrin coreceptor activity
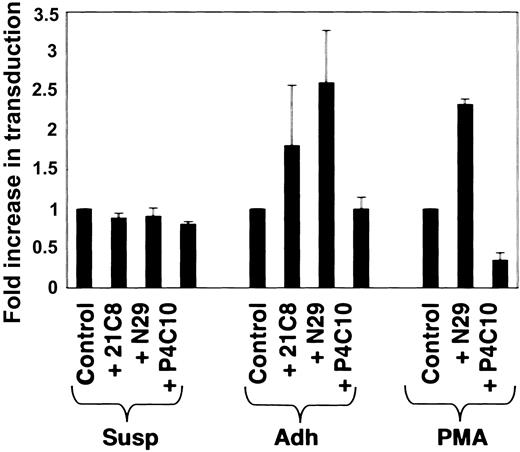
Prolonged PMA treatment causes pleiotropic changes in K562 cells.18 The studies reported thus far document a dependence of α5β1 integrin coreceptor function on β1 integrin affinity and avidity in the context of PMA-induced K562 cell differentiation. To test whether α5β1 integrin coreceptor function could also be elicited by antibody-mediated stabilization of the divalent ion-induced high-affinity conformation of β1 integrin in undifferentiated K562 cells, we compared the effects of 2 different β1 integrin–activating antibodies in untreated (Susp), spontaneously adherent (Adh), and PMA-treated K562 cells (PMA). No transduction could be observed in suspension K562 cells even in the presence of divalent ions and 2 different high-affinity–stabilizing β1 integrin antibodies (Figure 4). Basal transduction of suspension, adherent, and PMA-treated K562 cells were 11.63 ± 0.24, 392.61 ± 75.54, and 4581.13 ± 74.21 relative light units per microgram (RLU/μg) of protein, respectively, and were set at 1 for comparison. Transduction of spontaneously adherent and PMA-treated K562 cells, however, was increased substantially by antibody-mediated stabilization of high-affinity β1 integrins (Figure 4). Interestingly, function-blocking β1 integrin antibodies (P4C10) had an effect on PMA-treated cells but not on spontaneously adherent cells. Considering the overall low transduction efficiency in spontaneously adherent cells, these data suggest that most α5β1 integrins expressed on these cells are not functionally active in the presence of divalent ions, but stabilization of the high-affinity conformational state by monoclonal antibodies can activate these integrins to promote parvovirus B19 internalization. These data suggest that α5β1 integrin coreceptor function is dependent on both high-affinity receptor conformation and functional linkage of β1 integrins to postreceptor events that are present only in cells expressing adhesion-competent α5β1 integrins.
β1 integrin coreceptor function is dependent on postreceptor events. No transduction could be observed in suspension (Susp) K562 cells, even after pretreatment with divalent ions (1 mM Mn2+, 1 mM Mg2+) and high-affinity conformation-stabilizing β1 integrin antibodies (N29, 21C8). In contrast, transduction of spontaneously adherent (Adh) and PMA-differentiated K562 cells by recombinant parvovirus B19-Luc vectors was increased by pretreatment with high-affinity conformation-stabilizing β1 integrin antibodies (N29, 21C8) and decreased with inhibitory (P4C10) β1 integrin antibodies. Transduction without antibody treatment (Control) was set at 1 for comparison. Firefly luciferase activity was detected in cell extracts 24 hours after infection. Data are representative of 3 independent experiments; error bars represent SD.
β1 integrin coreceptor function is dependent on postreceptor events. No transduction could be observed in suspension (Susp) K562 cells, even after pretreatment with divalent ions (1 mM Mn2+, 1 mM Mg2+) and high-affinity conformation-stabilizing β1 integrin antibodies (N29, 21C8). In contrast, transduction of spontaneously adherent (Adh) and PMA-differentiated K562 cells by recombinant parvovirus B19-Luc vectors was increased by pretreatment with high-affinity conformation-stabilizing β1 integrin antibodies (N29, 21C8) and decreased with inhibitory (P4C10) β1 integrin antibodies. Transduction without antibody treatment (Control) was set at 1 for comparison. Firefly luciferase activity was detected in cell extracts 24 hours after infection. Data are representative of 3 independent experiments; error bars represent SD.
Mature human red blood cells lack expression of coreceptor for parvovirus B19
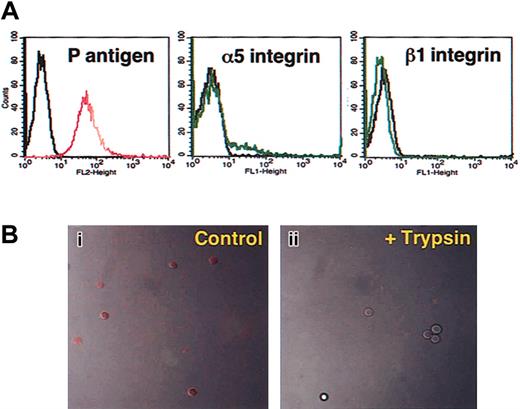
Parvovirus B19 replication takes place in the cell nucleus, making mature red cells (RBCs) poor targets for parvovirus B19 infection. However, RBCs express high levels of P antigen, the primary receptor for parvovirus B19.8 Flow cytometric analysis of normal donor RBCs (Figure 5A) confirmed high-level expression of P antigen (Figure 5A, left) and documented the complete absence of α5 (Figure 5A, middle) and β1 (Figure 5A, right) integrins. Furthermore, although parvovirus B19 bound efficiently to these cells, as detected by confocal microscopy (Figure 5Bi), treatment with trypsin almost completely removed parvovirus B19 particles bound to these cells, suggesting that most viral particles did not enter the RBCs (Figure 5Bii). It is important to emphasize that we have previously documented complete removal of uninternalized parvoviral particles by the trypsin treatment,17 and this treatment had no adverse effects on RBC viability, as determined by trypan blue exclusion assays (2.97% trypan blue–positive untreated RBCs versus 3.34% trypan blue–positive trypsin-treated RBCs, respectively). These results substantiate our hypothesis that α5β1 integrin serves as a coreceptor for parvovirus B19 entry into human cells.
Mature human RBCs lack expression of the coreceptor for parvovirus B19 and do not internalize the virus. (A) Flow cytometric analyses of surface expression of P antigen (left), α5 (middle), and β1 integrins (right) in pooled normal donor RBCs. Control cells stained with secondary antibodies only are shown in black. (B) Confocal microscopy of RBCs with bound Cy3-labeled (red) parvovirus B19 (Control; i) and that following treatment with trypsin (+Trypsin; ii). Image acquisition was achieved using a Zeiss confocal microscope detecting red fluorescence in the presence of transmission light to visualize cell contour/morphology.
Mature human RBCs lack expression of the coreceptor for parvovirus B19 and do not internalize the virus. (A) Flow cytometric analyses of surface expression of P antigen (left), α5 (middle), and β1 integrins (right) in pooled normal donor RBCs. Control cells stained with secondary antibodies only are shown in black. (B) Confocal microscopy of RBCs with bound Cy3-labeled (red) parvovirus B19 (Control; i) and that following treatment with trypsin (+Trypsin; ii). Image acquisition was achieved using a Zeiss confocal microscope detecting red fluorescence in the presence of transmission light to visualize cell contour/morphology.
Primary human erythroid progenitor cells express P antigen as well as α5β1 integrins and internalize parvovirus B19 via a β1 integrin–dependent mechanism
To directly examine the role of β1 integrin in internalization of the wt parvovirus B19 into primary human erythroid progenitor cells, we used highly purified (more than 99% purity as determined by flow cytometry) CD71+ early erythroid progenitor cells derived from human bone marrow. Flow cytometric analyses, shown in Figure 6A, confirmed high-level expression of P antigen (Figure 6A, left) as well as α5 (Figure 6A, middle) and β1 (Figure 6A, right) integrins on these cells. Southern blot analysis, shown in Figure 6B, indicated that whereas no signal was detected in uninfected erythroid progenitor cells (Figure 6B, lane 1), the single-stranded wt parvovirus B19 genomes were readily detected in untreated cells (Figure 6B, lane 2). Pretreatment of cells with inhibitory β1 integrin antibody (P4C10) (Figure 6B, lane 3) or pretreatment of wt parvovirus B19 with parvovirus B19 anti-VP2 antibodies (lane 5) abolished viral internalization. Preincubation of cells with inhibitory β1 integrin antibodies and secondary goat antimouse IgG antibodies (JB1a+sec. Ab) did not affect parvovirus B19 internalization (Figure 6B, lane 4), suggesting that most β1 integrins expressed on primary erythroid progenitor cells are in a functionally activated state and antibody-induced β1 integrin clustering does not result in further stimulation of the coreceptor activity. Thus, activated α5β1 integrins also mediate parvovirus B19 entry into primary human erythroid progenitor cells.
Primary human erythroid cells express both receptor and coreceptor for parvovirus B19 and internalize the virus. (A) Flow cytometric analyses of surface expression of P antigen (left), α5 (middle), and β1 (right) integrins in primary human erythroid progenitor cells. Control cells stained with secondary antibodies only are shown in black. Cell surface expression of P antigen, α5, and β1 integrins was analyzed by flow cytometry as described in “Materials and methods.” (B) Southern blot analysis of low Mr DNA isolated from uninfected (lane 1) and wt parvovirus B19-infected primary human erythroid progenitor cells (lane 2), which showed the presence of internalized single-stranded (ss) viral genomes. Inhibitory β1 integrin antibodies (lane 3) and parvovirus B19 anti-VP2 antibodies (lane 5) abrogated viral entry, whereas cross-linking of β1 integrin-inhibitory antibodies with secondary antibodies (JB1a+sec. Ab) had no significant effect (lane 4).
Primary human erythroid cells express both receptor and coreceptor for parvovirus B19 and internalize the virus. (A) Flow cytometric analyses of surface expression of P antigen (left), α5 (middle), and β1 (right) integrins in primary human erythroid progenitor cells. Control cells stained with secondary antibodies only are shown in black. Cell surface expression of P antigen, α5, and β1 integrins was analyzed by flow cytometry as described in “Materials and methods.” (B) Southern blot analysis of low Mr DNA isolated from uninfected (lane 1) and wt parvovirus B19-infected primary human erythroid progenitor cells (lane 2), which showed the presence of internalized single-stranded (ss) viral genomes. Inhibitory β1 integrin antibodies (lane 3) and parvovirus B19 anti-VP2 antibodies (lane 5) abrogated viral entry, whereas cross-linking of β1 integrin-inhibitory antibodies with secondary antibodies (JB1a+sec. Ab) had no significant effect (lane 4).
Discussion
Since the discovery of blood group P antigen as a cellular receptor for parvovirus B19 in 1993,8 it has been debated whether P antigen is necessary and sufficient for a successful infection by parvovirus B19 of its target erythroid progenitor cell. It would seem counterintuitive for the virus to evolve a strategy to use a cellular receptor that is expressed abundantly on mature erythrocytes, which lack nuclei. This would be tantamount to suicide, for parvovirus B19 replication is absolutely dependent on the nucleus and all the replication machinery contained therein. In our previously published studies, we had shown that P antigen is necessary for binding but not sufficient for B19 entry.17 We also observed that parvovirus B19-susceptible cells had an adherent phenotype, whereas resistant cells grew as suspension cultures.17 We document here that a phenotype shift from nonadherent to adherent growth renders the erythroleukemia cell line K562 susceptible to parvovirus B19 infection. Using PMA treatment, divalent ions (Mn2+), and high-affinity conformation-stabilizing and -destabilizing β1 integrin antibodies, we demonstrate that activated β1 integrins facilitate parvovirus B19 entry into K562 cells. Mn2+ ions activate β1 integrins by overcoming structural restraints that maintain β1 integrins in a low-affinity state,22,23 and function-activating antibodies stabilize this high-affinity conformation.20
Extended PMA treatment is known to have pleiotropic effects on cell function. In the K562 cell line, which was originally isolated from a patient with chronic myeloid leukemia (CML) and expresses the BCR-ABL oncogene product, prolonged (more than 24 hours) treatment with PMA has been shown to induce a differentiation program along the megakaryocyte lineage accompanied by changes in morphology, cell growth arrest, and increased cell-cell and cell-matrix interactions.24 Although the exact mechanism of PMA-induced K562 cell differentiation is not completely understood, activation and translocation of specific protein kinase C (PKC) subtypes,24,25 the rapid and sustained activation of extracellular signal-regulated kinase mitogen-activated protein kinase (ERK MAPK),26,27 and the subsequent activation of an ERK/90-kDa ribosomal S6 kinase 1/nuclear factor (NF)–κB pathway28 seem to play a role. Interestingly, PKCα was found to be up-regulated during PMA differentiation of K562 cells, and PKCϵ was expressed only in differentiated cells.25 In addition, PKCα has been demonstrated to physically interact with β1 integrin and to be involved in its internalization,29 and PKCϵ has been shown to play a role in β1 integrin recycling.30 Prolonged PMA treatment of K562 cells has also been demonstrated to result in a down-regulation of the kinase activity of the BCR-ABL gene product,26,31 and inhibition of BCR/ABL tyrosine kinase function has been shown to restore β1 integrin–mediated adhesive and growth control functions.32,33 Although we have not yet identified the nature of the PMA-induced effect on β1 integrin that elicits coreceptor function in K562 cells, we have not detected a change in the total levels or a conformational change in surface-expressed β1 integrins following PMA treatment (K.A.W.-K., M.C.Y., A.S., unpublished results, February 2002), suggesting that the induction of β1 integrin coreceptor activity by PMA treatment may also involve events at the postreceptor level.
α5β1 integrins expressed on suspension K562 cells have been shown to bind to fibronectin-coated surfaces and to promote phagocytosis of fibronectin-opsonized beads in the presence of either Mn2+ ions or function-activating antibodies.19 However, we could not observe virus entry into suspension K562 cells in the presence of Mn2+ ions alone or in combination with high-affinity–stabilizing antibodies. Parvovirus B19 internalization was not sensitive to inhibition by RGD peptides, corroborating the notion that parvovirus B19 uptake into K562 cells does not occur through phagocytosis of fibronectin-opsonized particles. In fibroblasts, ligand occupancy of α5β1 integrins with soluble, monovalent ligand (RGD peptides) triggers integrin localization to pre-existing focal contacts but does not induce phosphorylation of focal adhesion kinase (FAK) at tyrosine residues, a hallmark of β1 integrin–dependent signaling. Direct integrin aggregation even in the absence of ligand occupancy, however, induces a prompt transmembrane accumulation of a large class of at least 20 signal transduction molecules.34 Interestingly, α5β1 integrin coreceptor function in PMA-treated K562 cells could be enhanced either by stabilizing the high-affinity conformation of β1 integrins or by inducing β1 integrin clustering, suggesting that β1 integrin–mediated signaling might be important for parvovirus B19 internalization. In addition, β1 integrin movement within the plasma membrane might contribute to its coreceptor function because the BCR/ABL tyrosine kinase has been shown to restrict β1 integrin mobility in CML cells35 and inhibition of BCR/ABL tyrosine kinase activity by PMA treatment could have restored β1 integrin mobility and function in K562 cells.
Although at first glance it would seem somewhat perplexing for parvovirus B19 to utilize a surface macromolecule as a coreceptor that is involved in cell adhesion, it is known that its target erythroid progenitor cell in the bone marrow exhibits an adherent phenotype at early stages of erythropoiesis that is mediated by the interaction of surface-expressed integrins of the β1 family with extracellular matrix fibronectin in the marrow microenvironment.36 The β1 integrin–mediated adhesion has been implicated in the regulation of progenitor anchoring, trafficking, proliferation, and differentiation.37,38 At the stage of enucleating erythroblast/reticulocyte the β1 integrins are lost from the cell surface and circulating RBCs do not express these receptors.39 In addition, β1 integrins, especially α4β1 and α5β1, are expressed on human CD34+ hematopoietic stem and progenitor and long-term culture-initiating cells (LTC-ICs) and are involved in fibronectin binding and transendothelial migration40 as well as engraftment of human hematopoietic-repopulating cells in nonobese diabetic/severe combined immunodeficient (NOD/SCID) mice.41 Our data demonstrate that parvovirus B19 internalization into purified human erythroid progenitor cells can be completely inhibited by antibodies blocking β1 integrin function, corroborating the notion that β1 integrins play a crucial role in parvovirus B19 infection of its natural target cells.
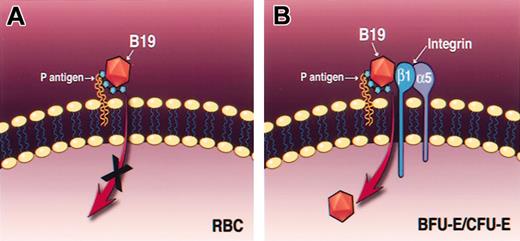
Based on our current studies, we propose a model, shown in Figure 7, for parvovirus B19 infection where binding of parvovirus B19 to RBCs, which only express the primary binding receptor, P antigen, can occur (Figure 7A); however, viral entry is restricted to progenitor cells within the erythroid lineage, because these cells express high levels of P antigen as well as the coreceptor, activated β1 integrin (Figure 7B). Further studies are required to gain a better understanding of the underlying mechanism of viral transport within the blood circulation, transition into the bone marrow cavity, and mechanism of internalization into its target erythroid progenitor cell, which, in turn, will be useful in utilizing the β1 integrin–mediated target cell–selective entry of parvovirus B19 using recombinant parvovirus B19 vectors in gene therapy in general and of parvovirus B19-globin vectors in gene therapy of human hemoglobinopathies in particular.
A model for parvovirus B19 binding and entry into primary human erythroid cells. Mature human RBCs, which express high levels of P antigen receptor, allow virus binding but not viral entry because they lack the α5β1 integrin coreceptor (A), whereas erythroid progenitor cells, which express both P antigen receptor and α5β1 integrin coreceptor, are permissive for parvovirus B19 entry (B).
A model for parvovirus B19 binding and entry into primary human erythroid cells. Mature human RBCs, which express high levels of P antigen receptor, allow virus binding but not viral entry because they lack the α5β1 integrin coreceptor (A), whereas erythroid progenitor cells, which express both P antigen receptor and α5β1 integrin coreceptor, are permissive for parvovirus B19 entry (B).
Prepublished online as Blood First Edition Paper, August 7, 2003; DOI 10.1182/blood-2003-05-1522.
Supported in part by Public Health Service grants R01 EB-002073 (A.S.), R01 HL-65570 (A.S.), and R01 HL-63169 (M.C.Y.) from the National Institutes of Health (NIH). K.A.W.-K. was supported by a postdoctoral training grant T32 HL-07910 from the NIH (A.S.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Ms Merica Gellerman and Ms Pamela Minick for their expert technical assistance and Drs Ghalib Alkhatib, Asok Antony, Hal Broxmeyer, Randy Brutkiewicz, Johnny He, and Jacqueline Hobbs for a critical reading of this manuscript. We are grateful to Kent Dupuis for generously supplying the parvovirus B19 sera.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal