Abstract
The complement system plays an important role in the initial defense against Neisseria meningitidis. In contrast, uncontrolled activation in meningococcal sepsis contributes to the development of tissue damage and shock. In a novel human whole blood model of meningococcal sepsis, we studied the effect of complement inhibition on inflammation and bacterial killing. Monoclonal antibodies (mAbs) blocking lectin and alternative pathways inhibited complement activation by N meningitidis and oxidative burst induced in granulocytes and monocytes. Oxidative burst was critically dependent on CD11b/CD18 (CR3) expression but not on Fcγ-receptors. Specific inhibition of C5a using mAb 137-26 binding the C5a moiety of C5 before cleavage prohibited CR3 up-regulation, phagocytosis, and oxidative burst but had no effect on C5b-9 (TCC) formation, lysis, and bacterial killing. An mAb-blocking cleavage of C5, preventing C5a and TCC formation, showed the same effect on CR3, phagocytosis, and oxidative burst as the anti-C5a mAb but additionally inhibited TCC formation, lysis, and bacterial killing, consistent with a C5b-9-dependent killing mechanism. In conclusion, the anti-C5a mAb 137-26 inhibits the potentially harmful effects of N meningitidis-induced C5a formation while preserving complement-mediated bacterial killing. We suggest that this may be an attractive approach for the treatment of meningococcal sepsis. (Blood. 2003;102:3702-3710)
Introduction
The gram-negative bacterium Neisseria meningitidis is an important human pathogen worldwide. It can cause meningitis or fulminant meningococcal sepsis (FMS); the latter is an overwhelming and often lethal condition that may lead to death within 24 hours. The pathogenic mechanism leading to FMS is a breakdown of homeostasis by a massive activation of diverse inflammatory systems such as the cytokine network and plasma cascades such as the complement, coagulation, fibrinolytic, and kinin/kallikreinin systems.1,2
The importance of the complement system in meningococcal disease is emphasized by several observations. Complement deficiencies are defined risk factors for meningococcal infections, indicating that complement is crucial in the initial defense against this bacterium.3-5 On the other hand, during FMS, disease severity, tissue damage, and outcome are closely related to the degree of complement activation.6,7 Thus, with respect to the pathogenesis of meningococcal disease, the complement system has been aptly named a double-edged sword.8
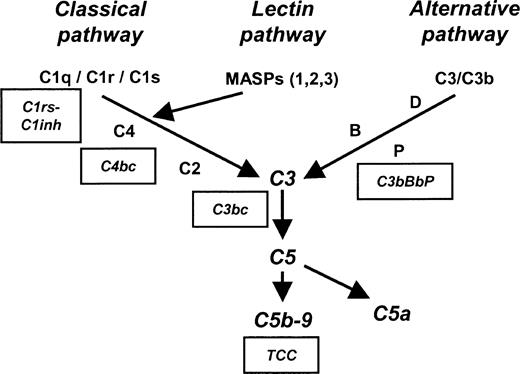
Complement is activated on the surfaces of meningococci by one or more of the 3 initial complement-activating pathways—classical, lectin, and alternative. After the activation of complement factor-3 (C3) by any of these 3 pathways, a C5 convertase (C4b2a3b through the classical and lectin pathways or C3bBbC3b through the alternative pathway) is formed, and the pivotal C5 molecule is cleaved into C5a and C5b. C5b is the initial molecule in the formation of the terminal C5b-9 complement complex (TCC). Membrane-associated TCC, also designated the C5b-9 membrane attack complex (MAC), plays a key role in lysis of the bacterium, whereas the fluid-phase SC5b-9 is lytically inactive after binding to vitronectin and clusterin. C5a is an important anaphylatoxin that has a wide range of proinflammatory effects, including endothelium activation, vascular permeability induction, histamine release, coagulation system activation, chemotaxis, cytokine modulation, CR3 up-regulation, and oxidative burst induction.9-11 A schematic overview of the complement system is shown in Figure 1.
Schematic overview of the complement system. Boxes indicate specific complement activation products measured by enzyme-linked immunosorbent assay (ELISA) and their relation to specific complement activation pathways. C1rs-C1inh complexes reflect classical pathway activation, C4bc reflects both classical and lectin pathway activation, and C3bBbP reflects alternative pathway activation. C3bc and terminal complement complex (TCC) are activation products of the final common and terminal pathways. MBL indicates mannose-binding lectin; MASP, MBL-associated serine protease.
Schematic overview of the complement system. Boxes indicate specific complement activation products measured by enzyme-linked immunosorbent assay (ELISA) and their relation to specific complement activation pathways. C1rs-C1inh complexes reflect classical pathway activation, C4bc reflects both classical and lectin pathway activation, and C3bBbP reflects alternative pathway activation. C3bc and terminal complement complex (TCC) are activation products of the final common and terminal pathways. MBL indicates mannose-binding lectin; MASP, MBL-associated serine protease.
In systemic meningococcal disease, there is a continuous search for a treatment modality that abrogates the deleterious inflammatory response. Unfortunately, to date no effective regimen has been found. Modulation of the complement system may be an attractive approach in the treatment of FMS or other inflammatory diseases.12,13 A main challenge of reducing disease severity by inhibiting complement in FMS is to preserve the beneficial bactericidal effect of complement while attenuating the detrimental systemic inflammatory effects, thus blunting one edge of the double-edged sword while sharpening the other.
The aim of the present study was to identify such a strategy. For this purpose we developed a human whole blood model of meningococcal sepsis based on a newly described method11 to determine the effect of inhibiting N meningitidis-induced complement activation on bactericidal activity and the inflammatory response.
Materials and methods
Equipment and reagents
All materials used in the stimulation experiments were endotoxin free. Polypropylene tubes were either NUNC cryotubes (Nalgene NUNC, Roskilde, Denmark) or Falcon (Becton Dickinson, Franklin Lakes, NJ) tubes. Phosphate-buffered saline (PBS) was produced in the laboratory, Dulbecco medium was obtained from Invitrogen (Paisley, Scotland), and lepirudin (Refludan) was from Hoechst (Frankfurt am Main, Germany). Flow cytometry was performed with FACScalibur (Becton Dickinson, San Jose, CA) and with an Epics XL (Coulter, Hialeah, FL) apparatus. MRX microplate reader (Dynex Technologies, Denkendorf, Germany) was used to determine optical density (OD).
Inhibitory antibodies
Mouse immunoglobulin G1 (IgG1) mAbs to human C2 (clone 175-26), factor D (clone 166-32), C5 (clone 137-76), C5a (clone 137-26), and the isotype-matched control (clone G3-519, anti-HIV1 gp 120) were produced and purified under identical conditions in the laboratory of one of the coauthors (M.F.).14 The anti-C5 antibody 137-76 binds C5 and prevents its cleavage, blocking the formation of C5a and that of C5b-9.15 Anti-C5a 137-26 blocks free C5a directly and preneutralizes its biologic effects by binding to the C5a moiety on native C5 without interfering with C5 cleavage. The anti-C5a antibody 137-26 is highly human specific, and no cross-reactivity has been observed with nonprimate species.16
Purified mouse IgG1 mAb to human CD11b (clone ICRF 44) was obtained from Serotec (Oxford, United Kingdom). Mouse mAb (F(ab′)2) to human CD16/FcγRIII (clone 3G8) recognizes both allelic forms of CD16 and blocks the binding of immune-complexed IgG to CD16. Mouse mAb (F(ab′)2) to human CD32/FcγRII (clone 7.3) reacts with the domain 2 epitope of all CD32 isoforms and blocks IgG immune-complexed binding. Mouse mAb (F(ab′)2) to human CD64/FcγRI (clone 10.1) recognizes the CD64 molecule of 72 kDa from gene FcγRIA and blocks binding of FcγRIA to IgG opsonized cells. All were obtained from Ancell Corp (Bayport, MN).
Purified mouse IgG1 mAb to human mannose-binding lectin (MBL) (clone HYB131-01), which reacts with human MBL both in its polymeric conformation and as a single subunit, was obtained from the AntibodyShop (Copenhagen, Denmark) and was documented by the manufacturer to block MBL function. All antibodies were used in concentrations ranging from 6.25 to 25 μg/mL because preliminary experiments showed maximum inhibition in this concentration range, except for the anti-CD11b and anti-MBL antibodies, which had to be used in concentrations up to 50 μg/mL (anti-CD11b) or 100 μg/mL (anti-MBL) to obtain maximum inhibition.
Bacterial strains
N meningitidis H44/76 is an isolate of a patient with invasive meningococcal disease.17 H44/76 is the production strain of the Norwegian group B OMV vaccine and an international reference strain. It has been serologically classified as B:15:P1.7,16, immunotype L3,7,9. N meningitidis was grown overnight on Kellog medium and resuspended in Hanks balanced salt solution (HBSS) (Gibco Invitrogen). Heat inactivation occurred at 60°C for 40 minutes. Escherichia coli and fluorescein isothiocyanate (FITC)-labeled, opsonized E coli strain LE 392 (American Type Culture Collection 33572) were obtained from Orpegen (Pharma, Heidelberg, Germany) and were used as controls. The concentration of bacteria was determined by measuring OD at 630 nm. The variant H44/76-1, more strongly expressing the Opc protein, was used in the serum-bactericidal activity and whole blood bactericidal activity assays.
N meningitidis was FITC labeled as described previously.18 In brief, 2 × 109 meningococci/mL was incubated with 0.16 mg/mL FITC (isomer 1, 90% high-performance liquid chromatography [HPLC]; Sigma Chemical, St Louis, MO) for 30 minutes at 37°C. Bacteria were washed 4 times and resuspended in PBS. The concentration of bacteria after washing was determined by measuring OD at 630 nm.
For the experiments using live meningococci, bacteria were grown overnight on brain-heart infusion (BHI) agar, reseeded on BHI agar plates, grown for 4 hours at 37°C in 5% CO2 into mid-log-phase, and harvested in HBSS with 0.1% bovine serum albumin (BSA).
Donors
Seven healthy donors were used throughout the experiments. Concentration of specific antibodies in the sera of the controls against N meningitidis H44/76 was determined by flow cytometry, as described previously.19 In brief, 5 μL of 109N meningitidis/mL was incubated with 50 μL of dilutions of serum for 1 hour at room temperature, washed twice, and developed with FITC-labeled goat antihuman IgG (Cappel; Organon Teknika, Turnhout, Belgium) for 1 hour followed by one washing. A 2-fold dilution series of reference plasma was used for the standard curve. For the serum bactericidal assay and the whole blood bactericidal activity assay, 2 separate donors were used with known high bactericidal titers to N meningitidis in the serum bactericidal assay and a high concentration of antibodies after vaccination. Informed consent was obtained from all donors before the experiments were performed, and the human experimentation guidelines of the local Ethics Committee of Rikshospitalet University Hospital were followed in the conduct of the research.
Whole blood model of inflammation
A recently developed whole blood model to study the role of complement in E coli-induced inflammation was modified for N meningitidis.11 The model is based on anticoagulation with lepirudin, a recombinant hirudin, which is a highly specific thrombin inhibitor not influencing complement activation. Whole blood was collected in polypropylene tubes containing lepirudin (50 μg/mL). Whole blood was preincubated with PBS or antibody for 4 minutes at 37°C in a water bath. For CR3 (CD11b/CD18) expression, phagocytosis, and oxidative burst, incubation with the stimulants was for 10 minutes at 37°C. After incubation, samples were processed immediately for flow cytometry. For the detection of complement activation, samples were incubated for 1 hour at 37°C, and activation was stopped by adding 20 mM (final concentration) ethylenediaminetetraacetic acid (EDTA).
Oxidative burst, phagocytosis, and CR3 (CD11b/CD18) expression
Oxidative burst and phagocytosis were measured using commercially available Burst-test and Phago-test kits (Orpegen Pharma, Heidelberg, Germany). FITC-labeled meningococci were used in the phagocytosis assay. Using a forward/side scatter (FSC/SSC) dot plot, granulocytes and monocytes were analyzed separately with regard to median fluorescence intensity (MFI). CR3 expression was measured after incubation with inhibitors and stimulants after cells were fixed with 0.5% (vol/vol) paraformaldehyde. Cells were stained with anti-CD-14 FITC (Becton Dickinson, San Jose, CA) to distinguish monocytes from granulocytes, the nuclear dye LDS-751 (FL-3) (Molecular Probes, Eugene, OR), anti-CD11b-PE, or isotype control (γ2a).
Complement activation enzyme immunoassays
Activation of the classical complement pathway was determined as described previously,20 using the monoclonal antibody Kok-12 specific to a neoepitope exposed only when the C1 inhibitor (C1inh) is in complex with its substrates. This antibody was a kind gift from Prof Dr C. E. Hack (Amsterdam, the Netherlands). Activation of the alternative pathway was detected by quantifying the alternative convertase C3bBbP, as was recently described in detail.11 Activation of the classical and lectin pathways was determined by an assay using a mAb specific for a neoepitope exposed in activated C4 detecting C4b and C4c (C4bc).21 The antibody was a kind gift from Prof Dr C. E. Hack. Activation of the final common pathway was quantified using the monoclonal antibody bH6 specific for a neoepitope expressed in C3b, iC3b, and C3c (C3bc) as described previously.22 Activation of the terminal pathway was quantified using the monoclonal antibody aE11 specific for C9 incorporated in the fluid-phase SC5b-9 complex (TCC), as described previously.23 An overview of these complement-activation markers can be seen in Figure 1.
Lysis of sensitized SRBCs in human whole blood
Lytic activity of the terminal complement complex in human whole blood was established using a modified classical pathway hemolytic assay. Sheep red blood cells (SRBCs) (hemolytic system; Virion/Serion, Würtzburg, Germany) were incubated at 37°C for 30 minutes, spun down at 1400g, and resuspended to a 5% solution in sterile veronal buffered saline (bioMerieux SA, Nancy l'Étoile, France) supplemented with 0.01% BSA (Sigma Chemical). SRBCs (20 μL) were incubated in the whole blood (120 μL) samples for 20 minutes at 37°C in a waterbath. Then 20 mM EDTA was added to stop ongoing complement activation, and the samples were spun down at 1400g. Supernatants were collected and read at 405 nm.
Serum bactericidal activity
The effect of the anti-C5 137-76 and anti-C5a 137-26 mAbs on serum bactericidal activity was tested using an agar overlay method, as described previously.24,25 In brief, human complement, as plasma, was used at a final concentration of 25%. A 2-fold dilution series, in HBSS with 0.1% BSA of the sera to be examined, were inoculated with approximately 100 colony-forming units (CFUs) per well of logarithmic phase-growth meningococci. This mixture was incubated for 60 minutes at 37°C in air; after that agar was added, and plates were incubated overni at 37°C in 5% CO2. The number of CFUs was counted with the use of a magnifyinghtg glass. Log2 titer represents the highest dilution of sera or the lowest antibody concentration at which more than 50% of the inoculum was killed.
Whole blood bactericidal activity
N meningitidis H44/76, grown overnight on BHI agar, was subcultured and grown into log-phase for 4 hours. Approximately 5000 CFUs were added to 1.1 mL lepirudin-anticoagulated whole blood samples preincubated for 5 minutes with PBS or antibody. Immediately after inoculation (t = 0) and after certain time periods, 100 μL whole blood was seeded on microbiologic Petri dishes containing blood agar and incubated for 24 hours at 37°C and 5% CO2. Bacterial growth was expressed as CFU/100 μL whole blood added. Blood was mixed thoroughly before each sampling on a whirl mixer.
Statistics
The nonparametric Mann-Whitney U test for unpaired data was used to analyze comparisons for statistical significant differences. P values less than .05 were considered statistically significant.
Results
Complement activation by N meningitidis in human whole blood
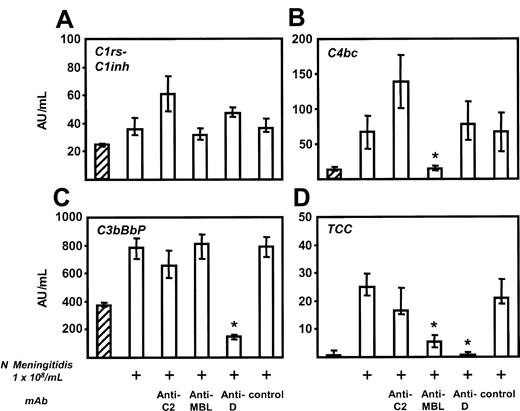
To determine the relative contributions of the classical, alternative, and lectin pathway on complement activation by N meningitidis, monoclonal antibodies blocking C2 (classical and lectin pathways), MBL (lectin pathway), and factor D (alternative pathway) were tested for their ability to inhibit the formation of specific complement activation products in lepirudin-anticoagulated human whole blood incubated with heat-inactivated bacteria for 1 hour. C1rs-C1inh complexes (classical pathway), C4bc (classical and lectin pathway), and C3bBbP (alternative pathway) were used as markers of initial pathway activation; TCC was used as a marker of total complement activation (Figure 1).
N meningitidis (1 × 108/mL) induced some classical pathway activation, seen as a modest, but significant, increase in C1rs-C1inh complexes. As expected, no effect of any of the antibodies on C1rs-C1inh could be observed (Figure 2A). A marked and significant increase in C4bc formation was seen after stimulation with N meningitidis. Anti-C2 had no effect; however, anti-MBL completely inhibited C4bc formation (Figure 2B). Noticeable alternative pathway activation was also seen after stimulation with meningococci, and anti-factor D inhibited all this alternative pathway activation (Figure 2C). N meningitidis induced a marked increase in fluid-phase TCC, which was completely abolished by anti-factor D, markedly reduced by anti-MBL (75% reduction), and modestly but not significantly reduced by anti-C2 (Figure 2D). There was no relation between antibody titers against N meningitidis H44/76 in the donors used for these experiments and the degree of complement activation or inhibition. Taken together, these data support mainly lectin and alternative pathway-dependent mechanisms of complement activation by N meningitidis H44/76 in this whole blood assay.
Complement activation byN meningitidis. Lepirudin-anticoagulated human whole blood was incubated with 1 × 108 heat-inactivated N meningitidis H44/76 for 1 hour at 37°C in the presence or absence of the following complement-inhibiting mAbs: anti-C2 (25 μg/mL), anti-MBL (100 μg/mL), and anti-factor D (25 μg/mL). C1rs-C1inh complexes (classical pathway; panel A), C4bc (classical and lectin pathways; panel B), and C3bBbP (alternative pathway; panel C) were used as markers of initial pathway activation, and fluid-phase terminal complement complex (TCC) was measured as an indicator of total complement activation (D). Minor spontaneous formation of all complement-activation products occurred in unstimulated blood (hatched columns). Meningococci (1 × 108) induced a minor increase of C1rs-C1inh complex formation but significant C4bc, C3bBbP, and TCC formation (second columns). Anti-C2 had no statistically significant effect on any of the activation products, whereas anti-MBL completely inhibited C4bc formation, and anti-factor D completely inhibited alternative pathway activation. The increase in TCC was completely abolished by anti-factor D and was markedly reduced by anti-MBL. Median ± IQRs of 4 separately performed experiments are presented. *P < .05 compared with N meningitidis-stimulated sample (second columns).
Complement activation byN meningitidis. Lepirudin-anticoagulated human whole blood was incubated with 1 × 108 heat-inactivated N meningitidis H44/76 for 1 hour at 37°C in the presence or absence of the following complement-inhibiting mAbs: anti-C2 (25 μg/mL), anti-MBL (100 μg/mL), and anti-factor D (25 μg/mL). C1rs-C1inh complexes (classical pathway; panel A), C4bc (classical and lectin pathways; panel B), and C3bBbP (alternative pathway; panel C) were used as markers of initial pathway activation, and fluid-phase terminal complement complex (TCC) was measured as an indicator of total complement activation (D). Minor spontaneous formation of all complement-activation products occurred in unstimulated blood (hatched columns). Meningococci (1 × 108) induced a minor increase of C1rs-C1inh complex formation but significant C4bc, C3bBbP, and TCC formation (second columns). Anti-C2 had no statistically significant effect on any of the activation products, whereas anti-MBL completely inhibited C4bc formation, and anti-factor D completely inhibited alternative pathway activation. The increase in TCC was completely abolished by anti-factor D and was markedly reduced by anti-MBL. Median ± IQRs of 4 separately performed experiments are presented. *P < .05 compared with N meningitidis-stimulated sample (second columns).
CR3 up-regulation and induction of oxidative burst by live and heat-inactivated meningococci
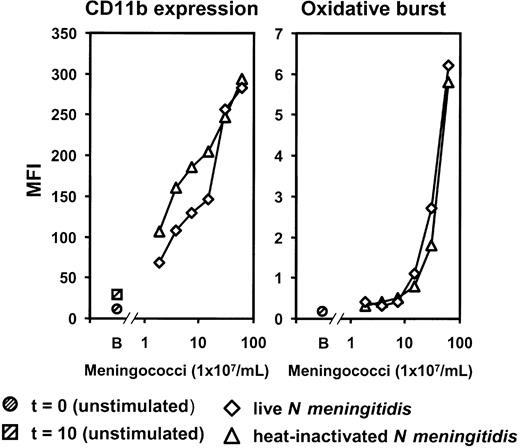
To validate the use of heat-inactivated meningococci in the inflammatory response experiments, live log-phase N meningitidis H44/76 and heat-inactivated N meningitidis H44/76 were compared with respect to CR3 (CD11b/CD18) expression and oxidative burst. A dose-dependent increase in granulocyte CR3 and oxidative burst was observed, with no difference between live and heat-inactivated bacteria (Figure 3). Results in monocytes showed a similar pattern to those seen in granulocytes. Based on these results the following experiments were performed with heat-inactivated meningococci.
Comparison of live and heat-inactivated bacteria. A dose-dependent increase of granulocyte CR3 (CD11b/CD18) expression and oxidative burst was observed in lepirudin-anticoagulated human whole blood after stimulation for 10 minutes at 37°C. Results for live log-phase and heat-inactivated meningococci were similar. MFI indicates median fluorescence intensity; B, baseline (unstimulated sample) at time of incubation (t = 0) and after 10 minutes (t = 10). The experiment was repeated once with virtually identical results.
Comparison of live and heat-inactivated bacteria. A dose-dependent increase of granulocyte CR3 (CD11b/CD18) expression and oxidative burst was observed in lepirudin-anticoagulated human whole blood after stimulation for 10 minutes at 37°C. Results for live log-phase and heat-inactivated meningococci were similar. MFI indicates median fluorescence intensity; B, baseline (unstimulated sample) at time of incubation (t = 0) and after 10 minutes (t = 10). The experiment was repeated once with virtually identical results.
Effect of initial complement pathway inhibition on oxidative burst
N meningitidis-induced oxidative burst in human whole blood was essentially dependent on complement activation because mAbs blocking the initial complement pathways markedly inhibited oxidative burst in granulocytes (Figure 4, left panel) and monocytes (Figure 4, right panel). Anti-C2 and anti-factor D partially reduced the granulocyte oxidative burst, whereas the monocyte oxidative burst was only marginally affected. However, when anti-C2 and anti-factor D were combined, the granulocyte oxidative burst was completely abolished and the monocyte oxidative burst was markedly reduced. Finally, anti-MBL alone markedly inhibited oxidative burst in both cell types, an effect that was further enhanced when anti-MBL and anti-factor D were combined. These data indicate that oxidative burst induced by the meningococcus in whole blood is dependent on complement activation through the lectin and alternative pathways.
Effect of complement inhibition on oxidative burst.N meningitidis H44/76-induced granulocyte and monocyte oxidative burst was measured in whole human blood after a 10-minute incubation with 2 × 108 meningococci/mL in the presence or absence of complement inhibitory mAbs. Antibody concentrations ranged from 6.25 to 25 μg/mL for anti-C2 and anti-D and from 25 to 100 μg/mL for anti-MBL and the isotype control. Note that combining anti-factor D with anti-MBL or anti-C2 enhanced the inhibitory effect. B indicates baseline (unstimulated sample) incubated for 10 minutes (t = 10). Median values of 4 separate experiments are presented.
Effect of complement inhibition on oxidative burst.N meningitidis H44/76-induced granulocyte and monocyte oxidative burst was measured in whole human blood after a 10-minute incubation with 2 × 108 meningococci/mL in the presence or absence of complement inhibitory mAbs. Antibody concentrations ranged from 6.25 to 25 μg/mL for anti-C2 and anti-D and from 25 to 100 μg/mL for anti-MBL and the isotype control. Note that combining anti-factor D with anti-MBL or anti-C2 enhanced the inhibitory effect. B indicates baseline (unstimulated sample) incubated for 10 minutes (t = 10). Median values of 4 separate experiments are presented.
Effect of inhibiting C5 and C5a on CR3 expression, phagocytosis, and oxidative burst
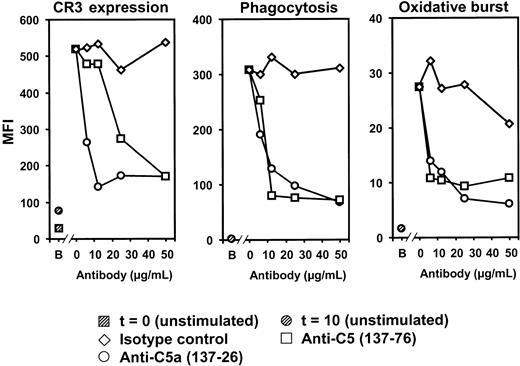
The effect of inhibiting C5 and C5a on N meningitidis-induced CR3 expression, phagocytosis of FITC-labeled meningococci, and oxidative burst by N meningitidis in granulocytes and monocytes was examined in human whole blood using anti-C5 and anti-C5a mAbs. Both antibodies efficiently abolished, in a dose-dependent fashion, CR3 up-regulation, phagocytosis, and oxidative burst in granulocytes, whereas the isotype control antibody had no effect (Figure 5). Monocyte CR3 expression, phagocytosis, and oxidative burst were also inhibited by the anti-C5 and anti-C5a antibodies, though this inhibition was less pronounced than in granulocytes. The data suggest that the inflammatory responses observed, particularly in granulocytes, were largely dependent on C5 activation, and the observation that both antibodies were equally efficient indicates that the response was mediated by C5a and not by TCC (C5b-9).
Effect of anti-C5 and anti-C5a on granulocyte responses.N meningitidis H44/76-induced CR3 (CD11b/CD18) expression, phagocytosis of FITC-labeled meningococci, and oxidative burst by N meningitidis were measured in whole human blood after a 10-minute incubation with 2 × 108 meningococci/mL in the presence or absence of the mAbs anti-C5 (clone 137-76, preventing C5 cleavage), anti-C5a (clone 137-26), and an isotype control (6.25-50 μg/mL). Anti-C5 and anti-C5a markedly, and to the same extent, inhibited N meningitidis-induced granulocyte CR3 up-regulation, phagocytosis, and oxidative burst. B indicates baseline (unstimulated sample) at time of incubation (t = 0) or after 10 minutes of incubation at 37°C (t = 10). Results from 1 of 3 representative experiments are shown.
Effect of anti-C5 and anti-C5a on granulocyte responses.N meningitidis H44/76-induced CR3 (CD11b/CD18) expression, phagocytosis of FITC-labeled meningococci, and oxidative burst by N meningitidis were measured in whole human blood after a 10-minute incubation with 2 × 108 meningococci/mL in the presence or absence of the mAbs anti-C5 (clone 137-76, preventing C5 cleavage), anti-C5a (clone 137-26), and an isotype control (6.25-50 μg/mL). Anti-C5 and anti-C5a markedly, and to the same extent, inhibited N meningitidis-induced granulocyte CR3 up-regulation, phagocytosis, and oxidative burst. B indicates baseline (unstimulated sample) at time of incubation (t = 0) or after 10 minutes of incubation at 37°C (t = 10). Results from 1 of 3 representative experiments are shown.
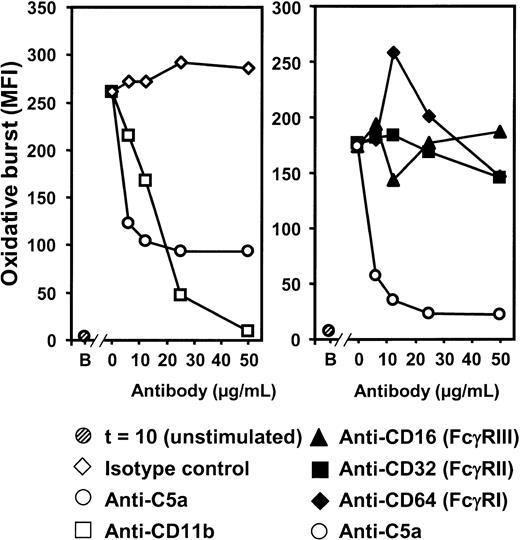
Effect of inhibition of CR3 and Fcγ receptors on the oxidative burst
The substantial complement-dependent increase in the expression of CR3 led us to investigate the relative roles of CR3 and Fcγ receptor (FcγR) in the oxidative burst induced by N meningitidis. Inhibition of CR3 using a mAb blocking CD11b showed that oxidative burst in granulocytes (Figure 6, left panel) was critically dependent on CR3 because anti-CD11b inhibited oxidative burst to baseline values. In contrast, F(ab′)2 antibodies blocking the interaction of FcγRI, FcγRII, and FcγRIII (CD64, CD32, and CD16, respectively) with their ligands had no effect on granulocyte oxidative burst (Figure 6, right panel). Results obtained for monocyte oxidative burst showed a similar pattern.
Effect of CR3 and FcγR inhibition on granulocyte oxidative burst. The anti-CD11b-blocking antibody ICRF44 (6.25-50 μg/mL) completely inhibited oxidative burst by N meningitidis H44/76 (2 × 108/mL, 10-minute incubation) (left panel). In contrast, blocking of FcγR using anti-CD16 (FcγRIII), anti-CD32 (FcγRII), and anti-CD64 (FcγRI) (6.25-50 μg/mL) had no effect (right panel). Anti-C5a (clone 137-26) was included in both experiments for comparison. B indicates baseline (unstimulated sample) after a 10-minute incubation (t = 10). Results from 1 of 3 representative experiments are shown.
Effect of CR3 and FcγR inhibition on granulocyte oxidative burst. The anti-CD11b-blocking antibody ICRF44 (6.25-50 μg/mL) completely inhibited oxidative burst by N meningitidis H44/76 (2 × 108/mL, 10-minute incubation) (left panel). In contrast, blocking of FcγR using anti-CD16 (FcγRIII), anti-CD32 (FcγRII), and anti-CD64 (FcγRI) (6.25-50 μg/mL) had no effect (right panel). Anti-C5a (clone 137-26) was included in both experiments for comparison. B indicates baseline (unstimulated sample) after a 10-minute incubation (t = 10). Results from 1 of 3 representative experiments are shown.
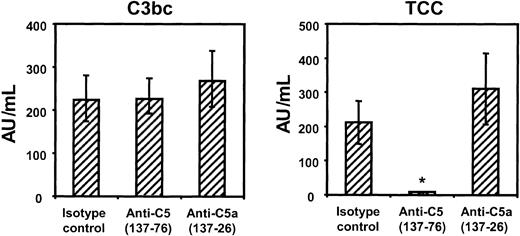
Effect of inhibiting C5 and C5a on red cell lysis and bactericidal activity
The effect of the anti-C5 and anti-C5a antibodies on the formation of fluid-phase C3 activation products (C3bc) and TCC in human whole blood was first investigated. As expected, neither of the antibodies had any effect on initial complement pathway activation because C3bc formation was unaffected (Figure 7, left panel). Blocking C5 activation by anti-C5 137-76 completely abrogated the formation of TCC, whereas anti-C5a 137-26 and the isotype control had no effect on TCC formation (Figure 7, right panel).
Effect of anti-C5 and anti-C5a onN meningitidis-induced complement activation. Generation of C3bc (left panel) and TCC (right panel) in lepirudin-anticoagulated human whole blood after incubation for 1 hour at 37°C with 1 × 108 heat-inactivated N meningitidis H44/76 in the presence of anti-C5 (clone 137-76, 50 μg/mL), anti-C5a (clone 137-26, 50 μg/mL), or isotype control antibody (50 μg/mL). Formation of TCC was completely blocked by anti-C5 but unaffected by anti-C5a. Median ± IQRs of 4 separate experiments are presented. *P < .05 compared with isotype control.
Effect of anti-C5 and anti-C5a onN meningitidis-induced complement activation. Generation of C3bc (left panel) and TCC (right panel) in lepirudin-anticoagulated human whole blood after incubation for 1 hour at 37°C with 1 × 108 heat-inactivated N meningitidis H44/76 in the presence of anti-C5 (clone 137-76, 50 μg/mL), anti-C5a (clone 137-26, 50 μg/mL), or isotype control antibody (50 μg/mL). Formation of TCC was completely blocked by anti-C5 but unaffected by anti-C5a. Median ± IQRs of 4 separate experiments are presented. *P < .05 compared with isotype control.
The ability of the anti-C5 mAbs to interfere with the formation of a functional lytic C5b-9 MAC was investigated using an adapted CH-50 hemolytic assay to study complement-lytic activity of human whole blood on sensitized SRBCs. Anti-C5 137-76 inhibited the lysis of SRBCs in whole blood by 89% (interquartile range [IQR], 96%-73%), whereas anti-C5a 137-26 had no effect. Furthermore, in a standard test for serum bactericidal activity (SBA), anti-C5 137-76 completely inhibited SBA of human sera and isolated anti-meningococcal antibody supplemented with exogenous complement, whereas anti-C5a 137-26 and the isotype control antibody showed no effect (Table 1).
Effect of anti-C5 and anti-C5a on serum bactericidal activity
Bactericidal activity . | No antibody . | Isotype control . | Anti-C5a 137-26 . | Anti-C5 137-76 . |
|---|---|---|---|---|
| L3, 7, 9 (positive control) | 14 | 15 | 15 | < 8 |
| L8 (negative control) | < 8 | < 8 | < 8 | < 8 |
| Subject 1 | 6 | 5 | 5 | < 1 |
| Subject 2 | 7 | 8 | 7 | < 1 |
Bactericidal activity . | No antibody . | Isotype control . | Anti-C5a 137-26 . | Anti-C5 137-76 . |
|---|---|---|---|---|
| L3, 7, 9 (positive control) | 14 | 15 | 15 | < 8 |
| L8 (negative control) | < 8 | < 8 | < 8 | < 8 |
| Subject 1 | 6 | 5 | 5 | < 1 |
| Subject 2 | 7 | 8 | 7 | < 1 |
Bactericidal activity (agar overlay method) of serum of the 2 control subjects and of anti-LPS L3,7,9 lgG2a antibody (positive control) and anti-LPS L8 lgG1 antibody (negative control) expressed as log2 titer of the last reciprocal serum dilution at which more than 50% of the inoculum was killed. Both control subjects showed excellent serum bactericidal activity against N meningitidis H44/76-1. Anti-C5 completely inhibited all bactericidal activity, whereas anti-C5a and isotype control had no effect.
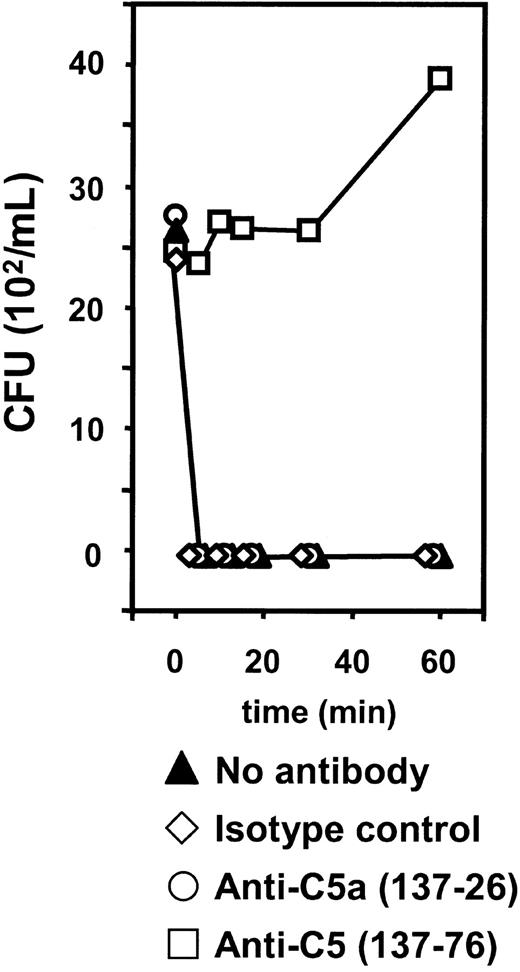
Finally, live N meningitidis H44/76 was incubated in human whole blood. Samples from the same 2 control subjects used in the SBA measurements were used. After 5 minutes of incubation no growth of meningococci was seen (Figure 8). Anti-C5a 137-26 and the isotype control antibody had no effect on the bactericidal activity. Inhibition with anti-C5 137-76, however, completely abrogated the ability of human whole blood to eliminate meningococci. Here, bacterial counts after shorter periods of incubation remained equivalent to amounts at the start of incubation, whereas after 60 minutes bacterial counts had increased almost 2-fold, indicating active bacterial growth in these samples (Figure 8). These data indicate that the elimination of serogroup B N meningitidis from this human whole blood model at the concentration tested is dependent on the lytic C5b-9 pathway of complement and that this pathway is kept functionally open in the presence of the anti-C5a mAb 137-26.
Effect of anti-C5 and anti-C5a on bacterial killing in whole blood. Bacterial counts on blood-agar plates 24 hours after seeding 100 μL whole blood incubated for different time intervals with live log-phase N meningitidis H44/76 in the absence or presence of anti-C5 (clone 137-76), anti-C5a (clone 137-26), and isotype control antibody. Median results of duplicate experiments with 2 different donors are presented.
Effect of anti-C5 and anti-C5a on bacterial killing in whole blood. Bacterial counts on blood-agar plates 24 hours after seeding 100 μL whole blood incubated for different time intervals with live log-phase N meningitidis H44/76 in the absence or presence of anti-C5 (clone 137-76), anti-C5a (clone 137-26), and isotype control antibody. Median results of duplicate experiments with 2 different donors are presented.
Discussion
N meningitidis is exclusively a human pathogen, and no animal model accurately simulating fulminant meningococcal sepsis is available. This mandates the use of an in vitro experimental system approaching as closely as possible the in vivo situation. Recently, an in vitro whole human blood model was developed to study the role of complement in E coli-induced inflammatory processes.11 The principle of this model is to keep all ambient inflammatory systems intact to be activated, and to mutually interact, but still avoid coagulation. Because most anticoagulants such as EDTA, citrate, and heparin interact with critical steps in the inflammatory network, the model uses the highly specific thrombin inhibitor lepirudin, a recombinant hirudin analog, as anticoagulant. In the present study the model was used to evaluate the in vitro inflammatory responses to N meningitidis. The main limitations of the model with regard to the in vivo situation are the absence of a vascular endothelium and the effect of lepirudin on thrombin. Thrombin is known to be an inflammatory mediator,26 and hirudin-based peptides were found to inhibit thrombin-induced inflammatory effects on endothelial cells.27 However, because whole blood models need anticoagulation, it is impossible to circumvent this problem completely. We suggest that lepirudin is the best alternative in complement activation studies because its effect is limited to thrombin inhibition, the final step of coagulation. Hirudin was found to be superior in studying platelet and monocyte activation in whole blood, and it did not modulate complement activation, interleukin-8 (IL-8) release, or tissue-factor expression, supporting lepirudin as the anticoagulant of choice for this model.28,29
For safety reasons heat-inactivated bacteria were used whenever possible. Because heat inactivation may influence the antigen exposure on bacteria, the interaction with the immune system may be altered.18,30 Therefore, we compared live and heat-inactivated bacteria with respect to inflammatory responses of importance for this study and showed that they elicited virtually identical responses. This validates the use of heat-inactivated N meningitidis to investigate the inflammatory response induced by these microorganisms.
In the whole blood model, a relatively high bacterial concentration (2 × 108) was used for the induction of CR3 up-regulation and oxidative burst. The central mechanism in the pathogenesis of fulminant meningococcal septic shock (FMS) is an unimpeded—and unmatched by any other infectious organism—outgrowth of meningococci within the bloodstream. Bacterial concentrations in blood during FMS can exceed 108 bacteria/mL,31-33 and these high bacterial loads are seen only in the most severe cases.34 The concentrations we used in the in vitro model resemble the concentration of meningococci seen in the bloodstream during severe FMS and reflect the levels of bacteremia that induce the deleterious systemic inflammatory response. The number of bacteria used in the serum and whole blood bactericidal assays (2.5 × 102) was much lower than the amount used to study CR3 up-regulation and oxidative burst. After invasion in the bloodstream by N meningitidis, the first line of defense is formed by complement-mediated, C5b-9-dependent direct bacterial lysis. Thus, this line of defense is engaged already at low concentrations of bacteria. When this defense fails, meningococci will multiply unimpeded, reaching concentrations (107-108 bacteria/mL) at which massive activation of the complement system causes tissue damage by granulocyte activation. For these reasons, we studied killing at relatively low concentrations, whereas the harmful effect of complement activation was studied at high concentrations of bacteria.
The aim of the present study was to evaluate with this model an anticomplement strategy that inhibits complement-mediated harmful processes and leaves bactericidal activity intact as a possible treatment option for meningococcal disease. It was shown that mAbs blocking the lectin pathway and alternative pathway, alone or in combination, inhibited complement activation by N meningitidis. In addition, it was found that blocking complement activation in this way abolished the oxidative burst in granulocytes and attenuated it in monocytes. The oxidative burst was dependent on CR3 (CD11b/CD18) expression, whereas the inhibition of Fcγ-receptors had no effect on oxidative burst, as previously demonstrated for E coli.11 The mAb 137-76, which blocks the cleavage of C5, averted CR3 up-regulation and inhibited phagocytosis and associated oxidative burst, but also inhibited the formation of TCC and bactericidal activity. However, the inhibition of C5a by the mAb 137-26, which binds both free C5a and the C5a moiety of native C5 without interfering with the cleavage of C5,16 had the same inhibitory effect on CR3 up-regulation, phagocytosis, and oxidative burst, but it preserved TCC formation and bactericidal activity.
Theoretically, the inflammatory response induced by C5 activation could be attributed to the potent anaphylatoxin C5a or to the C5b-9 complex given that the latter has been shown not only to be a lytic, bactericidal structure but is also able to induce inflammatory reactions by sublytic attack on nucleated cells.35 However, the inflammatory responses we observed, as caused by C5 activation, were solely mediated by C5a and not by the C5b-9 complex because exactly the same degree of inhibition was observed using either the anti-C5 or the anti-C5a antibody.
The initial pathways of complement activation after stimulation of whole blood by N meningitidis were studied by measuring specific complement activation products and by using monoclonal antibodies blocking specific complement components essential for each of these pathways. N meningitidis induced significant increases in all activation products, but they were more pronounced for C4bc and C3bBbP than for C1rs-C1inh complexes. We found that anti-MBL blocked C4bc formation and that anti-factor D blocked C3bBbP formation. Both anti-MBL and anti-factor D inhibited TCC formation by N meningitidis. This indicates that both the lectin and the alternative pathway are involved in complement activation by meningococci. The alternative pathway may be activated directly or as an amplification of the lectin pathway activation. These results also imply a limited role for the classical pathway in complement activation by N meningitidis, further underscored by the finding that the antibody titer against H44/76 meningococci did not correlate with the magnitude of classical pathway activation in this assay system. Our findings are supported by the findings of Bjerre et al,36 who suggested that complement activation by neisserial OMVs is largely dependent on the lectin and alternative pathways, and by Brandtzaeg et al,8 who found that during meningococcal septic shock, systemic complement activation is predominantly dependent on the alternative pathway. The latter study was performed before the contribution of the lectin pathway could be assessed, and it might be that the alternative pathway activation observed was partly the result of amplification from the lectin pathway. The observation that complement activation by the meningococcus is mostly lectin- and alternative-pathway-mediated seemingly contrasts with the increased SBA seen by specific bactericidal antibodies. Drogari-Apiranthitou et al,37 who also found that complement activation and assembly of the C5b-9 (MAC) on the meningococcal surface are for the most part independent of classical pathway activation, suggest that it is likely that proper MAC insertion mediated by antibody rather than the quantity of MAC formation is of importance for efficient bacterial killing.38,39 The observation that anti-C2 inhibited complement activation to a lesser extent than anti-MBL may be explained by a direct activation of C3 by MBL-associated serine proteases (MASPs), bypassing C2, as was suggested recently,40,41 though recent data contrast such a direct activation under physiologic conditions.42
Incubation of whole blood with N meningitidis induces the formation of reactive oxygen species such as the superoxide anion and hydrogen peroxide. These reactive oxygen species are reported to activate the nuclear transcription factor NFκB and are highly toxic to host tissues.43,44 When microbes intrude the body, granulocyte activation and oxidative burst—aimed at eliminating the invading agent—occur in a localized area with limited tissue damage. However, if systemic activation of the granulocytes occurs, this contributes to the development of organ failure, including acute respiratory distress syndrome (ARDS) and irreversible septic shock. Importantly, we showed that after incubation of whole blood with N meningitidis, the production of reactive oxygen species, as reflected by the oxidative burst, is completely dependent on complement activation because it could be fully inhibited by blocking complement. Remarkably, anti-MBL alone inhibited oxidative burst better than one would expect based on the effects of anti-MBL on complement activation. An additional function of MBL, the reported direct effect of this protein on opsonophagocytosis, may explain this observation.45
The oxidative burst process was dependent on C5a-mediated up-regulation of CR3, leading to increased phagocytosis of meningococci and increased oxidative burst, 2 closely related processes. This expands for N meningitidis our earlier observation that showed C5a and C5aR play essential roles in the E coli-induced up-regulation of CR3, phagocytosis, and oxidative burst.11 The divergent effect of C5a inhibition on CR3 expression, phagocytosis, and oxidative burst between granulocytes and monocytes, with a relatively decreased effect of C5a inhibition on these processes in the monocyte, suggests that alternative mediators are also of importance in the regulation of monocyte CR3 expression.
Invasive gram-negative bacteria can be cleared from the blood by C3b- or IgG-dependent phagocytosis or by lysis of the bacterium by the MAC.39,46 In the present study we showed that in whole human blood with strong anti-meningococcal SBA, meningococci are rapidly and completely killed. Inhibition of C5a, with potent inhibitory effects on CR3-mediated phagocytosis, had no effect on TCC formation, SBA, or bactericidal activity in whole blood. Of importance, however, the inhibition of C5 completely prevented TCC formation and the killing of meningococci. This implies that in the presence of a functional MAC, meningococci are rapidly killed, whereas in the absence of the MAC unimpeded growth occurs. Thus, the MAC is the main determinant of meningococcal killing in this human whole blood assay using a low inoculum of bacteria. Theoretically, the inhibition of phagocytosis by the anti-C5a antibody at high intravascular bacterial concentrations could be deleterious because this is also a primary host defense mechanism. However, if the anti-C5a antibody were used in the treatment of patients with FMS, the situation would differ from the one studied in our in vitro whole blood model. First, the mainstay of treatment of meningococcal infection is bactericidal antibiotic therapy, which kills the meningococci rapidly. Second, the main clearance mechanisms of gram-negative bacteria from the circulation in mammals are Kupffer cells in the liver and, to a lesser extent, spleen macrophages.47,48 The class A scavenger receptor of the macrophages has been implicated as a major receptor for phagocytosis of meningococci.49 Granulocytes appear to play a minor role in the intravascular clearance of meningococci,47 and the phagocytosis of meningococci by granulocytes intravascularly is a pathologic condition that presumably occurs only at high concentrations of bacteria because it is associated with fatal meningococcal septic shock.50 Therefore, the inhibition of systemic phagocytosis-related oxidative burst is likely to be advantageous in clinically severe sepsis.
In meningococcal disease, excessive activation of the complement system is closely related to disease severity and patient death, implicating a possible beneficial effect of complement inhibition on disease severity in FMS.6,7 In contrast to other mediators in FMS such as proinflammatory cytokines that immediately decline after the initiation of effective antibiotic therapy,51-53 complement activation is sustained and reaches a maximum at 12 to 15 hours after the initiation of treatment.6,34 Attempts to influence disease severity by modulating complement activation therefore have an increased time span for possible therapeutic intervention.
By preference, adjunctive treatment for FMS should be aimed at inhibiting the negative effects of inflammatory substances without decreasing the capacity of the immune system to eliminate the infectious organism. In addition, primary mediators that induce secondary inflammatory reactions, enhancing the systemic breakdown of homeostasis, are particularly important candidates for intervention. Inhibition of C5a is a treatment modality that could perform such a task. Previous studies have shown that neutralizing the effect of C5a, either by antibodies or by receptor antagonists, has beneficial effects on survival in a range of animal models of gram-negative septic shock.54-57 These favorable effects may be explained by the preservation of neutrophil function, attenuation of CR3 expression, and reduced production of reactive oxygen species, as we show for N meningitidis in the present study. In addition, other secondary C5a-mediated effects that may contribute to the degree of septic shock might be attenuated, such as the release of histamine, chemotactic proteins, and proinflammatory cytokines leading to vasodilation and capillary leakage9,10,58 and to direct effects on coagulation and fibrinolysis.57 In conclusion, the present study supports a beneficial effect of complement modulation on potentially harmful systemic inflammatory responses such as CR3 expression and oxidative burst induced by N meningitidis. This was achieved by selective inhibition of C5a with preserved bactericidal activity using the anti-C5a mAb 137-26. We suggest this is an attractive approach to the arduous treatment of meningococcal septic shock.
Prepublished online as Blood First Edition Paper, July 24, 2003; DOI 10.1182/blood-2003-03-0703.
Supported by The Research Council of Norway (International Scholarship Section), the Nederlandse Organisatie voor Wetenschappelijk Onderzoek (grant number 920-03-176), the Norwegian Foundation for Health and Rehabilitation, the Norwegian Council on Cardiovascular Disease, Odd Fellow Foundation, the EU MBL Project (QLG1-CT-2001-01039), and Tanox Inc, Houston, TX.
M.F. is an employee of Tanox Inc, the company whose product was studied in the present work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Hilde Fure, Gunni Ulvund, and Mohammed R. Mirlashari for excellent technical assistance.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal