Abstract
Platelet spreading on the subendothelium in response to vascular injury is fundamental to the regulation of physiologic hemostasis. Previously, we have shown that, when bound to glycoprotein IIb (GPIIb), calcium- and integrin-binding protein (CIB) regulates platelet spreading on immobilized fibrinogen (Fg). In this study, we investigated the signaling events that occur downstream of CIB in the absence of signaling that occurs as a result of granular secretion. Using Chinese hamster ovary (CHO) cells as a model, we demonstrate that CIB induces cell migration. Immunofluorescence analysis of CIB localization indicates that endogenous CIB accumulates in areas of focal adhesions, and its overexpression up-regulates the formation of focal adhesion complexes compared with control cells. Immunoprecipitation analysis indicates that CIB associates with focal adhesion kinase (FAK), a key regulator in focal complex formation, and up-regulates its activity. Overexpression of dominant-negative FAK, FRNK, along with CIB in CHO cells completely inhibits CIB-induced cell migration. Further, confirmation of these data in the platelet system indicates that CIB and FAK associate throughout all stages of platelet spreading but only on Fg binding to GPIIb/IIIa. Taken together, our results suggest that CIB regulates platelet spreading through the regulation of FAK activation. (Blood. 2003;102: 3629-3636)
Introduction
Platelet adhesion to and spreading on the subendothelial matrix is integral to the maintenance of physiologic hemostasis and thrombosis. Glycoprotein IIb/IIIa (GPIIb/IIIa) is the platelet fibrinogen (Fg) receptor, which mediates both platelet aggregation and platelet spreading on vascular matrices at sites of vascular injury.1-5 However, the exact signaling mechanism that takes place on GPIIb/IIIa binding to immobilized Fg that leads to platelet spreading, broadly classified as outside-in signaling, has only been partially defined.
The nonreceptor tyrosine kinases Syk and Src were shown to be involved in this signaling cascade.4,6-8 On activation of Syk, Src, and possibly a number of other kinases as a result of GPIIb/IIIa binding to Fg, discoid platelets undergo a rapid shape change during which adherent platelets transiently exhibit filopodia-like structures. This fleeting event involves rapid actin depolymerization and repolymerization events, resulting in a spiky morphology. Dynamic reorganization of the platelet cytoskeleton triggers a signaling cascade that results in secretion of platelet agonists such as adenosine diphosphate (ADP) and serotonin from platelet dense granules and adhesive proteins such as platelet factor 4, Fg, and von Willebrand factor from α-granules.9 Binding of agonists to their receptors initiates extensive signaling events that lead to a second wave of cytoskeletal reorganization and culminate in a fully spread platelet morphology. However, the signaling events required to achieve each of these distinct morphologies have not yet been clearly elucidated.
Recently, we have reported that the interaction between calcium and integrin binding protein (CIB) and GPIIb/IIIa is required for the process of platelet spreading on immobilized Fg.10 We find that, on ligand binding, most CIB binds to GPIIb/IIIa to form a complex. Inhibition of complex formation by introduction of either anti-CIB antibody or GPIIb cytoplasmic tail peptide into platelets blocks platelet spreading but not adhesion or filopodia formation. However, a spread morphology can be recovered by the introduction of recombinant CIB into antibody- or GPIIb peptide-inhibited platelets. The identity of the signaling components downstream of CIB in this process is not yet known.
In this report, we identify focal adhesion kinase (FAK) as a downstream component of CIB-induced signaling. FAK activation has been shown to require actin polymerization events, implicating its role in the second wave of cytoskeletal rearrangement.11,12 In fact, it has been shown that secretion of ADP controls activation of both FAK and phosphatidylinositol 3-kinase (PI3K),13,14 which is essential for platelet spreading. To determine the downstream effects of CIB signaling that lead to platelet spreading without the complications from signaling events because of secretion, we reconstituted CIB-mediated signaling in Chinese hamster ovary (CHO) cells. We find that overexpression of CIB in CHO cells induces migration, a process which requires cell adhesion and spreading. Immunofluorescence analysis of such cells indicates that overexpression of CIB enhances focal adhesion formation and, thus, cell spreading, and that CIB localizes in the areas of focal contacts along the cell periphery. Further, we find that CIB associates with FAK in these cells, and that FAK activity is up-regulated on CIB overexpression. Blockade of FAK activation by the introduction of FAK-related nonkinase (FRNK) in these cells inhibits CIB-induced cell migration on fibronectin. Furthermore, we confirm that these events occur in the platelet system by showing that CIB and FAK indeed colocalize along the membranous protrusions of platelets spreading on immobilized Fg. Coimmunoprecipitation analysis indicates that CIB and FAK associate on platelet adhesion to Fg, or on platelet activation with thrombin receptor-activating peptide (TRAP). We further show that association of CIB with FAK requires Fg binding to GPIIb/IIIa. Thus, these data implicate CIB in the signaling pathway that regulates the focal adhesion dynamics required for platelet spreading on immobilized Fg or agonist-induced platelet aggregation.
Materials and methods
Antibodies and reagents
Production of anti-CIB monoclonal antibodies UN2 and UN7.79 was described previously.15 Function blocking anti-α5β1 monoclonal antibody was a generous gift from Dr Rudolph Juliano (University of North Carolina, Chapel Hill). Polyclonal antibody against FAK was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Antiphosphotyrosine antibody, PY20, was purchased from Chemicon (Temecula, CA). Antivinculin antibody was a kind gift from Dr Keith Burridge (University of North Carolina, Chapel Hill). The construct for dominant-negative FAK, FRNK, was kindly provided by Dr Patricia Keely (University of Wisconsin, Madison). Human fibronectin was purchased from Collaborative Biomedical Research (Bedford, MA). All other reagents used were analytical grade from Sigma (St Louis, MO).
Cell culture and transfection
Human umbilical cord vein endothelial cells (HUVECs) were obtained from Cambrex Bio Science (Walkersville, MD) and maintained in the medium provided by the manufacturer. CHO cells and murine fibroblasts NIH3T3 were obtained from the American Type Culture Collection (ATCC; Manassas, VA). Cells were maintained in Dulbecco modified Eagle medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin (Invitrogen, Carlsbad, CA). CHO cells were transfected using Lipofectamine (Invitrogen) following the manufacturer's protocol as described previously.16 Geneticin (G418) was added to the culture medium 24 hours after transfection at a final concentration of 500 μg/mL. The resistant colonies were isolated to obtain single cell clones. Thereafter, cells were maintained in culture medium containing 300 μg/mL G418. CIB overexpression levels were determined by immunoblotting as described.15 In separate cotransfection experiments, transiently transfected cells were harvested after 48 hours of transfection as indicated.
Migration assays
Scratch assay. CIB-induced cell migration on fibronectin was monitored by 2 different assays. In a qualitative wound-healing assay,17,18 mock- and CIB-transfected cells were serum starved, and then plated on 35-mm fibronectin precoated Petri dishes overnight at 37°C to form a monolayer. The confluent monolayer was scratched with the use of a sterile disposable yellow pipette tip and rinsed with serum-free medium. Photographs were taken at time zero using a phase contrast microscope, and cells were allowed to migrate under serum-free conditions at 37°C for an additional 24 hours. The extent of cell migration toward the denuded area was documented.
Transwell migration assay. A haptotatic transwell motility assay was performed as described previously.19 Briefly, the undersides of 8- μm pore size polycarbonate transwell insert membranes were coated with fibronectin (10 μg/mL) in phosphate-buffered saline (PBS) overnight at 4°C, and, simultaneously, appropriate cells were serum starved overnight. After harvesting, 50 000 cells were added to the upper transwell compartment in triplicates. Migration through the pores was allowed to proceed for 5 hours at 37°C under serum-free conditions. In the case of the addition of inhibitors or function-blocking antibodies, cells in suspension were incubated with the appropriate reagents for 20 minutes, and then allowed to migrate in the presence of inhibitors as described earlier. Unmigrated cells were removed, whereas cells that migrated through the pores to the underside were fixed with freshly prepared 4% paraformaldehyde in PBS for 10 minutes. Migrated cells were stained with Diff-Quik (Dade Behring, Newark, DE) staining kit, following the manufacturer's instructions. Ten random views per insert in triplicate were counted and scored by different individuals. Statistical analyses were performed using the Student paired t test.
Immunofluorescence
Immunofluorescence studies were conducted with indicated cells according to procedures described previously.16 Briefly, indicated primary antibodies (1:100) were added to fixed and permeabilized cells at 4°C overnight. After incubation, cells were washed 3 times with 3% bovine serum albumin (BSA) in PBS and incubated with rhodamine X-conjugated donkey antimouse immunoglobulin G (IgG) secondary antibody (1:300) or fluorescein isothiocyanate (FITC)-conjugated donkey antirabbit antibody (1:300) (Jackson ImmunoResearch, West Grove, PA) for 1 hour at room temperature. For staining of F-actin, Alexa 488-conjugated phalloidin (1:500) (Molecular Probes, Eugene, OR) was used where indicated. Cells were rinsed with PBS, and Slowfade (Molecular Probes) was added to minimize fading of the fluorescence intensity. A Zeiss LSM 510 laser-scanning microscope (Thornwood, NY) was used for confocal microscopy.
Isolation of human platelets
Blood was drawn into 6:1 (vol/vol) citric acid/citrate/dextrose (pH 4.5) by venipuncture from healthy, drug-free volunteers older than 18 years under informed consent. Approval was obtained from the Univeristy of Delaware Institutional review board for these studies according to the Declaration of Helsinki. Platelets were washed as described previously.20 To obtain fixed discoid platelets, platelet-rich plasma was diluted with an equal volume of 8% paraformaldehyde in PBS as fixative for 10 minutes at room temperature. For localization studies, an aliquot of 15 μL washed platelet suspension (1.5 × 107/mL) was added to cover slide chambers precoated with 10 μg/mL fibrinogen that contained 250 μL Tyrode buffer. Platelets were allowed to adhere for 45 minutes at 37°C. Cells were then fixed and processed as described in “Immunofluorescence.” All experiments using platelets were performed at least 3 times with the use of platelets from several different donors. Platelets were counted using a Beckman-Coulter Z2 counter (Miami, FL).
FAK activation in CHO cells
Serum-starved mock- and CIB-transfected CHO cells (3 × 105) were plated on fibronectin precoated dishes for various time points at 37°C. Adherent cells were rinsed with PBS and then immediately lysed in 1 mL RIPA buffer (150 mM NaCl, 1% NP40, 0.05% deoxycholate, 0.1% SDS (sodium dodecyl sulfate), 10 mM sodium orthovanadate, and a cocktail of protease inhibitors in 50 mM Tris (tris(hydroxymethyl)aminomethane) (pH 8.0) on ice for 30 minutes. Lysates were then subjected to immunoprecipitation as described in “Immunoprecipitation.”
FAK activation in platelets
Washed platelets (4.5 × 108 cells/mL) were plated on 10 μg/mL Fg-coated or 5 mg/mL BSA-coated 100-mm Petri dishes for 45 minutes at 37°C and monitored occasionally to ensure spreading. The adherent platelets were rinsed with PBS and lysed in 1 mL RIPA buffer as described. In another set of experiments, platelets (2 × 108 cells/mL) in suspension were activated with 10 μM TRAP, in the absence or the presence of 1 mM RGDS peptide as indicated. Platelets were immediately lysed on ice for 30 minutes using 2 × RIPA buffer. Lysates were subjected to immunoprecipitation analysis as described in “Immunoprecipitation.”
Immunoprecipitation
Lysates were precleared with isotype-specific antibody (HB67; ATCC) and protein G-Sepharose beads (Amersham Pharmacia, Piscataway, NJ). Precleared lysates containing 500 to 800 μg protein were incubated at 4°C with either anti-FAK or UN7.79 antibodies as indicated. The immunocomplexes were captured by using protein G-Sepharose beads and washed 3 times with RIPA buffer. Samples were then either processed for immunoblotting as previously described15 or for in vitro kinase assay (FAK autophosphorylation) as previously described.20 To determine FAK activity, immunocomplexes were subjected to γ-32P ATP (2 μCi [0.074MBq], 10 μM)] (Amersham Pharmacia). Samples were resolved by SDS-polyacrylamide gel electrophoresis (PAGE) and further processed for autoradiography.
Results
CIB sequence and localization is conserved among species
We have previously shown that through binding GPIIb, CIB regulates the process of platelet spreading on immobilized Fg.10 To more clearly elucidate the signaling events downstream of CIB action, we sought to reconstitute a mammalian expression model that would highlight CIB-induced signaling without the interference from the signaling events that occur because of platelet granular secretion. To choose an appropriate host system, we first sought to determine the homology of CIB protein among species. A database search of the human CIB protein sequence resulted in a large number of potential orthologues with sequence similarity to CIB. CIB, as its name suggests, is a calcium-binding protein; as such, the large number of hits is most likely because of the highly conserved calcium-binding domain sequences (known as EF-hand motifs) that exist in most calcium-binding proteins. Therefore, only those proteins similar in size to human CIB that also contain 2 EF-hand motifs located at the C-terminus and that show high sequence similarity were selected as human CIB orthologues. Proteins matching such criteria were found in Mus musculus (AAG38960), Rattus novegicus (NP_112407), and Gallus gallus (BG709937), and a partial transcript was found in Fugu rubripres (SINFRUP00000051614). Mouse and rat CIB sequences are 85% identical to human CIB and 90% identical to each other (Figure 1A). Similarly, chicken and fish CIB sequences are approximately 63% identical to human CIB and 60% identical to each other (Figure 1A). This relationship is also observed in evolution (Figure 1B). The high level of sequence conservation across species strongly suggests that CIB plays an important role in the regulation of cellular function and also makes it possible to reconstitute a mammalian expression system in a variety of hosts.
CIB sequence and areas of localization are highly conserved among species. (A) Amino acid sequences of CIB in humans, rats, mice, chicken, and fish were analyzed by multiple sequence alignment. Those amino acids that differ from the human sequence are highlighted in red, and EF-hand motifs are boxed; asterisks indicate aminoacid identity. (B) Multiple sequence alignment dendrogram of CIB showing the evolutionary relationship between the orthologues. (C) Immunofluorescence images of CIB localization in a variety of cell types. Platelets were allowed to attach on fibrinogen-coated glass cover slides for 45 minutes, then fixed and stained with UN2 for CIB (red) and FITC-conjugated phalloidin for F-actin (green). Scale bar, 5 μm. HUVECs, CHO, and NIH3T3 cells were allowed to spread on fibronectin-coated cover glass for 45 minutes, then fixed and stained as above. Scale bar, 20 μm.
CIB sequence and areas of localization are highly conserved among species. (A) Amino acid sequences of CIB in humans, rats, mice, chicken, and fish were analyzed by multiple sequence alignment. Those amino acids that differ from the human sequence are highlighted in red, and EF-hand motifs are boxed; asterisks indicate aminoacid identity. (B) Multiple sequence alignment dendrogram of CIB showing the evolutionary relationship between the orthologues. (C) Immunofluorescence images of CIB localization in a variety of cell types. Platelets were allowed to attach on fibrinogen-coated glass cover slides for 45 minutes, then fixed and stained with UN2 for CIB (red) and FITC-conjugated phalloidin for F-actin (green). Scale bar, 5 μm. HUVECs, CHO, and NIH3T3 cells were allowed to spread on fibronectin-coated cover glass for 45 minutes, then fixed and stained as above. Scale bar, 20 μm.
We next performed immunofluorescence studies to examine endogenous CIB distribution in a variety of cell lines such as HUVECs and CHO and NIH3T3 cells and to compare such distribution with CIB localization in platelets. In platelets allowed to adhere to fibrinogen, endogenous CIB distribution was concentrated at the tips of the filopodia of partially spread platelets and at the membrane periphery of fully spread platelets, where it also colocalized with F-actin (Figure 1C). HUVECs fully spread on fibronectin exhibit multiple filopodia where CIB and F-actin appear to colocalize (Figure 1C). In the fibroblastic cell lines CHO and NIH3T3, CIB appears at the peripheral membrane ruffles of cells adhering to fibronectin where it again colocalizes with F-actin (Figure 1C). Because the morphologies of the tested cell lines are different, CIB staining in these cells appears slightly different. However, it is apparent that in all situations, CIB uniquely and specifically localizes to the membranous extensions or to the cell periphery, suggesting that CIB may play a role in the spreading of these cells. Further, its colocalization with F-actin, whose reorganization is fundamental for the cellular mobile dynamics required for platelet spreading, also suggests such a phenotype.
Overexpression of CIB induces cell migration
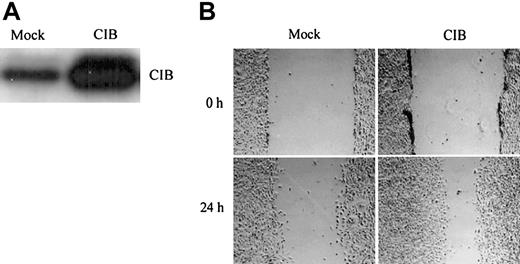
We chose CHO cells as our mammalian expression model, as these cells have been widely used as a simplified model to study platelet-related events,21-26 and thus overexpressed recombinant human CIB in CHO cells to better understand its physiologic role. Although CHO cells are not of human origin, the fact that CIB is so highly conserved among species (Figure 1A) suggests that overexpression of human CIB may complement the endogenous signaling. Western blot analysis of total cell lysates from CIB- and mock-transfected (control) cells indicated that CIB was substantially overexpressed over the endogenous level (Figure 2A). Additionally, the cellular localization patterns of endogenous and overexpressed CIB remained the same (data not shown). Because a synergistic relationship between cell migration and cell adhesion is well established,19,27 a wound-induced cell migration assay was performed.17,18 Cell migration on fibronectin in both mock- and CIB-transfected wounded monolayers was observed at 0 hours and 24 hours after making a wound. Within a 24-hour period, CIB-overexpressed cells migrated across almost three fourths of the wounded area compared with mock-transfected cells (Figure 2B), indicating that CIB induces cell spreading and migration.
CIB overexpression induces cell migration. (A) Western blot analysis of the extent of CIB overexpression compared with endogenous levels. (B) Serum-starved mock- and CIB-transfected CHO cells were grown to confluency on fibronectin to form a monolayer, which was then wounded and observed at 0 and 24 hours. Original magnification, × 100.
CIB overexpression induces cell migration. (A) Western blot analysis of the extent of CIB overexpression compared with endogenous levels. (B) Serum-starved mock- and CIB-transfected CHO cells were grown to confluency on fibronectin to form a monolayer, which was then wounded and observed at 0 and 24 hours. Original magnification, × 100.
We further investigated CIB-induced cell migration in a more quantitative way by using a transwell motility assay19 on a variety of extracellular matrix proteins. Compared with mock-transfected cells, CIB induced CHO cell migration and spreading on a fibronectin matrix (Figure 3A). Quantitation of these data indicated that CIB induced CHO cell migration about twice that of controls (Figure 3B). However, no significant CIB-induced increase was observed on a matrix of other extracellular matrix (ECM) proteins such as vitronectin, collagen, or gelatin (data not shown). These results, thus, indicated that CIB-induced migration was clearly specific to and dependent on fibronectin. To determine whether the signaling pathway involved in CIB-induced migration is specific to the fibronectin receptor, integrin α5β1, we treated the cells with an anti-α5β1 function-blocking antibody. The resultant cell migration was inhibited in both mock- and CIB-overexpressed cells in a dose-dependent manner (Figure 3B). We, thus, conclude that CIB-induced cell migration on fibronectin is specifically mediated through integrin α5β1. However, it should be noted that previous studies using the yeast 2-hybrid system have indicated that CIB does not directly interact with either α5 or β1 integrin subunits.15 Thus, this mediation is most likely through activation of a downstream signaling component.
CIB induces cell migration through integrin α5β1 (A) Mock- and CIB-overexpressed cells were allowed to migrate for 5 hours across transwell inserts coated on the underside with fibronectin. Unmigrated cells were removed, and migrated cells were fixed and stained. Original magnification, × 200. (B) Mock- and CIB-overexpressed cells were incubated with increasing concentrations of function-blocking anti-α5β1 for 20 minutes and were then allowed to migrate across fibronectin-coated inserts in the presence of function-blocking antibody for 5 hours. Error bars indicate mean ± SEM of at least 3 independent experiments.
CIB induces cell migration through integrin α5β1 (A) Mock- and CIB-overexpressed cells were allowed to migrate for 5 hours across transwell inserts coated on the underside with fibronectin. Unmigrated cells were removed, and migrated cells were fixed and stained. Original magnification, × 200. (B) Mock- and CIB-overexpressed cells were incubated with increasing concentrations of function-blocking anti-α5β1 for 20 minutes and were then allowed to migrate across fibronectin-coated inserts in the presence of function-blocking antibody for 5 hours. Error bars indicate mean ± SEM of at least 3 independent experiments.
CIB localizes at the focal adhesions of CHO cells
Once integrin binding to its respective extracellular matrix proteins has occurred, further points of adhesion resulting from integrin clustering form to increase the adhesive strength of the cell to the substratum. These points of adhesion mature into focal adhesions, the formation and degradation of which promotes the rapid adhesion and release required for cell motility.28 Because CIB promotes CHO migration, we sought to determine the specific localization of CIB in these cells as compared with focal adhesions. Focal adhesion structures contain a number of kinases and cytoskeletal proteins. We labeled CHO cells by using immunofluorescence techniques to observe a known focal adhesion component, vinculin, as well as phosphotyrosine, to determine whether CIB also localizes to these structures. As expected, CIB appears to colocalize with both vinculin and phosphotyrosine, most predominantly at the nascent focal contacts that form at the membrane periphery of these cells (Figure 4). Interestingly, although focal adhesions are present in the center of the cell, CIB does not appear to associate with these internal contact sites (Figure 4). Together, these data suggest that CIB plays a role in peripheral focal adhesion physiology.
CIB localizes at the focal adhesions. Immunofluorescent images of CHO cells plated on fibronectin for 60 minutes and immunostained for endogenous CIB, vinculin, or phosphotyrosine (p-Tyrosine). The merged image shows that CIB colocalizes at areas of focal adhesions. Scale bar, 10 μm.
CIB localizes at the focal adhesions. Immunofluorescent images of CHO cells plated on fibronectin for 60 minutes and immunostained for endogenous CIB, vinculin, or phosphotyrosine (p-Tyrosine). The merged image shows that CIB colocalizes at areas of focal adhesions. Scale bar, 10 μm.
Overexpression of CIB increases focal adhesions
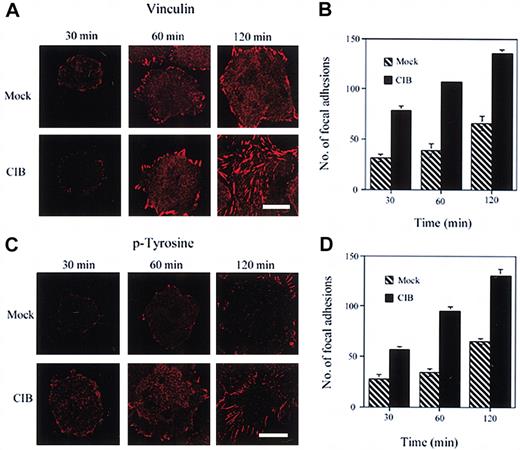
To determine more conclusively the role of CIB in this process, we overexpressed CIB in these cells and studied its effect on the formation of focal adhesions. In CIB-transfected cells, focal adhesions appear after 30 minutes of adhesion and are abundantly increased at both 60- and 120-minute periods (Figure 5A). In comparison, in mock-transfected cells, very few adhesions form after 30 minutes of adhesion and are slightly increased after 60 to 120 minutes of adhesion (Figure 5A). Quantitation of these focal adhesions shows that the number of focal adhesions formed in CIB-overexpressing cells is greatly and significantly (P < .05) increased compared with the focal adhesions formed in mock-transfected cells at any given time (Figure 5B). When a similar experiment was performed by using anti-phosphotyrosine antibody, a similar trend was noted, in that the number of focal adhesions formed in CIB-overexpressing cells was significantly (P < .05) increased (Figure 5C-D). Thus, these data suggest that CIB-mediated adhesion on fibronectin might be regulated through a CIB-induced formation of focal adhesion complexes.
CIB induces focal adhesion formation. (A) Immunofluorescence images of mock- and CIB-overexpressed CHO cells adhered to fibronectin and stained for vinculin. Cells were observed for focal adhesion formation at 30, 60, and 120 minutes. (B) Quantitation of the number of focal adhesions formed per cell in both mock- and CIB-transfected cells at each time point as observed by vinculin staining. CIB overexpression significantly (P < .05) increases focal adhesion formation. (C) Immunofluorescent images as in panel A stained for phosphotyrosine (p-Tyrosine). (D) Quantitation of the number of focal adhesions formed in both mock- and CIB-transfected cells at each time point as observed by phosphotyrosine staining, in that CIB overexpression significantly (P < .05) increased the number and rate of development of focal adhesions. Scale bar (A,C), 10 μm. Error bars (B, D) indicate mean ± SEM of at least 3 independent experiments.
CIB induces focal adhesion formation. (A) Immunofluorescence images of mock- and CIB-overexpressed CHO cells adhered to fibronectin and stained for vinculin. Cells were observed for focal adhesion formation at 30, 60, and 120 minutes. (B) Quantitation of the number of focal adhesions formed per cell in both mock- and CIB-transfected cells at each time point as observed by vinculin staining. CIB overexpression significantly (P < .05) increases focal adhesion formation. (C) Immunofluorescent images as in panel A stained for phosphotyrosine (p-Tyrosine). (D) Quantitation of the number of focal adhesions formed in both mock- and CIB-transfected cells at each time point as observed by phosphotyrosine staining, in that CIB overexpression significantly (P < .05) increased the number and rate of development of focal adhesions. Scale bar (A,C), 10 μm. Error bars (B, D) indicate mean ± SEM of at least 3 independent experiments.
CIB associates with FAK in CHO cells
It is known that CIB interacts with several protein kinases, including DNA-protein kinase,29 and pololike kinase family members Plk2 and Plk3.30 In addition, it has been recently shown that CIB regulates pololike kinases 231 and 3 (Naik et al, manuscript submitted, 2003) through inhibition of their kinase activities. In this study, we find that CIB appears to colocalize with phosphotyrosine associated with focal adhesions in CHO cells (Figure 4), which increase on overexpression of CIB (Figure 5C-D). Because FAK is tyrosine phosphorylated and is key to the processes of cell migration and platelet spreading, we next sought to investigate whether CIB also associates with FAK in CHO cells. We immunoprecipitated CIB from both mock- and CIB-transfected CHO cell lysates, then blotted with anti-FAK antibody, and found that CIB and FAK indeed associate. We found that this association is increased on increased expression of CIB (Figure 6A). This increase could be because in mock-transfected cells endogenous CIB forms interactions with a number of different molecules and only on increased expression of CIB is the interaction with FAK evident. An immunoprecipitate kinase assay using the same cells indicated that the kinase activity of FAK also increased on overexpression of CIB compared with its activity in mock-transfected cells (Figure 6B), suggesting that CIB up-regulates FAK activity in these cells, attesting to the physiologic relevance of this interaction. To investigate more conclusively the physiologic nature of this putative relationship, we introduced dominant-negative FAK, or FRNK, into CHO cells, either by itself or along with CIB, and performed a transwell migration assay on fibronectin as in Figure 3. Again, we find that CIB induces CHO cell migration on fibronectin in these cells compared with mock-transfected cells, but FRNK-overexpressing cells migrate to the same extent as control cells (Figure 6C). Interestingly, when both CIB and FRNK were overexpressed, the CIB-induced migration observed previously was inhibited (Figure 6C). Taken together, these data suggest that CIB induces CHO cell migration on fibronectin through both focal adhesion formation and FAK activation. It is, therefore, possible that a similar process occurs in platelets, in that CIB and GPIIb regulate platelet spreading on Fg through the regulation of FAK activity.
CIB interacts with and regulates FAK activity. (A) Immunoprecipitation of CIB from mock- and CIB-transfected CHO cell lysates. Western blot analysis with anti-FAK that shows CIB interacts with FAK. (B) Immunoprecipitate kinase assay using mock- and CIB-transfected CHO cell lysates. Although FAK expression levels are similar (WB: FAK), the amount of phosphotyrosine is up-regulated on CIB overexpression (WB: pY). FAK activity is also up-regulated on CIB cell overexpression (Autoradiogram). (C) Haptotactic transwell migration assay performed on fibronectin using mock-, CIB-, and FRNK-transfected CHO cells, as well as CIB + FRNK-cotransfected cells. FRNK inhibits CIB-induced CHO migration on fibronectin. Error bars indicate mean ± SEM of at least 3 independent experiments.
CIB interacts with and regulates FAK activity. (A) Immunoprecipitation of CIB from mock- and CIB-transfected CHO cell lysates. Western blot analysis with anti-FAK that shows CIB interacts with FAK. (B) Immunoprecipitate kinase assay using mock- and CIB-transfected CHO cell lysates. Although FAK expression levels are similar (WB: FAK), the amount of phosphotyrosine is up-regulated on CIB overexpression (WB: pY). FAK activity is also up-regulated on CIB cell overexpression (Autoradiogram). (C) Haptotactic transwell migration assay performed on fibronectin using mock-, CIB-, and FRNK-transfected CHO cells, as well as CIB + FRNK-cotransfected cells. FRNK inhibits CIB-induced CHO migration on fibronectin. Error bars indicate mean ± SEM of at least 3 independent experiments.
CIB associates with FAK in human platelets
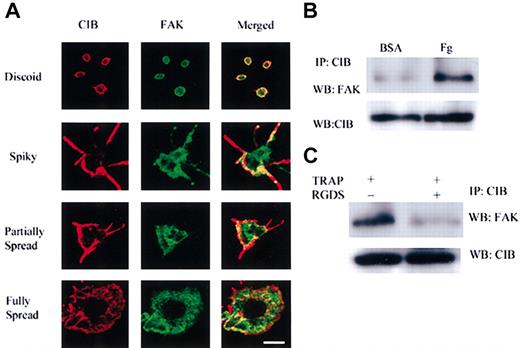
We next sought to confirm that CIB indeed regulates FAK in the platelet system. We first compared the localization of these molecules at various stages of platelet spreading. Immunofluorescence analysis of CIB and FAK localization in platelets adhering to Fg indicates that CIB and FAK colocalize throughout the stages of platelet spreading, but specifically at areas of focal contacts such as at the transiently formed filopodia of spiky and partially spread platelets, and at the membrane periphery of fully spread platelets (Figure 7A). Interestingly, a few filopodia in the spiky and partially spread platelets do not exhibit FAK staining, although CIB staining remains (Figure 7A). In fact, these filopodia have not made contact with the immobilized Fg, thus emphasizing the necessity for Fg binding for the association of these molecules. We next sought to confirm whether CIB and FAK associate by immunoprecipitation analysis. A substantial amount of FAK associates with CIB on platelet adhesion to immobilized Fg (Figure 7B). We also observed a slight association between CIB and FAK in platelets exposed to BSA (Figure 7B); however, this association could be due to an unintentional activation that could have occurred during platelet preparation. Because the activation of FAK requires cytoskeletal rearrangement, we asked whether the association of CIB with FAK is dependent on this process. When washed platelets were pretreated with cytochalasin D prior to adhesion to Fg, interaction between CIB and FAK was completely abolished (data not shown). We also tested whether activation of platelets by an agonist such as TRAP also results in the association of CIB and FAK. Immunoprecipitation from TRAP-activated platelet lysates using anti-CIB antibody confirms this association; however, when Fg binding is inhibited by RGDS peptide, this association fails to occur (Figure 7C). These immunoprecipitation studies again emphasize the necessity for ligand binding for the formation of this complex. Together, these data suggest that CIB and FAK associate on Fg binding as a result of outside-in signaling. Moreover, we can conclude that CIB may regulate platelet spreading on Fg through the regulation of FAK activity.
CIB associates with FAK in human platelets. (A) Immunofluorescence analysis of CIB and FAK localization in platelets throughout the stages of platelet adhesion to immobilized Fg. Scale bar, 1 μm. (B) Immunoprecipitation of CIB from platelet lysates of cells adhered to Fg or BSA. Western blot analysis with anti-FAK shows the association between CIB and FAK increases on platelet adhesion to Fg. (C) Immunoprecipitation of CIB from TRAP-activated platelet lysates in the presence or absence of RGDS. Western blot analysis with anti-FAK shows the association between CIB and FAK is blocked on inhibition of the ligand binding.
CIB associates with FAK in human platelets. (A) Immunofluorescence analysis of CIB and FAK localization in platelets throughout the stages of platelet adhesion to immobilized Fg. Scale bar, 1 μm. (B) Immunoprecipitation of CIB from platelet lysates of cells adhered to Fg or BSA. Western blot analysis with anti-FAK shows the association between CIB and FAK increases on platelet adhesion to Fg. (C) Immunoprecipitation of CIB from TRAP-activated platelet lysates in the presence or absence of RGDS. Western blot analysis with anti-FAK shows the association between CIB and FAK is blocked on inhibition of the ligand binding.
Discussion
Platelet spreading on subendothelial matrix proteins that are exposed following injury to the vascular endothelium is one of the crucial primary events that mediate physiologic hemostasis. For this reason, it is important to understand the molecular signaling events that are involved to develop remedies for pathologies in which this process is deregulated. We have previously shown that formation of the CIB-GPIIb/IIIa complex is required for platelet spreading on immobilized Fg.10 FAK activation is also required for spreading.22,32 In this study, we investigated CIB as a novel mediator of FAK activity that leads to focal adhesion formation and platelet spreading. We used immunofluorescence analysis and found that CIB localizes to the membrane periphery of many cell types and colocalizes with components of focal adhesions. On CIB overexpression, the number of focal adhesions increases, as does FAK activity. Through immunoprecipitation analysis, CIB was also found to associate with FAK on platelet binding to Fg. We, thus, conclude that CIB may regulate platelet spreading through the regulation of FAK activity.
Our previous findings suggested that CIB interaction with GPIIb is required for platelet spreading.10 However, myriad other molecules are present in platelet granules, such as ADP, serotonin in the platelet dense granules, and adhesive proteins such as platelet factor 4, Fg, and von Willebrand factor in α-granules.9 Therefore, granular secretion not only initiates ADP-related signaling, but, on binding of these other molecules to their respective receptors, also initiates numerous other signaling events downstream. As such, we sought to examine downstream events of CIB signaling without complications from secretory signaling events. Thus, we chose to reconstitute a mammalian expression model to study platelet-related CIB-induced signaling in cells that do not support granular secretion. Additionally, because platelets are anucleate, a number of molecular biologic techniques routinely used to study such topics cannot be done in platelets. CHO cells have been used frequently by a number of established researchers to model aspects of platelet signaling.21-26 A wealth of knowledge concerning platelet-related events has been derived from studies in this particular host system. For example, mutational studies of platelet-specific components such as GPIIb/IIIa, GPVI, and GPIb-IX, are virtually impossible to perform in platelets using current molecular biologic techniques. CHO cells are often transfected with appropriate constructs so that the effect of these mutations can be studied and extrapolated back to the platelet system.21-26 These mutational studies have yielded a great deal of information about platelet function, as specific sequences of these primary functional molecules are being studied as targets for therapeutic approaches.33
FAK is widely regarded as an integral component of platelet signaling that leads to platelet spreading.11,32 Integrin engagement with their respective ligands initiates integrin clustering. The site of convergence of these adhesive receptors initiates FAK recruitment and activation, leading to the formation of a mature focal adhesion.24 In this study, we find that the association between CIB and FAK occurs only on cell attachment to Fg (Figure 7A). In fact, as noted in Figure 7A, filopodia that do not contact Fg fail to exhibit FAK staining, and, hence, CIB and FAK colocalization is not observed. Further, CIB and FAK immunoprecipitate only on platelet binding of Fg (Figure 7B). When ligand binding is disrupted by the presence of RGDS peptide, these proteins fail to associate (Figure 7C). Taking into account the fact that CIB binds GPIIb/IIIa directly, these data are consistent with the fact that FAK activation requires integrin occupancy and suggest that perhaps the CIB/GPIIb complex is required for FAK activation. Investigation of the presence of FAK in the CIB/GPIIb complex or of GPIIb in the CIB/FAK complex is of great interest and is ongoing.
FAK has not only been characterized as a protein tyrosine kinase but is also a well-known adaptor protein, as it can bind several different signaling proteins together. The major site of FAK phosphorylation, Y397, serves as a binding site for the Src homology domain 2 (SH2) domains of Src,34,35 and PI 3-kinase.36 These interactions are known to be the key to platelet spreading, as pretreatment of platelets with PI3K or Src inhibitors arrests platelet spreading,8,37 in a morphology similar to that which we see on inhibition of CIB.10 In addition, FAK associates with a number of other structural and signaling molecules, such as Grb2, p130Cas, paxillin, and Graf.38-42 Graf (GTPase [guanosine triphosphatase] regulator associated with FAK) localizes with the actin cytoskeleton and its GTPase-activating protein (GAP) domain stimulates the GTPase activity of both Rho and Cdc42.42 However, roles of Rho and Cdc42 in the formation of focal adhesions are decidedly different. The focal complexes formed by Cdc42 and Rac at the membrane periphery are regarded as vinculin- and phosphotyrosine-positive structures and are morphologically distinct from Rho-regulated focal adhesions formed at the end of stress fibers.43-45 We find that CIB localizes with vinculin- and phosphotyrosine-positive structures specifically at the membrane periphery (Figure 5) and that the formation of these structures is up-regulated on CIB overexpression (Figure 6). As such, investigation of the FAK/CIB association for the presence of Cdc42 or Rac would be of great interest to further define the signaling pathway in this spreading mechanism. Consistent with this idea, it has been shown that CIB interacts with Rac3 and induces GPIIb/IIIa-dependent cell spreading.46 Investigation of this complex for the presence of Graf in platelets would also be of great interest, with regard to the apparent association of both CIB and Graf with the actin cytoskeleton, and the regulation of actin dynamics in platelet spreading.
A surprising finding in this study was the involvement of CIB in cell migration. Although we interpreted these data as a way to investigate platelet spreading, because of the complimentary effect between cell spreading and migration that is the key to effective motility, this finding also inspires another line of investigation concerning the role of CIB. CIB has already been implicated in a wide variety of cell functions other than in platelets, such as the regulation of cytokinesis through interaction with pololike kinase 3 (Naik et al, unpublished observation, 2003), in Alzheimer disease,47 and in the repair of DNA damage.48 These data may extend the role of CIB into vascular biology. We find, not only in platelets but also in CHO cells, that CIB induces FAK activity which leads to cell spreading and migration. This role was indicated by the localization of CIB to the membrane periphery of these cells. Notably, we also discovered that CIB localizes to the membrane periphery and lamellipodial structures of human endothelial cells (Figure 1C). It is, thus, possible that CIB may also induce endothelial cell spreading and migration through FAK activation and focal adhesion formation as well. Confirmation of this hypothesis would be of great interest to the area of vascular biology, as CIB may contribute to the regulation of a number of physiologic and pathologic events related to vasculogenesis.
Prepublished online as Blood First Edition Paper, July 24, 2003; DOI 10.1182/blood-2003-05-1703.
Supported by grants from the National Institutes of Health (HL57630) (U.P.N.) and the National Center for Research Resources (1P20RR155801) (U.P.N.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank K. Eckfeld for her untiring help in preparing the manuscript.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal