Abstract
γδ T cells are inadequately defined both in terms of their migration potential and contribution to antimicrobial immunity. Here, we have examined the migration profile of human blood γδ T cells and related cell lines and correlated these findings with their distribution in secondary lymphoid tissues and their function in B-cell cocultures. We find that resting γδ T cells are characterized by an inflammatory migration program similar to cells of the innate immune system. However, T-cell receptor (TCR) triggering resulted in the rapid but transient induction of a lymph node (LN)-homing program, as evidenced by functional CCR7 expression and concomitant reduction in expression and function of CCR5 and, to a lesser degree, CCR2. Moreover, the LN-homing program was reflected by the presence of γδ T cells in gastrointestinal lymphoid tissues, notably in clusters within germinal centers of B-cell follicles. In line with these findings, VγVδ-TCR triggering resulted in prominent expression of essential B-cell costimulatory molecules, including CD40L, OX40, CD70, and ICOS. Furthermore, γδ T cells were shown to provide potent B-cell help during in vitro antibody production. Collectively, our findings agree with a role for γδ T cells in humoral immunity during the early phase of antimicrobial responses. (Blood. 2003; 102:3693-3701)
Introduction
The extraordinary conservation of the subset of T cells expressing VγVδ-T-cell receptors (TCRs) suggests a nonredundant but still poorly understood role in immune defense.1-5 The 2 major γδ T-cell subsets in human beings, defined by TCRs containing Vδ1 or Vδ2 chains, differ in their anatomic localization; Vδ1+ T cells predominate within mucosal epithelia and skin whereas Vδ2+ T cells are most numerous in peripheral blood. A function in antimicrobial immunity is supported by the dramatic expansion of human γδ T cells in response to a wide range of pathogens and by reduced γδ T-cell numbers and function during disease progression.4,5 Infectious disease models demonstrated a 2-stage involvement of γδ T cells, the first taking place during the innate phase of primary immune responses and the second being effective during wound repair after pathogen elimination.4,5 Recently, γδ T cells were postulated to contribute also to memory responses against mycobacteria.6
Lysates from a large variety of bacteria, parasites, and plants were shown to induce oligoclonal expansion of human Vδ2+ T cells in vitro.3,4 However, γδ T cells differ fundamentally from αβ T cells in the types of antigens (Ags) they recognize. Human γδ T cells recognize small molecular weight nonpeptide Ags, stress- or tumor-induced self Ags, and the CD1 nonpolymorphic lipid Ag-presenting molecules.3,4,7 The principal pathogen-derived γδ T-cell Ags are isopentenyl pyrophosphate (IPP) and one of its metabolic precursors, (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate (HMBPP).8-10 The “simple” mode of Ag recognition (apparent lack of requirement for Ag processing and major histocompatibility complex [MHC] restriction) and the chemical nature of these essential pathogen-specific metabolites suggest a “pattern recognition” type of Ag selectivity by human Vδ2+ T cells.3,4,9 CD1-restricted Ag recognition mainly involves Vδ1-TCRs and appears to participate in self-recognition of nonpolymorphic CD1 that may contribute to dendritic cell (DC) maturation11 (M.B.B. and D. Leslie, personal written communication, July 2002).
Responsiveness to microbial metabolic intermediates points to an immediate (as opposed to proliferation/differentiation-dependent) involvement of γδ T cells in antimicrobial processes. Therefore, the migration properties of γδ T cells may resemble those of monocytes and granulocytes that appear early at sites of infection. Chemokines are best known for controlling leukocyte localization during hematopoiesis, initiation of immune responses, and inflammation.12-14 Traffic patterns and the inflammatory function in leukocytes are largely defined by their chemokine receptor expression and migration profile. Chemokines are divided into 2 major subfamilies, the “homeostatic” and the “inflammatory” chemokines, and their distinctive roles are best exemplified in mature T cells.14-18 The homeostatic chemokines SLC/CCL21 13 and ELC/CCL19 are constitutively produced in T zones of secondary lymphoid tissues (such as lymph nodes [LNs], Peyerpatches [PPs], and spleen) and selectively attract CCR7-bearing leukocytes, including resting naive/memory T cells as well as Ag-loaded/mature DCs.19-23 Ag-driven T-cell priming and differentiation induces sequential changes in chemokine responsiveness.14,15,17,18 First, T-cell priming induces a follicular migration program by down-modulating responsiveness to the T-zone chemokines SLC and ELC and concomitant up-regulation of CXCR5, the selective receptor for the follicular chemokine BCA-1/CXCL13.24,25 CXCR5 is the signature chemokine receptor for follicular B helper T (TFH) cells.26-28 Second, further differentiation into effector T cells correlates with an inflammatory migration program for responsiveness to chemokines induced at inflammatory sites. Effector T cells in peripheral blood do not express CCR7 but instead express distinct combinations of receptors for inflammatory chemokines (including CXCR3, CCR1, CCR2, CCR3, and CCR5).14,15 Naive and most memory T cells in peripheral blood do not respond to inflammatory chemokines and therefore are excluded from inflammatory processes in peripheral tissues. This migration profile clearly contrasts with the one seen in cells of the innate immunity, such as monocytes and granulocytes, which do not respond to T-zone or B-cell follicle chemokines but instead readily migrate in response to inflammatory chemokines.13-15
Here, we report the migration properties of peripheral blood γδ T cells and related lines. Inducible LN-homing program, clustering in follicles of secondary lymphoid tissues, and potent B-cell helper activity provide strong evidence for an unexpected involvement of γδ T cells in the control of humoral immunity.
Materials and methods
Reagents and antibodies
Synthetic chemokines (I-TAC, SDF-1, BCA-1, RANTES, MCP-1, EOTAXIN, MDC, MIP-1β, LARC, and SLC) were prepared as described29 and kindly provided by I. Clark-Lewis, Vancouver, BC, Canada. MicroBeads coated with monoclonal antibodies (mAbs) to CD4, CD14, or CD19 as well as with goat antimouse immunoglobulin G (IgG) and TCRγ/δ isolation Kit were from Miltenyi Biotec, Bergisch Gladbach, Germany. Mouse mAbs to CCR1 (53504.111), CCR2 (48607.211), CXCR5 (51505.111), and CCR7 (150503) and goat antibodies (Abs) to SLC (AF366) and BCA-1 (AF470) were from R&D Systems, Minneapolis, MN; mouse mAbs to CCR3 (7B11), CCR5 (LS78-5C7), CCR7 (7H12), CXCR1 (LS33-7D9), CXCR2 (7-6C6.1), and CXCR3 (LS77-1C6.2) were from LeukoSite, Cambridge, MA; rat mAb anti-CCR7 (3D12) was from M. Lipp, Berlin, Germany; rabbit Ab anti-CXCR5 is described26 ; mouse mAbs to CCR4 (1G1), CCR5 (2D7), CCR6 (11A9), CXCR3 (1C6), CXCR4 (12G5), Vδ2-TCR (B6.1), CD3 (UCHT1), CD4 (RPA-T4), CD8 (HIT8α), CD16 (3G8), CD19 (HIB19), CD20 (2H7), CD25 (M-A251), CD27 (M-T271), CD28 (CD28.2), CD29 (MAR4), CD45RA (HI100), CD45RO (UCHL-1), CD49d (9F10), CD56 (B159), CD62L (Dreg-56), CD69 (FN50), CDw70 (Ki-24), CD103 (Ber-Act8), and CD134 (ACT35) and rat mAbs to integrin β7 (FIB504) and CLA (HECA-452) were from BD PharMingen, San Diego, CA; mouse mAbs to pan-VγVδ-TCR (11F2), pan-VαVβ-TCR (WT31), and CD154 (89-76) were from Becton Dickinson, San Jose, CA; mouse mAbs to pan-VγVδ-TCR (5A6.E9) and Vδ1-TCR (TS8.2) were from Endogen, Woburn, MA; goat Ab anti-pan-VγVδ-TCR (A20) was from Santa Cruz Biotechnology, Santa Cruz, CA; mouse mAb anti-pan-VαVβ-TCR (BMA 031) was from Caltag Laboratories, Burlingame, CA; mouse mAb anti-CD49d (44H6) was from Accurate Chemical and Scientific, Westbury, NY; mouse mAb to integrin αL (TS1-22) and β2 (TS1-18) were from R. Pardi, Milano, Italy; mouse mAb anti-ICOS (F44) was from R. A. Kroczek, Berlin, Germany; mouse mAbs to Vδ1-TCR (A13) and Vδ2-TCR (BB3) were from M. B. Brenner, Boston, MA; and rabbit Ab anti-CD3 (A0452) and mouse mAb anti-CD21 (1F8) were from DAKO Diagnostics, Glastrup, Sweden. The following secondary Abs, conjugates, and control Ab were used: R-phycoerythrin (RPE)-conjugated goat antimouse IgG from DAKO; RPE-conjugated goat anti-rabbit IgG from Sigma-Aldrich (St Louis, MO); biotin-conjugated donkey anti-rabbit IgG from Amersham, Piscataway, NJ; biotin-conjugated donkey anti-rat IgG from Jackson ImmunoResearch Laboratories, West Grove, PA; RPE as well as RPE-cyanine 5 (RPE-Cy5)-conjugated streptavidine (SA) from DAKO; rabbit control IgG from Zymed Laboratories, South San Francisco, CA; mouse control IgG1 (MOPC21) and goat control IgG (I-5256) from Sigma-Aldrich; and all other isotype-matching control Abs from BD PharMingen. Samples were analyzed on a FACScan from Becton Dickinson, San Jose, CA.
Cell preparation and culture
Human peripheral blood lymphocytes (PBLs) were isolated as described.26 Peripheral blood γδ T cells and B cells were positively selected using the magnetic cell sorting system from Miltenyi Biotec. Tonsils from individuals undergoing tonsillectomy were mechanically dispersed, and lymphocytes were isolated by centrifugation on Ficoll-Paque. Tonsillar γδ T cells were isolated by positive selection, and other lymphocyte subpopulations were sorted by fluorescence-activated cell sorting (FACS) as described.26 All cultures were performed in RPMI 1640 supplemented with 2 mM l-glutamine, 1% nonessential amino acids, 1% sodium pyruvate, 50 μg/mL penicillin/streptomycin, 5 × 10-5 M 2-mercaptoethanol from GIBCO Invitrogen (Basel, Switzerland); 10% fetal calf serum (FCS) from Hyclone Laboratories (Logan, UT) or from GIBCO Invitrogen; and 1000 IU/mL interleukin-2 (IL-2). Phytohemagglutinin-P (PHA-P) (1:4000 final dilution) from Difco (Detroit, MI) or, alternatively, isopentenyl pyrophosphate (IPP) (50 μM) from Sigma-Aldrich presented by either Epstein-Barr virus (EBV)-transformed B cells or autologous primary B cells were used to activate and expand γδ T cells. γδ T-cell lines were generated by periodic in vitro restimulation.
Functional assays
Chemotaxis and Ca2+ mobilization assays were performed as described.24,26 Membranes with pore sizes of 3 μm and 5 μm were used for resting and recently activated γδ T cells, respectively.
The ability of γδ T cells to provide B-cell help was studied essentially as described for TFH cells.26 After positive selection γδ T cells were cultured overnight in the presence of stimulating anti-pan-VγVδ-TCR Ab; tonsillar B cells (CD4-CD45RO-CXCR5+) and αβ T cells (CD4+CXCR5+ and CD4+CXCR5-) were freshly prepared by FACS.26 Cocultures (96-well plates at 105 cells per well of each T and B cells) were preformed for 10 days in culture medium without addition of cytokines, and IgM, IgG, IgA, and IgE contents in the culture supernatants were determined by enzyme-linked immunosorbent assay (ELISA).
Immunohistochemistry
Biopsies from healthy donors and patients suffering from bacterial infections (yersiniosis, diverticulitis), Crohn disease, or tumor comprised samples of appendix, small intestine, colon, gastrointestinal draining LNs, and spleen. Tonsils were from patients undergoing tonsillectomy. Tissue samples were formaldehyde fixed and paraffin embedded. Freshly prepared 5 μm tissue sections were dewaxed and rehydrated. After pretreatment with 1 mg/mL proteinase from Streptomyces griseus (Sigma) in Tris (tris(hydroxymethyl)aminomethane)-buffered saline (TBS) for 6 minutes at 37°C, blocking with human immunoglobulins (3 mg/mL; Sandoglobuline; Novartis, Basel, Switzerland), swine or rabbit serum (10%; DAKO), and Tween 20 (0.01%), sections were incubated overnight at 4°C with primary Ab against pan-VγVδ-TCR (A20, 5A6.E9), Vδ1-TCR (A13), Vδ2-TCR (BB3), CD3 (A0452), CD21 (1F8), SLC (AF366), BCA-1 (AF470), or isotype control Ab mouse IgG1 (MOPC21), goat IgG (I-5256), and rabbit IgG. Subsequent detection and visualization of bound Abs was performed by the ABC method as described26 or, alternatively, with a combination of secondary reagents using Texas red-, CyChrome- or fluorescein isothiocyanate (FITC)-labeled conjugates for double-fluorescence microscopy. Mounted slides were analyzed with a Nikon Y-FL EPI-Fluorescence microscope (Melville, NY).
Results
Rapid migration reprogramming during activation of in vitro-cultured γδ T cells
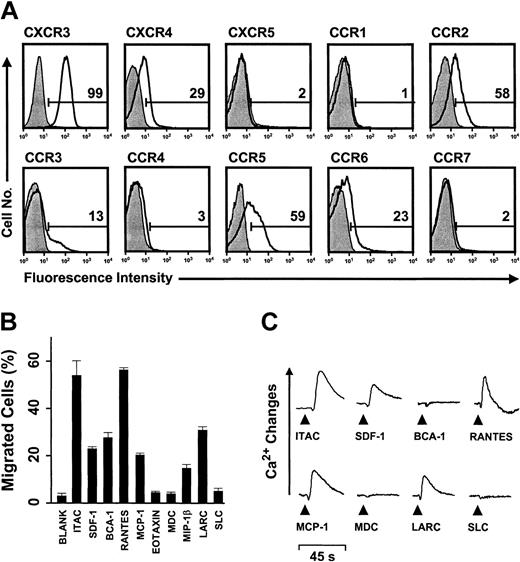
The migration potential of human γδ T cells was first determined in IPP-selective or PHA-responding γδ T-cell lines derived from PBLs. By contrast to freshly isolated blood γδ T cells, which contained 7% to 37% Vδ1-TCR cells and 59% to 70% Vδ2-TCR cells, IPP treatment selectively enriched for Vδ2-TCR cells whereas the lines expanded by PHA stimulation still contained minor fractions (10% to 17%) of Vδ1-TCR cells. Importantly, activation markers and chemokine receptor expression were similarly regulated in both types of cell lines (IPP- or PHA-responding) and, for sake of clarity, the data obtained with IPP-selective γδ T cells are shown. γδ T-cell lines uniformly expressed memory (CD45RO) and activation (CD69) markers but had low levels (less than 25% CD28, ICOS, CD70) or even lacked (CD40L, OX40) costimulatory molecules (not shown). Also, these γδ T cells expressed receptors for inflammatory chemokines that are typically found on effector/memory αβ T cells, most prominently CXCR3 (93% to 99%), CCR2 (15% to 58%), and CCR5 (59% to 98%) (Figure 1A).14 By contrast, homeostatic chemokine receptors CXCR4 (14% to 29%), CXCR5 (less than 5%), CCR4 (less than 5%), and CCR7 (less than 5%) that control lymphocyte traffic in primary and secondary lymphoid tissues were low or undetectable.14,16-18 In agreement with this pattern of chemokine receptor expression, γδ T cells showed vigorous migration and intracellular Ca2+ mobilization responses to inflammatory chemokines, notably I-TAC/CXCL11 (CXCR3) and RANTES/CCL5 (CCR1, CCR3, CCR5) but not lymphoid tissue chemokines, including SLC (CCR7) and MDC/CCL22 (CCR4) (Figure 1B-C). MIP-1β/CCL4, MCP-1/CCL2, LARC/CCL20, SDF-1/CXCL12, and BCA-1 showed intermediate activity. Of note, migration but lack of Ca2+ mobilization responses was observed for BCA-1. Migration in response to BCA-1 was solely due to CXCR3 and could be blocked completely by anti-CXCR3 Abs (see the supplemental material on the Blood website; follow the Supplemental Materials link at the top of the online article). CXCR3 was recently shown to function as an alternative, low-affinity BCA-1 receptor in the absence of the primary receptor CXCR5.30 Together, resting (previously activated but postproliferating) γδ T cells are well equipped for rapid recruitment to inflammatory sites but not secondary lymphoid tissues. This situation was dramatically changed during γδ T-cell activation with IPP (or PHA; not shown). Receptors for secondary lymphoid tissue chemokines (CCR4, CCR7) were rapidly induced, reaching maximal levels at 1 to 2 days of culture, and these changes in chemokine receptor expression were fully matched by corresponding migration and Ca2+ mobilization responses (Figure 2A-B). Of note, maximal levels of these homeostatic chemokine receptors were reached well before the onset of cell proliferation (Supplemental Materials) and then declined to base levels during progression of cell culture. As in resting cells, CXCR5 was not detected during γδ T-cell stimulation. Conversely, responses to the inflammatory chemokines MCP-1, RANTES, and MIP-1β (but not I-TAC) were reduced. Together, γδ T-cell activation resulted in an immediate acquisition of secondary lymphoid tissue-homing capabilities, which is the first indication that γδ T cells may have a role in adaptive immune responses.
nflammatory migration program in human peripheral blood-derived γδ T-cell lines. (A) Flow cytometric analysis of chemokine receptors (open histograms) on resting (previously activated, nonproliferating), IPP-selective γδ T-cell lines derived from human peripheral blood. Filled histograms represent control staining with isotype-matched Abs, and numbers refer to the percent receptor-positive cells. (B) Chemotactic migration and (C) Ca2+ mobilization responses in resting IPP-selective γδ T cells. Migration data, expressed as the fraction (percent) of input cells that have migrated (± SD), correspond to maximal responses determined by titration of chemokine concentrations; BCA-1 was only active at the highest concentration (3 μM) tested. Maximal chemotaxis responses were obtained with 1 or 10 nM I-TAC, SDF-1, MCP-1, RANTES, MDC, and MIP-1β; 100 nM eotaxin and LARC; 1 μM SLC; and 3 μM BCA-1. Ca2+ mobilization data correspond to 100 nM of chemokines. Functional data are representative of at least 3 independent experiments.
nflammatory migration program in human peripheral blood-derived γδ T-cell lines. (A) Flow cytometric analysis of chemokine receptors (open histograms) on resting (previously activated, nonproliferating), IPP-selective γδ T-cell lines derived from human peripheral blood. Filled histograms represent control staining with isotype-matched Abs, and numbers refer to the percent receptor-positive cells. (B) Chemotactic migration and (C) Ca2+ mobilization responses in resting IPP-selective γδ T cells. Migration data, expressed as the fraction (percent) of input cells that have migrated (± SD), correspond to maximal responses determined by titration of chemokine concentrations; BCA-1 was only active at the highest concentration (3 μM) tested. Maximal chemotaxis responses were obtained with 1 or 10 nM I-TAC, SDF-1, MCP-1, RANTES, MDC, and MIP-1β; 100 nM eotaxin and LARC; 1 μM SLC; and 3 μM BCA-1. Ca2+ mobilization data correspond to 100 nM of chemokines. Functional data are representative of at least 3 independent experiments.
Stimulation of cultured γδ T cells rapidly induces an LN-homing program. (A) Change in chemokine receptor expression during stimulation of peripheral blood-derived γδ T-cell lines with IPP. Positivity for CCR7 (□), CCR4 (▵), and CXCR4 (○) was determined by flow cytometry as in Figure 1 and is expressed as the fraction (percent) of receptor-positive cells. (B) Chemotactic migration profile (±SD) of activated γδ T cells after 3 days of stimulation with IPP was examined as described in Figure 1. Asterisk points out that the migration response to LARC was examined with γδ T cells after 6 days instead of 3 days of stimulation, reflecting a delay in maximal CCR6 expression. One of 3 independent experiments is shown.
Stimulation of cultured γδ T cells rapidly induces an LN-homing program. (A) Change in chemokine receptor expression during stimulation of peripheral blood-derived γδ T-cell lines with IPP. Positivity for CCR7 (□), CCR4 (▵), and CXCR4 (○) was determined by flow cytometry as in Figure 1 and is expressed as the fraction (percent) of receptor-positive cells. (B) Chemotactic migration profile (±SD) of activated γδ T cells after 3 days of stimulation with IPP was examined as described in Figure 1. Asterisk points out that the migration response to LARC was examined with γδ T cells after 6 days instead of 3 days of stimulation, reflecting a delay in maximal CCR6 expression. One of 3 independent experiments is shown.
Peripheral blood γδ T and αβ T cells are distinguished by opposite migration patterns
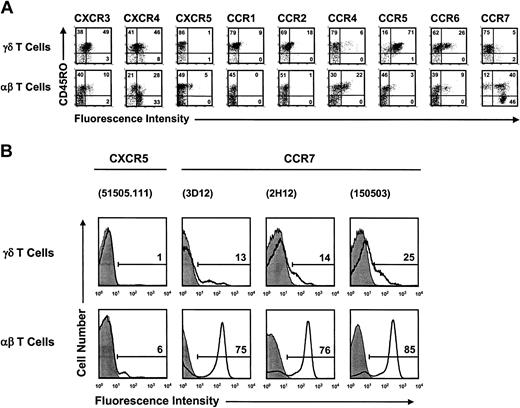
Next, we examined freshly isolated peripheral blood γδ T and αβ T cells. We found that the chemokine receptor profile in fresh γδ T cells closely resembled the one seen in resting γδ T-cell lines but differed fundamentally from that of fresh αβ T cells (Figure 3A). Receptors for inflammatory chemokines, notably CXCR3, CCR5, and to a lesser extent CCR2, were expressed at significantly higher levels in γδ T cells than in αβ T cells. In contrast, the LN-homing chemokine receptor CCR7 and follicular-homing chemokine receptor CXCR5 were expressed at lower levels or were absent in γδ T cells. CCR7 serves as a marker for LN-homing αβ T cells and is characteristic for peripheral blood naive and nonpolarized memory (termed “central memory,” or “TCM”) T cells 31,32 ; CCR7+ T cells with undefined migration properties do also exist in peripheral tissues and may reflect recently activated effector T cells.33-38 Accordingly, most peripheral blood αβ T cells have the ability to continuously recirculate through secondary lymphoid tissues, whereas most peripheral blood γδ T cells are excluded from these sites. Further, we found that CXCR5 was absent on freshly isolated γδ T cells, similar to cultured γδ T cells (Figure 1A) but in contrast to peripheral blood αβ T (TFH) cells.26,27 Our CCR7 and CXCR5 expression data contrast with a recent report showing uniform but low-level expression of both LN-homing chemokine receptors in γδ T cells.39 The same antibody reagents as those used in this report39 plus 2 additional anti-CCR7 antibody reagents resulted in our hands in low but distinct (CCR7) or no (CXCR5) positivity (Figure 3B). Also, peripheral blood αβ T cells used in control stainings showed the expected fractions of receptor-positive cells (Figure 3A). Finally, most blood γδ T cells expressed the necessary adhesion molecules (α4β1, α4β7, αLβ2, CD62L) for extravasation into peripheral mucosal tissues and associated lymphoid structures (Table 1).12 Thus, most peripheral blood γδ T cells are characterized by an inflammatory migration program, which differs fundamentally from the LN-homing potential seen in most peripheral blood αβ T cells.
γδ T cells and αβ T cells in peripheral blood differ fundamentally in their respective chemokine receptor expression profiles. (A) Dot plot diagrams show double staining data for CD45RO and chemokine receptors in freshly isolated peripheral blood γδ T cells (upper row) and αβ T cells (lower row). The data are representative of a study that included peripheral blood from a total of 19 donors. (B) γδ T cells and αβ T cells from the same donor blood differ substantially in their levels of cell surface CCR7 and CXCR5. Chemokine receptor staining data (open histograms) are plotted over control stainings with isotype-matched Abs (filled histograms). Bars define gates for receptor-positive cells, and numbers refer to the percent of receptor-positive cells. The Abs used in flow cytometric analysis are listed in parentheses and include 1 rat mAb (3D12) and 2 mouse mAbs (2H12, 150503) against CCR7 and 1 mouse mAb (51505.111) against CXCR5. The data are representative of a comparative analysis with peripheral blood T cells from 3 healthy individuals.
γδ T cells and αβ T cells in peripheral blood differ fundamentally in their respective chemokine receptor expression profiles. (A) Dot plot diagrams show double staining data for CD45RO and chemokine receptors in freshly isolated peripheral blood γδ T cells (upper row) and αβ T cells (lower row). The data are representative of a study that included peripheral blood from a total of 19 donors. (B) γδ T cells and αβ T cells from the same donor blood differ substantially in their levels of cell surface CCR7 and CXCR5. Chemokine receptor staining data (open histograms) are plotted over control stainings with isotype-matched Abs (filled histograms). Bars define gates for receptor-positive cells, and numbers refer to the percent of receptor-positive cells. The Abs used in flow cytometric analysis are listed in parentheses and include 1 rat mAb (3D12) and 2 mouse mAbs (2H12, 150503) against CCR7 and 1 mouse mAb (51505.111) against CXCR5. The data are representative of a comparative analysis with peripheral blood T cells from 3 healthy individuals.
Expression of adhesion molecules in fresh and IPP-treated peripheral blood γδ T cells
. | Freshly isolated cells . | 36-hour activation with IPP . |
|---|---|---|
| Adhesion molecules . | % of positive cells,*mean ± % SD (no. donors) . | % of positive cells, mean ± % SD (no. donors) . |
| CD62L | 61 ± 17 (8) | 53 ± 12 (6) |
| CLA | 12 ± 13 (6) | 16 ± 11 (4) |
| LFA-1 | 99 ± 1 (3) | 98 ± 2 (2) |
| α4-integrin | 98 ± 1 (5) | 97 ± 3 (3) |
| αe-integrin | 1 ± 1 (2) | 1 ± 1 (2) |
| β1-integrin | 91 ± 12 (5) | 91 ± 6 (3) |
| β7-integrin | 74 ± 10 (8) | 82 ± 8 (3) |
. | Freshly isolated cells . | 36-hour activation with IPP . |
|---|---|---|
| Adhesion molecules . | % of positive cells,*mean ± % SD (no. donors) . | % of positive cells, mean ± % SD (no. donors) . |
| CD62L | 61 ± 17 (8) | 53 ± 12 (6) |
| CLA | 12 ± 13 (6) | 16 ± 11 (4) |
| LFA-1 | 99 ± 1 (3) | 98 ± 2 (2) |
| α4-integrin | 98 ± 1 (5) | 97 ± 3 (3) |
| αe-integrin | 1 ± 1 (2) | 1 ± 1 (2) |
| β1-integrin | 91 ± 12 (5) | 91 ± 6 (3) |
| β7-integrin | 74 ± 10 (8) | 82 ± 8 (3) |
Flow cytometric analysis of adhesion molecules on γδ T cells. Percentage refers to specifically labeled cells above isotype-matched control stainings.
Importantly, short-term stimulation altered the migration properties in several ways, and the corresponding changes in γδ T-cell chemokine receptor profiles are summarized in Table 2. As in cultured cells, stimulation of peripheral blood γδ T cells with IPP (or PHA; not shown) rapidly induced CCR7 and instead dramatically down-modulated CCR5 and, to a lesser extent, CCR2 (Figure 4A). Further agreement with γδ T-cell lines is seen in unchanged high level of CXCR3 and lack of CXCR5 expression, whereas obvious differences relate to the prominent expression of CXCR4 on freshly isolated blood γδ T cells, which remained unchanged during stimulation with IPP, and the moderate induction of CCR4 expression. Of note, CXCR4 is well expressed on freshly isolated blood γδ T cells, whereas resting, cultured γδ T cells have low level of functional CXCR4 (Figure 1). Importantly, and in agreement with stimulated γδ T-cell lines, maximal expression of CCR7 was reached early (12 to 36 hours) during γδ T-cell activation and declined to base levels during subsequent cell culture, indicating that this chemokine receptor marks early activated as opposed to proliferating γδ T cells (Supplemental Materials). Of note, the reverse relationship in the regulation of inflammatory versus LN-homing chemokine receptors as a function of γδ T-cell stimulation (eg, CCR5 versus CCR7) is fully matched by changes in the migration responses to the corresponding chemokines (Figure 4B). Stimulation for 3 days did not affect the level and repertoire of adhesion molecules on γδ T cells (Table 1).
Chemokine receptor expression in fresh and IPP-treated peripheral blood γδ T cells
. | Freshly isolated cells* . | . | IPP-activated cells, 36 to 50 h . | . | ||
|---|---|---|---|---|---|---|
| Chemokine receptor . | n . | % of positive cells,†mean ± SD . | n . | % of positive cells, mean ± SD . | ||
| CXCR3 | 10 | 66.5 ± 8.2 | 9 | 65.9 ± 18.9 | ||
| CXCR4 | 7 | 53.1 ± 13.8 | 6 | 57.2 ± 21.6 | ||
| CXCR5 | 7 | 2.3 ± 1.8 | 5 | 2.0 ± 2.9 | ||
| CCR1 | 5 | 20.6 ± 10.7 | 4 | 24.5 ± 16.9 | ||
| CCR2 | 9 | 23.0 ± 7.2 | 7 | 14.1 ± 5.3 | ||
| CCR4 | 12 | 13.3 ± 10.2 | 12 | 29.9 ± 10.8 | ||
| CCR5 | 12 | 61.2 ± 10.4 | 9 | 2.5 ± 5.4 | ||
| CCR6 | 6 | 20.1 ± 11.1 | 4 | 15.2 ± 14.7 | ||
| CCR7 | 23 | 18.9 ± 9.7 | 14 | 77.6 ± 14.7 | ||
. | Freshly isolated cells* . | . | IPP-activated cells, 36 to 50 h . | . | ||
|---|---|---|---|---|---|---|
| Chemokine receptor . | n . | % of positive cells,†mean ± SD . | n . | % of positive cells, mean ± SD . | ||
| CXCR3 | 10 | 66.5 ± 8.2 | 9 | 65.9 ± 18.9 | ||
| CXCR4 | 7 | 53.1 ± 13.8 | 6 | 57.2 ± 21.6 | ||
| CXCR5 | 7 | 2.3 ± 1.8 | 5 | 2.0 ± 2.9 | ||
| CCR1 | 5 | 20.6 ± 10.7 | 4 | 24.5 ± 16.9 | ||
| CCR2 | 9 | 23.0 ± 7.2 | 7 | 14.1 ± 5.3 | ||
| CCR4 | 12 | 13.3 ± 10.2 | 12 | 29.9 ± 10.8 | ||
| CCR5 | 12 | 61.2 ± 10.4 | 9 | 2.5 ± 5.4 | ||
| CCR6 | 6 | 20.1 ± 11.1 | 4 | 15.2 ± 14.7 | ||
| CCR7 | 23 | 18.9 ± 9.7 | 14 | 77.6 ± 14.7 | ||
Chemokine receptor expression was analyzed in freshly isolated as well as in cultured γδ T cells that were stimulated with IPP for 36 hours to 50 hours.
Chemokine receptor positivity by flow cytometric analysis was expressed as mean plus SD of the fraction of positive cells in percent, and n refers to the number of donors analyzed. Percent positivity equals the fraction of antibody-stained cells after substraction of the isotype-matched control stainings.
Rapid switch in the migration program during activation of freshly isolated peripheral blood γδ T cells. (A) Chemokine receptor expression is shown in freshly isolated peripheral blood γδ T cells (dashed lines) and activated γδ T cells after 36 hours of stimulation with IPP (bold lines). The control isotype Ab stainings (filled histograms) are shown for activated γδ T cells. Note the inverse regulation of CCR5 and CCR7 expression during γδ T-cell activation. (B) Chemotactic migration profile of activated peripheral blood γδ T cells after 36 hours of stimulation with IPP was examined as described in Figure 1. Maximal migration responses (±SD) were observed at 1 or 10 nM I-TAC, SDF-1, RANTES, and MDC; 1 μM SLC; and 3 μM BCA-1. One of 3 independent experiments is shown.
Rapid switch in the migration program during activation of freshly isolated peripheral blood γδ T cells. (A) Chemokine receptor expression is shown in freshly isolated peripheral blood γδ T cells (dashed lines) and activated γδ T cells after 36 hours of stimulation with IPP (bold lines). The control isotype Ab stainings (filled histograms) are shown for activated γδ T cells. Note the inverse regulation of CCR5 and CCR7 expression during γδ T-cell activation. (B) Chemotactic migration profile of activated peripheral blood γδ T cells after 36 hours of stimulation with IPP was examined as described in Figure 1. Maximal migration responses (±SD) were observed at 1 or 10 nM I-TAC, SDF-1, RANTES, and MDC; 1 μM SLC; and 3 μM BCA-1. One of 3 independent experiments is shown.
Vδ1+ T cells represent a smaller proportion (7% to 37%) of γδ T cells in peripheral blood.1-5 They showed a similar migration profile as the majority fraction of γδ T cells, although the level of receptors for inflammatory chemokines showed a marked donor-to-donor variation (Supplemental Materials). Also, as seen in bulk γδ T cells, the LN-homing program was rapidly induced during stimulation of Vδ1+ T cells. In summary, peripheral blood γδ T cells acquire the ability to enter secondary lymphoid tissues during contact with Ag, thereby differing strikingly from most peripheral blood αβ T cells, which enter these sites by default.
Localization of γδ T cells in epithelia-associated secondary lymphoid tissues
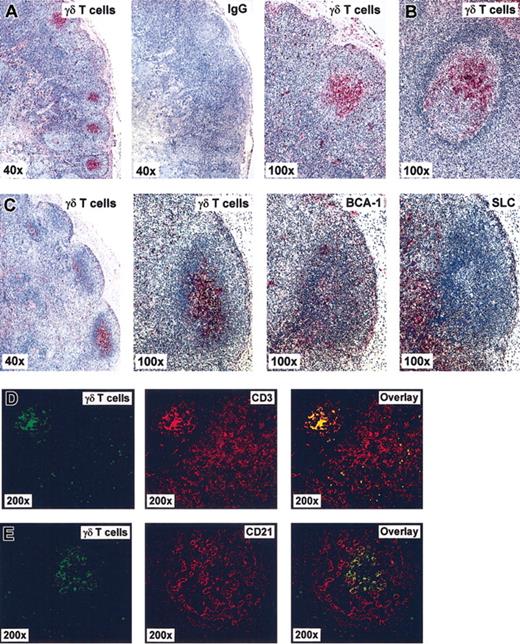
Ag-dependent induction of migration responses to the LN chemokines suggests that local activation of γδ T cells is reflected by their presence in “involved” secondary lymphoid tissues. This hypothesis was examined by immunohistochemical analysis of gastrointestinal LNs and intestinal mucosa-associated follicles of healthy or diseased donors as well as tonsils from tonsillectomy patients and of healthy spleen (Table 3). Indeed, γδ T cells were frequently detected in these tissues as demonstrated by immunohistochemical staining with pan-VγVδ-TCR Ab of sections from a normal abdominal LN, and mucosa-associated lymphoid follicles, and a reactive LN from patients with Yersinia infection (Figure 5). Of note, single γδ T cells were broadly scattered in the T zone and marginal sinus area but, most conspicuously, were clustered in germinal centers as opposed to the mantle zone of B-cell follicles. γδ T cells in germinal centers of mucosal follicles were often oriented toward the epithelium. For comparison, control staining using Ab against the chemokines BCA-1 and SLC, which characterize follicular areas and T zone, respectively, revealed the expected patterns (Figure 5).19-21,25,26 A similar distribution profile was observed by using 2 different pan-VγVδ-TCR-specific Abs as well as Vδ1- and Vδ2-TCR-specific Abs (“Materials and methods”), indicating that the 2 major γδ T-cell subsets do not differ significantly in their microanatomic localization within LNs (Supplemental Materials). γδ T cells clustering within the follicular dendritic cell (FDC) network of germinal centers and singular appearance among CD3+ cells in the T zone were also demonstrated by double-fluorescence microscopy (Figure 5D-E). γδ T cells were not detected in primary follicles (ie, follicles lacking germinal centers) but were present in some but not all reactive follicles of secondary lymphoid tissues (Table 3). Within healthy gut tissue, germinal centers of mucosa-associated lymphoid follicles were frequently positive for γδ T-cell clusters, and acute infections did not further increase their incidence, which may be related to the constant immune activities within this compartment against commensal bacteria and food Ag. However, reactive LNs of Yersinia-infected donors contained markedly more γδ T cells in their draining secondary follicles than LNs from healthy individuals. γδ T cells were always found in T zones of gastrointestinal LNs, and their distribution between T zone and germinal centers appears to be inversely related. LN tissue samples from 2 patients suffering from Morbus Crohn also contained follicular γδ T cells. Three of 5 tonsils were positive (ie, contained 1 or more γδ T cell-positive germinal center), and only 1 of 3 spleen specimens had secondary follicles, and some but not all of these secondary follicles contained γδ T-cell clusters.
γδ T-cell distribution in gastrointestinal mucosa and draining lymph nodes, tonsil, and spleen
. | . | Secondary follicles . | . | T zone . | |
|---|---|---|---|---|---|
| Tissue* . | No. samples†(no. donors) . | No. analyzed GCs, (range of no. of GCs per sample) . | γδ T-cell-positive GCs, %‡(range per sample) . | γδ T-cell density,§cells per mm2 ± SD . | |
| Mucosa | |||||
| Normal | 9 (5) | 70 (3-26) | 53 (0-85) | ND | |
| Yersinia | 14 (3) | 286 (4-42) | 48 (0-100) | ND | |
| Morbus Crohn | 3 (3) | 37 (8-18) | 37 (0-67) | ND | |
| LN | |||||
| Normal | 32 (7) | 847 (2-85) | 25 (0-88) | 75 ± 30 | |
| Yersinia | 12 (3) | 305 (2-87) | 72 (38-100) | 22 ± 5 | |
| Morbus Crohn | 2 (2) | 121 (60-61) | 63 (58-67) | 73 ± 6 | |
| Tonsil | |||||
| Tonsillectomy | 5 (5) | 172 (16-50) | 20 (0-59) | 57 ± 28 | |
| Spleen | |||||
| Normal | 3 (3) | 87 (0-87) | 24∥ | ND | |
. | . | Secondary follicles . | . | T zone . | |
|---|---|---|---|---|---|
| Tissue* . | No. samples†(no. donors) . | No. analyzed GCs, (range of no. of GCs per sample) . | γδ T-cell-positive GCs, %‡(range per sample) . | γδ T-cell density,§cells per mm2 ± SD . | |
| Mucosa | |||||
| Normal | 9 (5) | 70 (3-26) | 53 (0-85) | ND | |
| Yersinia | 14 (3) | 286 (4-42) | 48 (0-100) | ND | |
| Morbus Crohn | 3 (3) | 37 (8-18) | 37 (0-67) | ND | |
| LN | |||||
| Normal | 32 (7) | 847 (2-85) | 25 (0-88) | 75 ± 30 | |
| Yersinia | 12 (3) | 305 (2-87) | 72 (38-100) | 22 ± 5 | |
| Morbus Crohn | 2 (2) | 121 (60-61) | 63 (58-67) | 73 ± 6 | |
| Tonsil | |||||
| Tonsillectomy | 5 (5) | 172 (16-50) | 20 (0-59) | 57 ± 28 | |
| Spleen | |||||
| Normal | 3 (3) | 87 (0-87) | 24∥ | ND | |
ND indicates not determined.
Secondary follicles containing germinal centers (GCs) and T-zone areas of the listed samples were examined by immunohistochemistry using the A20 pan-VγVδ-TCR Ab.
No. samples refers to the number of individual lymphoid organs analyzed, which originated from the number of donors as indicated in parentheses.
The average of γδ T-cell-positive GCs in percent and the range in percent of γδ T cell-positive GCs within a tissue sample are indicated.
γδ T-cell density in the T zone refers to the number of γδ T cells per mm2 ± SD (5 × 0.31 mm2 areas per sample were counted).
Only 1 of 3 samples from 3 individual donors contained secondary follicles with germinal centers (GCs).
γδ T cells cluster in B-cell follicles within secondary lymphoid tissues. Immunohistochemical analysis of tissue section from human biopsy specimen by means of bright field (A-C) and fluorescence microscopy (D-E). (A) Normal abdominal LN, (B) gut follicle from a patient with ileitis (Yersinia), and (C) draining LN from a patient with appendicitis (Yersinia). Red staining in panels A-C depicts positivity for γδ T cells using a goat Ab against pan-VγVδ-TCR or BCA-1 and SLC as indicated using mouse mAb, and IgG refers to negative control staining with isotype-matched unrelated Ab. (D-E) Assessment of γδ T-cell distribution in sections of an abdominal LN from a patient with yersiniosis. Double-fluorescence images show localization of γδ T cells in green, CD3+ T cells (D) and CD21+ FDCs (E) in red, and the corresponding superimposed images (overlays).
γδ T cells cluster in B-cell follicles within secondary lymphoid tissues. Immunohistochemical analysis of tissue section from human biopsy specimen by means of bright field (A-C) and fluorescence microscopy (D-E). (A) Normal abdominal LN, (B) gut follicle from a patient with ileitis (Yersinia), and (C) draining LN from a patient with appendicitis (Yersinia). Red staining in panels A-C depicts positivity for γδ T cells using a goat Ab against pan-VγVδ-TCR or BCA-1 and SLC as indicated using mouse mAb, and IgG refers to negative control staining with isotype-matched unrelated Ab. (D-E) Assessment of γδ T-cell distribution in sections of an abdominal LN from a patient with yersiniosis. Double-fluorescence images show localization of γδ T cells in green, CD3+ T cells (D) and CD21+ FDCs (E) in red, and the corresponding superimposed images (overlays).
Collectively, γδ T cells are readily detected in secondary lymphoid tissues, notably in tight clusters within germinal centers of gastrointestinal LNs and mucosa-associated lymphoid follicles. Their relocation to secondary lymphoid tissues may be initiated at sites of microbial infections and suggests a role for γδ T cells in the initiation of antimicrobial LN activities.
γδ T cells provide help to follicular B cells
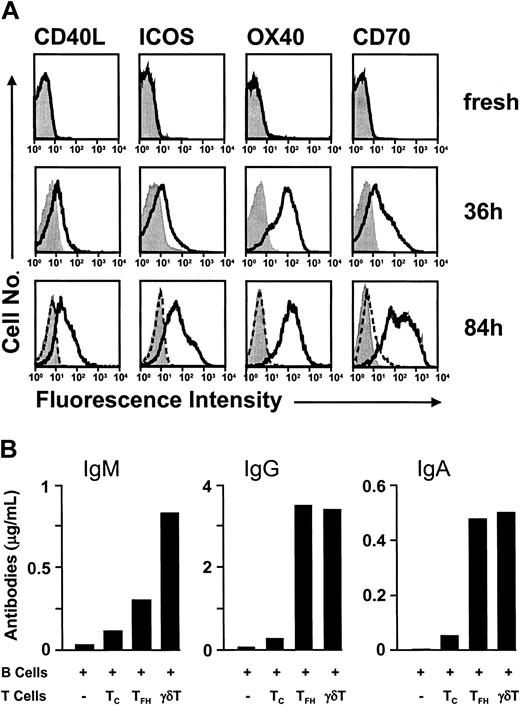
Clustering in germinal centers implies a role for γδ T cells in humoral immune responses. γδ T cells produce substantial amounts of cytokines, including tumor necrosis factor-α (TNF-α) and interferon-γ (IFN-γ) and, in some cases IL-4, which may affect B-cell responses.3-5 Here, we demonstrate that the costimulatory make-up on activated γδ T cells equips them well to provide cell-cell contact-dependent B-cell help. Freshly isolated peripheral blood γδ T cells lack the T-cell activation marker CD69 and the costimulatory molecules CD40L (CD154), OX40 (CD134), ICOS, and CD70 (CD27 ligand) (all less than 5% positive) and showed weak expression of the lymphoblast marker CD25 (5% to 15%). Stimulation with IPP for 36 hours resulted in 69% to 86% CD69+ cells containing more than 50% blasts with uniform (100%) expression of CD25 (not shown) and substantial expression of CD40L (44% ± 16% [± SD]; n = 7), OX40 (80% ± 17% [± SD]; n = 3), ICOS (46% ± 21% [± SD]; n = 3), and CD70 (49% ± 15% [± SD]; n = 3) (Figure 6A). Of note, further culturing maintained or even increased the level of costimulatory molecules, reaching 54% ± 14% (± SD; n = 3) CD40L+ cells, 91% ± 1% (± SD; n = 2) OX40+ cells, 63% ± 17% (± SD; n = 4) ICOS+ cells, and 81% ± 15% (± SD; n = 4) CD70+ cells by 3 to 4 days of IPP stimulation. The delay in CD40L and ICOS up-regulation in γδ T cells is in obvious contrast to their rapid expression in αβ T cells,40-43 and long-term maintenance of all 4 costimulatory molecules strongly suggests a direct involvement of activated γδ T cells in follicular activities.44-55 This idea was further examined in γδ T-cell-B-cell coculture experiments. Coculture with γδ T cells resulted in substantial amounts of IgM, IgG, and IgA production (Figure 6B). Significant donor-to-donor variation in the level of Ab production was observed, which may have been due to variations in the state of preactivation among tonsillar B-cell preparations; however, the γδ T-cell helper effect was consistently seen and was at least as potent as the one obtained with the positive control TFH cells.26,27 Tonsillar TFH cells were recovered in a state of preactivation,26 and the γδ T cells were stimulated by γδ-TCR cross-linkage with an antibody before use in the B-cell coculture experiments. γδ T cell-mediated yield in Ab production was in the order of 0.51 ± 0.49 μg/mL for IgM, 1.61 ± 1.43 μg/mL for IgG, and 0.23 ± 0.24 μg/mL IgA, while IgE production was below detection (less than 0.01 μg/mL). Both, autologous (tonsillar) and heterologous (peripheral blood) γδ T cells were equally capable of providing B-cell help.
γδ T cells provide potent B-cell help. (A) Activation-dependent expression of costimulatory molecules in γδ T cells is delayed but sustained. Flow cytometric analysis of costimulatory molecules on peripheral blood γδ T cells that were analyzed either immediately after isolation (top row) or after 36 hours (middle row) and 84 hours (lower row) of stimulation with IPP. Culturing of γδ T cells in the absence of TCR triggering (but in the presence of IL-2) did not result in marked expression of costimulatory molecules, as evidenced in cells at 84 hours of culture (dashed line). Control Ab stainings are shown in filled histograms. (B) γδ T cells induce Ab production during coculture with B cells. Tonsillar B cells were cultured alone (-)orin the presence of CXCR5-negative αβ T cells (TC), TFH cells (TFH), or peripheral blood γδ T cells (γδ T) without addition of exogenous cytokines; after 10 days the Ab titers in the culture supernatants were determined by ELISA. One of 4 representative experiments is shown.
γδ T cells provide potent B-cell help. (A) Activation-dependent expression of costimulatory molecules in γδ T cells is delayed but sustained. Flow cytometric analysis of costimulatory molecules on peripheral blood γδ T cells that were analyzed either immediately after isolation (top row) or after 36 hours (middle row) and 84 hours (lower row) of stimulation with IPP. Culturing of γδ T cells in the absence of TCR triggering (but in the presence of IL-2) did not result in marked expression of costimulatory molecules, as evidenced in cells at 84 hours of culture (dashed line). Control Ab stainings are shown in filled histograms. (B) γδ T cells induce Ab production during coculture with B cells. Tonsillar B cells were cultured alone (-)orin the presence of CXCR5-negative αβ T cells (TC), TFH cells (TFH), or peripheral blood γδ T cells (γδ T) without addition of exogenous cytokines; after 10 days the Ab titers in the culture supernatants were determined by ELISA. One of 4 representative experiments is shown.
Discussion
Our study provides strong evidence of a direct role for γδ T cells in the control of humoral immunity. This conclusion is supported by a detailed migration analysis of peripheral blood γδ T cells. Resting γδ T cells migrate very efficiently in response to the inflammatory chemokines I-TAC, MCP-1, and RANTES and uniformly express the corresponding receptors CXCR3, CCR2, and CCR5, whereas responsiveness to the LN chemokine SLC and expression of its receptor CCR7 are largely absent. CCR7 is the principal chemokine receptor for the steady-state circulation of peripheral blood T cells through secondary lymphoid tissues, as evidenced by mice lacking CCR7 or its specific ligands SLC and ELC.22,23 CCR7, together with CD62L, is used to define LN-homing T cells, including naive and TCM cells, which are characterized by their nonpolarized, noneffector phenotype.31,32 This paradigm applies to most peripheral blood αβ T cells, whose inflammatory involvement is secondary to their MHC-restricted recognition of Ag presented by T-zone DCs within secondary lymphoid tissues. By contrast, we show that most peripheral blood γδ T cells lack CCR7 and, consequently, are not expected to recirculate through peripheral LNs and PPs by default. Instead, peripheral blood γδ T cells are formidably equipped for instant relocation to inflammatory processes, which explains their early accumulation at sites of microbial infection.56,57 Low numbers of peripheral blood γδ T cells express CCR7 and bear signs of preactivation, suggesting that they reflect ongoing immune activities (not shown).
Unexpectedly, activation by IPP (plus primary or EBV-transformed B cells) or PHA resulted in a rapid and transient switch in the γδ T-cell migration profile. Responsiveness to inflammatory chemokines and expression of corresponding receptors is largely replaced by the transient up-regulation of migration responses to the LN chemokines SLC and MDC, together with the expression of the corresponding receptors CCR7 and CCR4. Invariant and high-level expression of CXCR3 suggests a dual role for this chemokine receptor in regulating the relocation of peripheral blood γδ T cells to sites of microbial infections as well as in recruiting activated γδ T cells to draining (reactive) LNs.14,15,58 In accordance with resting blood αβ T cells and tissue-derived mature DCs, we propose that activated γδ T cells find their way into reactive LNs via the expression of cell surface CCR7 and the responsiveness to the LN chemokines SLC and ELC.19-23 Their further relocation from the T zone into the B-cell compartments may be controlled by follicular chemokines, such as mantle zone BCA-1 targeting CXCR5 and, alternatively, CXCR3,26-28,30 and B cell-derived MDC acting on CCR4.59,60 We have no obvious explanation for the discrepancy in CCR7 and CXCR5 expression on peripheral blood γδ T cells between our study and a recent report.39 However, another study dealing with Vδ2+ T-cell numbers in immunocompromised and healthy individuals supports our finding about the low-level expression of CCR7 in peripheral blood γδ T cells61 ; unfortunately, changes in expression of CCR7 or modulation of migration properties during γδ T-cell activation were not examined. Collectively, we demonstrate that γδ T-cell activation resulted in the partial substitution of the inherent “inflammatory” homing capabilities for an immediate and transient LN-homing program. Possibly, the ability of γδ T cells to enter LNs is initiated during contact with microbial Ag at inflammatory sites.
What is the role of γδ T cells in secondary lymphoid tissues? γδ T cells may influence local immune processes through cell-to-cell contact or, alternatively, by providing cytokines. The former view is strongly supported by the remarkable level of costimulatory molecules present on activated but not resting γδ T cells, including CD40L, OX40, ICOS, and CD70, whose essential function in humoral immunity is well documented.44-55 By contrast to αβ T cells, costimulatory molecules are induced late during γδ T-cell activation and persist at high levels for more than 3 days, a finding that may be of crucial importance for the function of γδ T cells in controlling adaptive immune processes within germinal centers. Further, we show that γδ T cells are at least as effective as TFH cells in providing B-cell help,26,27 as evidenced by Ab production during coculture with B cells. Indeed, experiments in αβ T cell-deficient (TCRα-/-, TCRβ-/-) mice demonstrated a nonredundant role for γδ T cells in the generation of antimicrobial Abs62,63 and autoantibodies.62,64-66 Because γδ T cell-deficient mice do not show obvious defects in IgM and IgG Ab production, γδ T cells may have a modulatory as opposed to primary function in the control of humoral immunity. Ab production was also shown in in vitro cultures of human γδ T cells with B cells,67-70 but the outcome (level of Ab production) was moderate and the mechanisms underlying the observed B-cell help were not examined.
Contribution of γδ T cells to follicular activities may occur early during microbial infections, well before adaptive (αβ T cell-dependent) defenses come into play. Recently, we have observed that γδ T cells also induce DC maturation, further implicating their influence in early stages of antimicrobial immunity (M.B.B. and D. Leslie, personal written communication, July 2002). Follicular localization together with excellent provision of costimulation suggests that γδ T cells contribute to humoral immunity. We postulate that the “innate” response of γδ T cells, including their LN-homing and follicular activities, precedes the generation of an αβ T cell-driven immune response, which depends on a series of time-consuming steps, including Ag uptake and processing by tissue DCs, their relocation to draining LNs, and subsequent T-cell priming and effector cell development. In support, γδ T cells were found early at sites of infection, and their numbers decreased during disease progression.56,57
Thus, γδ T cells may influence the subsequent adaptive immune responses through interaction with peripheral DCs and B cells in reactive secondary lymphoid tissues. The rapid activation-dependent switch in homing characteristics provides new important insights into the role of γδ T cells in antimicrobial immune responses and clearly puts them apart from any other type of innate cells.
Prepublished online as Blood First Edition Paper, July 24, 2003; DOI 10.1182/blood-2003-04-1016.
Supported by grants from the Swiss National Science Foundation (31-055996.98 [B.M.] and 32-57616.99 [M.B.]) and from the Bundesamt für Bildung und Wissenschaft (99.0471-5) (B.M.).
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful to I. Clark-Lewis for providing chemokines, to LeukoSite for Abs to diverse chemokine receptors, and to R. Pardi, M. Lipp, and R. A. Kroczek for Abs to αLβ2, CCR7, and ICOS, respectively. We acknowledge the invaluable help by M. Caversaccio and L. Mazzucchelli for human tissue samples and K. Baltensperger for help in fluorescence microscopy.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal