Abstract
We here report that the arterial blood flow after endothelial injury in mice deficient in α2-antiplasmin (α2-AP-/- mice) was well maintained compared with that of wild-type mice. Moreover, the development of neointima 4 weeks after injury in α2-AP-/- mice was significantly decreased. Histologic observations showed a prompt recovery of endothelial cells with a much higher proliferating index in repaired endothelium in α2-AP-/- mice. The amount of secreted vascular endothelial growth factor (VEGF) by explanted vascular smooth muscle cells (SMCs) from α2-AP-/- mice was significantly increased. In separate experiments using a human endothelial cell (EC) line, we could demonstrate that plasminogen binds to ECs and that this binding can be prevented by α2-AP. Finally, an injection of either an anti-VEGF receptor-1 antibody or α2-AP reduced the prompt endothelial healing. α2-AP is the main inactivator of plasmin, which cleaves extracellular matrix-bound VEGF to release a diffusible proteolytic fragment. Lack of α2-AP, therefore, could lead to a local over-release of VEGF by the continuously active plasmin in the injured area, which could result in a prompt re-endothelialization after vascular injury. Our results provide new insight into the role of α2-AP and VEGF in the pathogenesis of re-endothelialization following vascular injury. (Blood. 2003;102: 3621-3628)
Introduction
The integrity of the endothelial surface is essential for maintaining homeostasis between blood and surrounding tissues. Vascular endothelial growth factor (VEGF) is a potent mitogen with high specificity for endothelial cells.1 It plays a major role in angiogenesis,2 and indeed mice lacking 1 of the 2 VEGF alleles die before birth and show defects in the development of the cardiovascular system.3 Moreover, VEGF mediates vascular permeability,4,5 endothelial chemotaxis,6 endothelium-derived relaxing factor-dependent vasodilatation,7 and thrombogenesity.8 Four different molecular isoforms of VEGF exist, having 121, 165, 189, and 206 amino acids, respectively (VEGF121, VEGF165, VEGF189, and VEGF206). Native VEGF is a basic heparin-binding glycoprotein of 4.5 kDa.9 These properties correspond to those of VEGF165, the major isoform. VEGF121 is a weakly acidic polypeptide that fails to bind to heparin.10 VEGF189 and VEGF206 are more basic and bind to heparin with greater affinity than VEGF165. Interestingly, the longer forms VEGF189 and VEGF206 are almost completely sequestered in the extracellular matrix and may be released by plasmin.10
α2-Antiplasmin (α2-AP) is a serpin (serine protease inhibitor) and is the main physiologic inhibitor of the fibrinolytic plasmin in mammalian plasma. It is synthesized in the liver and is present in plasma at a concentration of about 1.0 nmol/mL.11 Human and murine α2-AP with molecular weight of 65 to 70 kDa12 rapidly inactivate plasmin, resulting in the formation of a stable inactive complex, plasmin-α2-AP.13 Apart from the removal of fibrin, the fibrinolytic system also plays a pivotal role in phenomena such as embryogenesis, ovulation, intima formation, proliferation, migration, tumorigenesis, and metastasis.14 It has been reported that the levels of plasmin-α2-AP complex in plasma are elevated in acute stroke, myocardial infarction, unstable angina, and arterial fibrillation.15,16 These findings may reflect a self-defense system against the risk of ischemic events, which are caused by vascular stenosis after vascular injury. Endothelial cell proliferation and migration to repair the vascular surface and vessel wall follow vascular injury. Studies have shown that VEGF is highly expressed in smooth muscle cells after endothelial denudation and that it accelerates re-endothelialization.17,18 However, plasmin plays a role to cleave and release VEGF from smooth muscle cells (SMCs).9 We, therefore, investigated the role of α2-AP, a physiologic plasmin inhibitor, in vascular remodeling by using mice deficient in α2-AP. Here, we report for the first time a crucial role of α2-AP following endothelial injury.
Materials and methods
Animals
Reagents
Recombinant murine VEGF, antimouse VEGF, antimouse VEGF receptor-1 (Fit-1), and anti-VEGF receptor-2 (Flk-1) were purchased from R&D Systems (Minneapolis, MN). The other chemical substances were obtained from Sigma Chemical (St Louis, MO).
Experimental endothelial injury in mice
The experimental procedure to induce an endothelial injury has been described in detail previously.21,22 Mice (n = 8, each group) were anesthetized by intraperitoneal injection of 44 mg/kg sodium pentobarbital. In brief, the right common carotid artery, the left jugular vein, and the right femoral artery were exposed under anesthesia with pentobarbital. Catheters (internal diameter = 0.5 mm; outer diameter = 0.8 mm, polyethylene sp3; Natume, Tokyo, Japan) were connected to the left jugular vein and to the right femoral artery for the injection of Rose Bengal (50 mg/kg; Sigma Chemical) and for monitoring blood pressure and pulse rate using a pressure transducer (AP601G; Nihon Koden, Tokyo, Japan) during experiments on day 0. Blood flow in the carotid artery was continuously monitored using a Doppler flow probe (Model PDV-20; Crystal Biotech, Tokyo, Japan) positioned proximally to the injured area of the carotid artery. Irradiation by green light (540 nm) proximal to the flow probe was started, and then Rose Bengal was injected as a bolus 10 minutes after the observation of control blood flow. The irradiation was continued for 15 minutes after the injection of Rose Bengal. This procedure results in destruction of endothelial cells in the irradiated area by oxygen radicals induced by the photochemical reaction between Rose Bengal and green light. Our previous histologic observations have revealed that under such conditions, a platelet-rich thrombus including fibrin was formed.17 The flow probe was removed after the first observation (day 0) and replaced on each consecutive observation day (day 1, 2, and 3). The presence of an occlusive thrombus was detected when blood flow was zero. After recovery from anesthesia, the animals were kept in individual cages and fed standard chow (RC4; Oriental Yeast, Osaka, Japan).
Quantitative analysis of neointima formation
Four weeks after the endothelial injury, the mice (n = 5, each time point) were anesthetized by sodium pentobarbital (44 mg/kg, intraperitoneally), and the common carotid artery was excised, rinsed with saline, and frozen. After removal of the artery, the animal was killed by an intraperitoneal injection of an overdose of sodium pentobarbital. The frozen sections were cut transversely into 20 sections at 100-μm intervals, followed by staining with hematoxylin and eosin (Sigma Chemical) after perfusion fixation at constant physiologic pressure. The total areas within the internal elastic lamina (IELA) and lumen (LA) were measured by using a computerized image graphic analysis system. For this analysis, 5 consecutive carotid artery cross sections (4-5 μm thick) were taken at 100-μm intervals from the bifurcation of the carotid artery. The intima area (IA = IELA - LA) was then expressed proportional to IELA by averaging the 3 measurements performed for each cross section.23
Proliferation index in vivo
In separate experiments, proliferating SMCs were identified by the thymidine analog 5-bromo-2-deoxy-Uridine (BrdU) in both types of mice.24 BrdU tests were performed at day 1, 3, 7, and 14 after injury (n = 4 each time point). BrdU (50 mg/kg) was injected subcutaneously 1, 8, 16, and 24 hours prior to removal of the carotid arteries. Following removal of the arteries, frozen cross sections were prepared from these arteries. BrdU-positive cells were stained with a murine monoclonal antibody (Sigma), followed by goat antimouse immunoglobulin antibodies conjugated to peroxidase and detected with diaminobenzidine (DAB). Sections were also stained for background with hematoxylin. The numbers of positive and negative nuclei were counted in the media and newly formed intima. The BrdU-labeling index was calculated by using the following formula: (the number of positive nuclei stained with DAB)/(the number of total nuclei stained with hematoxylin) × 100.
Electron microscopic analysis
Mice (n = 3 each) were killed by injection of an overdose of pentobarbital 48 hours after the initiation of endothelial injury. At the time of death, mice were exsanguinated and 2 mL phosphate-buffered saline (PBS) was injected into the right jugular vein to perfuse the whole body. Carotid arteries were removed and then transferred into 4% formaldehyde or 2% glutaraldehyde for 24 hours and next into in 50 mM sodium phosphate buffer. The formaldehyde-fixed samples were paraffin embedded, cut in butterfly-shaped sections of 5-μm thickness, placed on glass slides, and stained with hematoxylin and eosin (HE). The other glutaraldehyde-fixed samples were cut open longitudinally to allow visual inspection for scanning electron microscopy (SEM) as described.24
Immunohistochemical staining of VWF
Staining for von Willebrand factor (VWF) was used to detect repairing endothelial cells in the injured area of the murine vessels. Mice (n = 4 each time point) were killed by injection of an overdose of pentobarbital before and 2 hours, 48 hours, and 4 weeks after the initiation of endothelial injury. At the time of death, mice were exsanguinated, and 2 mL saline was injected into the right jugular vein to perfuse the whole body. Following removal of the carotid artery, frozen cross sections were prepared. Endothelial cells were stained with a peroxidase-conjugated monoclonal anti-VWF antibody (P 0226; DAKO Japan, Kyoto, Japan) and detected with DAB. Sections were also stained for background with hematoxylin.
Binding study of plasminogen to endothelial cells
A real-time biomolecular interaction assay system, an optical biosensor (IAsys Auto+; Affinity Sensors, Cambridge, United Kingdom) was used for the binding assay.25 In this cuvette-based resonant mirror instrument a change in the refractive index is obtained when a molecule binds to the sensing surface, resulting in a shift in the resonant angle. The angle change detected by the instrument is displayed in arc seconds; higher arc seconds mean greater binding. Plasminogen or α2-AP (100 μg/mL, 50 μL volume) was immobilized on the surface of an IAsys carboxylate cuvette (Affinity Sensors) via an aminohexanoic acid linker according to the manufacturer's protocol. Briefly, after equilibration and obtaining a stable baseline with PBS, carboxylate on the cuvette surface was activated with 200 nmol/L N-hydroxysuccimide (NHS) and 50 nmol 1-ethyl-3 (3-dimethylaminopropyl) carbodiimide (EDC) for 7 minutes. The activation solution was removed and washed with PBS, and then 1 mol/L aminohexanoic acid (pH = 8.5) was added for 5 minutes to immobilize. After removal of the aminohexanoic acid solution and washing with PBS, the carboxylate of the immobilized aminohexanoic acid was activated with 200 nmol/L NHS and 50 nmol/L EDC. After washing with PBS, 50 μg/mL plasminogen or α2-AP was added for 15 minutes. Finally, after removal of the solution and washing with PBS, the sensor surface was blocked with 1 mg/mL bovine serum albumin. The immobilization of plasminogen or α2-AP on the cuvette surface was determined from the increasing arc seconds. A baseline was established with PBS. Binding of endothelial cells (5 × 104 to 5 × 105 cell/mL in PBS) to immobilized plasminogen or α2-AP was monitored. For competing studies, plasminogen that had been preincubated with α2-AP was used to determine the binding of the endothelial cells. All experiments in the IAsys instrument were carried out at 25°C.
Spontaneous secretion of VEGF in primary cultured cells
Vascular SMCs were obtained from the thoracic aorta of wild-type mice and mice deficient in α2-AP.26 The cultured cells (1 × 105) were seeded into 35-mm-diameter dishes and maintained in 2 mL Dulbecco modified Eagle medium (DMEM) containing 10% fetal calf serum (FCS) at 37°C in a humidified atmosphere of 5% CO2/95% air. After 6 days, the medium was exchanged for serum-free DMEM. The cells were used for experiments after 48 hours. VEGF in conditioned medium was measured by enzyme-linked immunosorbent assay.
Treatment of α2-AP-/- mice with anti-VEGF antibodies
An anti-VEGF antibody (25 μg per body) was injected as a bolus 5 minutes before the initiation of endothelial injury in α2-AP-/- mice (n = 3, each group). In separate mice (n = 3, each group), antibodies against VEGFR-1 (Flt-1) or VEGFR-2 (KDR), which are expressed almost exclusively on endothelial cells,27 were also injected as a bolus, and then endothelial injury was induced in the carotid artery of α2-AP-/- mice. Two days after the injury, the injured artery was removed and treated for electron microscopic analysis and for the measurement of BrdU-positive cells.
Treatment of α2-AP+/+ mice with VEGF
To more directly define the effect of VEGF after endothelial injury, VEGF (2, 4, or 8 ng per body, n = 3 each group) was injected as a bolus in wild-type mice a few minutes before the initiation of injury. Two days after the injury, the injured artery was removed and treated for electron microscopic analysis and for the measurement of BrdU-positive cells.
Treatment of α2-AP-/- mice with α2-AP
α2-AP (75 μg per body) was injected as a bolus 5 minutes before the initiation of endothelial injury in α2-AP-/- mice (n = 3). Two days after the injury, the injured artery was removed and treated for electron microscopic analysis. In separate α2-AP-/- mice, α2-AP (75 μg per body) was injected as a bolus 5 minutes before the initiation of endothelial injury via tail vein and continuously treated for 1 day, 3 days, 1 week, 2 weeks, or 4 weeks. Four weeks after the initiation of endothelial injury, vessels in each group were removed, and neointimal area was measured as mentioned in “Quantitative analysis of neointima formation.”
Statistical analysis
All data are expressed as the mean ± SEM. The significance of the effect of each treatment (*P < .01) was determined by analysis of variance (ANOVA) followed by the Student Newman-Keuls test.
Results
Lack of α2-AP well maintains the blood flow after endothelial injury
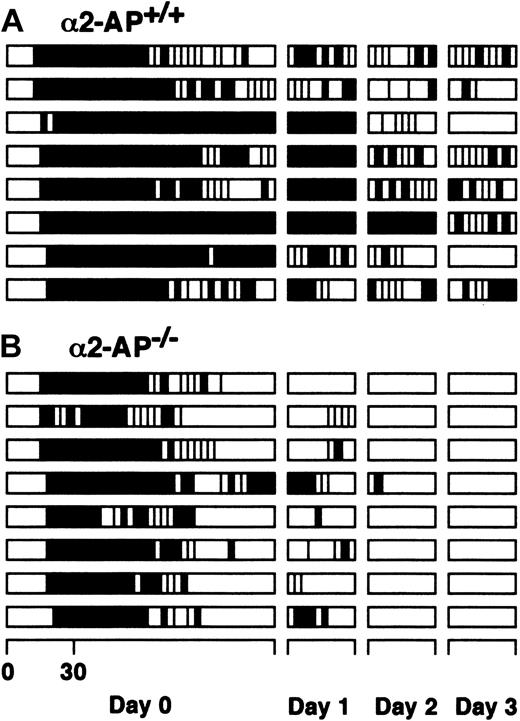
The time profiles of vascular patency after the initiation of endothelial injury by the photochemical reaction are schematically illustrated in Figure 1. The time to occlusion because of the development of a thrombus after endothelial injury in α2-AP-/- mice (16.6 ± 1.6 minutes) was slightly prolonged compared with that of wild-type mice (13.2 ± 0.9 minutes). In both types of mice, cyclic reocclusion and reflow after spontaneous reperfusion were clearly observed in the artery on the day of the endothelial injury (day 0). Spontaneous reperfusion was observed in 5 of 8 wild-type mice at the end of the first observation period 120 minutes after the initiation of injury, which was consistently associated with cyclic reocclusion and reflow. In the other mice, cyclic reocclusion and reflow were also observed. Twenty-four hours later (day 1), a persistent occlusion was seen in 4 mice; cyclic reocclusion and reflow in 4 others. These findings gradually changed until at day 3, persistent patency was observed in 2 mice, whereas in the others cyclic reocclusion and reflow were still present. Following reperfusion, the mean blood flow remained less than 58% of the baseline blood flow (Table 1). Spontaneous reperfusion was clearly observed in all arteries of α2-AP-/- mice within the first observation period, however, with prominent cyclic reocclusion/reflow. These flow patterns clearly changed at day 1 when cyclic reocclusion/reflow was diminished. Persistent patency was observed in all α2-AP-/- mice until day 3, and reperfused blood flow recovered to 86% of baseline flow. Mean arterial blood flows and vascular patency after spontaneous reperfusion are shown in Tables 1 and 2.
Alteration of arterial blood flow after endothelial injury. Vascular patency after spontaneous reperfusion in the carotid artery of α2-AP+/+ mice (A) and α2-AP-/- mice (B). The time profile of vascular patency after the endothelial injury was schematically illustrated for 120 minutes at the first observation (day 0) and for 30 minutes at day 1, 2, or 3. The black and open columns indicate the periods of vascular occlusion and of blood flow (more than 10% of the blood flow obtained before the initiation of vascular injury), respectively.
Alteration of arterial blood flow after endothelial injury. Vascular patency after spontaneous reperfusion in the carotid artery of α2-AP+/+ mice (A) and α2-AP-/- mice (B). The time profile of vascular patency after the endothelial injury was schematically illustrated for 120 minutes at the first observation (day 0) and for 30 minutes at day 1, 2, or 3. The black and open columns indicate the periods of vascular occlusion and of blood flow (more than 10% of the blood flow obtained before the initiation of vascular injury), respectively.
Alteration of arterial blood flow after injury
. | α2-AP+/+ mice . | α2-AP−/− mice . |
|---|---|---|
| Time to occlusion, min | 11.3 ± 1.2 | 16.1 ± 4.1 |
| Mean blood flow, % | ||
| Reperfusion | 56.3 ± 8.4 | 66.2 ± 7.2 |
| Day 1 | 49.9 ± 9.1 | 70.3 ± 8.3* |
| Day 2 | 59.4 ± 8.4 | 75.3 ± 4.6* |
| Day 3 | 63.3 ± 6.3 | 82.5 ± 3.6* |
. | α2-AP+/+ mice . | α2-AP−/− mice . |
|---|---|---|
| Time to occlusion, min | 11.3 ± 1.2 | 16.1 ± 4.1 |
| Mean blood flow, % | ||
| Reperfusion | 56.3 ± 8.4 | 66.2 ± 7.2 |
| Day 1 | 49.9 ± 9.1 | 70.3 ± 8.3* |
| Day 2 | 59.4 ± 8.4 | 75.3 ± 4.6* |
| Day 3 | 63.3 ± 6.3 | 82.5 ± 3.6* |
Blood flow is represented as a percentage of control blood flow (before the initiation of injury).
Indicates P < .05 versus corresponding time point in α2-AP+/+ mice.
Vascular patency after spontaneous reperfusion of artery in α2-AP+/+ mice
. | α2-AP+/+ mice . | . | . | α2-AP−/− mice . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
. | PO . | CR . | PP . | PO . | CR . | PP . | ||||
| Day 0 | 3 | 5 | 0 | 1 | 7 | 0 | ||||
| Day 1 | 4 | 4 | 0 | 0 | 6 | 1 | ||||
| Day 2 | 1 | 7 | 0 | 0 | 1 | 7* | ||||
| Day 3 | 0 | 6 | 2 | 0 | 0* | 8* | ||||
. | α2-AP+/+ mice . | . | . | α2-AP−/− mice . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
. | PO . | CR . | PP . | PO . | CR . | PP . | ||||
| Day 0 | 3 | 5 | 0 | 1 | 7 | 0 | ||||
| Day 1 | 4 | 4 | 0 | 0 | 6 | 1 | ||||
| Day 2 | 1 | 7 | 0 | 0 | 1 | 7* | ||||
| Day 3 | 0 | 6 | 2 | 0 | 0* | 8* | ||||
Vascular patency was judged to be the state at the end of the observation period on each time course. The carotid arterial or jugular vein patency was expressed as persistent occlusion (PO) when no reperfusion was observed at all; as cyclic flow reduction (CR) when the vascular reflow alternately showed stop and flows; and as persistent patency (PP) when the vascular flow was maintained until the end of the observation period.
P < .05 versus each wild-type mouse on the same time course. Data correspond to the number of vessels in each group.
Time-dependent re-endothelialization
Figure 2 shows endothelial cells before and after injury in both types of mice. Before the initiation of injury, endothelial cell layers in both types of mice were clearly observed (Figure 2A-B). A few endothelial cells remained on the vascular surface after injury (Figure 2C-D). No difference between α2-AP-/- mice and wild-type mice could be observed in the above conditions. In contrast, the re-endothelialization was markedly different 48 hours after injury. Indeed, the endothelial cell layer was much greater covering the injured area in α2-AP-/- mice (Figure 2F), whereas this covering was only partial in wild-type mice (Figure 2E). Development of neointima was next measured 4 weeks after injury in both types of mice (Figure 2G-H) and showed a complete re-endothelialization of the newly formed intima. However, a thicker reconstructed endothelial layer was seen in α2-AP-/- mice than in wild-type mice.
Histologic analysis of time-dependent re-endothelialization in murine carotid artery. Typical observations of endothelial cells stained by anti-VWF antibodies in the carotid artery of α2-AP-/- (B,D,F,H; original magnification, × 400) and in α2-AP+/+ mice (A,C,E,G; original magnification, × 400). Before the initiation of injury, an intact endothelial cell layer was observed (A-B). Endothelial cells were removed by the injury (C-D). Regenerated endothelial cells (arrows) only partly covered the injured area in wild-type mice (E); however, an obvious compact endothelial cell layer (arrows) developed on the injured surface in α2-AP-/- mice (F) 2 days after injury. Four weeks after injury, a neointimal thickening (*) had developed in the injured area. A regenerated endothelial cell layer (arrows) was observed on the neointima in wild-type mice (G) and in α2-AP-/- mice, in which the endothelial cell layer (arrows), however, was thicker (G). Scanning electron photomicrographs of regeneration of endothelial cells in the injured carotid artery 48 hours after injury in α2-AP+/+ mice (I,K) and in α2-AP-/- mice (J,L) showed prompt and intense re-endothelialization in α2-AP-/- mice. The white bar represents 10 μm.
Histologic analysis of time-dependent re-endothelialization in murine carotid artery. Typical observations of endothelial cells stained by anti-VWF antibodies in the carotid artery of α2-AP-/- (B,D,F,H; original magnification, × 400) and in α2-AP+/+ mice (A,C,E,G; original magnification, × 400). Before the initiation of injury, an intact endothelial cell layer was observed (A-B). Endothelial cells were removed by the injury (C-D). Regenerated endothelial cells (arrows) only partly covered the injured area in wild-type mice (E); however, an obvious compact endothelial cell layer (arrows) developed on the injured surface in α2-AP-/- mice (F) 2 days after injury. Four weeks after injury, a neointimal thickening (*) had developed in the injured area. A regenerated endothelial cell layer (arrows) was observed on the neointima in wild-type mice (G) and in α2-AP-/- mice, in which the endothelial cell layer (arrows), however, was thicker (G). Scanning electron photomicrographs of regeneration of endothelial cells in the injured carotid artery 48 hours after injury in α2-AP+/+ mice (I,K) and in α2-AP-/- mice (J,L) showed prompt and intense re-endothelialization in α2-AP-/- mice. The white bar represents 10 μm.
Electron microscopic observation
SEM at 2 days after injury confirmed that the injured vascular surface was not completely covered by repairing endothelial cells in wild-type mice (Figure 2I-J). However, in α2-AP-/- mice, saturation of endothelial cells was observed on the injured vascular surface (Figure 2K). The repaired endothelial surface of α2-AP-/- mice, surprisingly, was not smooth as expected but quite rough with extruding cells (Figure 2L).
Neointima formation in response to endothelial injury
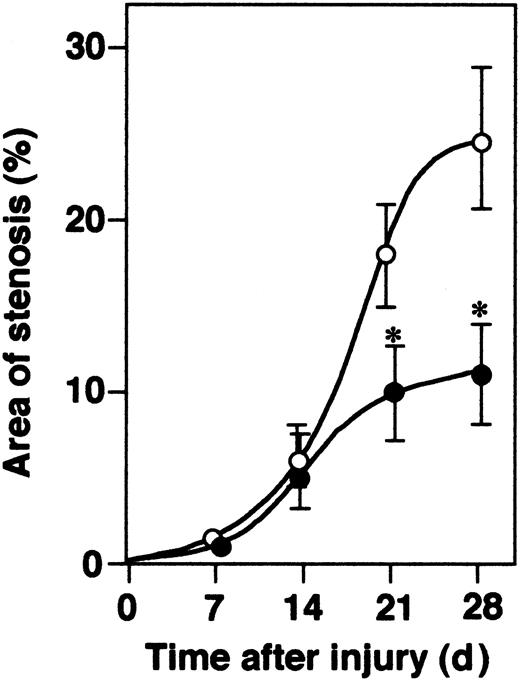
All mice developed concentric intimal lesions in response to endothelial injury. The ratios of time-dependent vascular occlusion by newly formed neointima are shown in Figure 3. In α2-AP-/- mice, the extent of vascular occlusion 4 weeks after the initiation of injury was significantly smaller compared with those of wild-type mice. Typical examples of newly formed neointima in both types of mice were shown in Figure 2G-H.
Time-dependent neointima formation. The development of neointima in α2-AP-/- (•) and α2-AP+/+ mice (○) is shown as the percentage of luminal stenosis by newly formed intima (n = 6 each time point). * indicates P < .05. Data represents mean ± SEM.
Time-dependent neointima formation. The development of neointima in α2-AP-/- (•) and α2-AP+/+ mice (○) is shown as the percentage of luminal stenosis by newly formed intima (n = 6 each time point). * indicates P < .05. Data represents mean ± SEM.
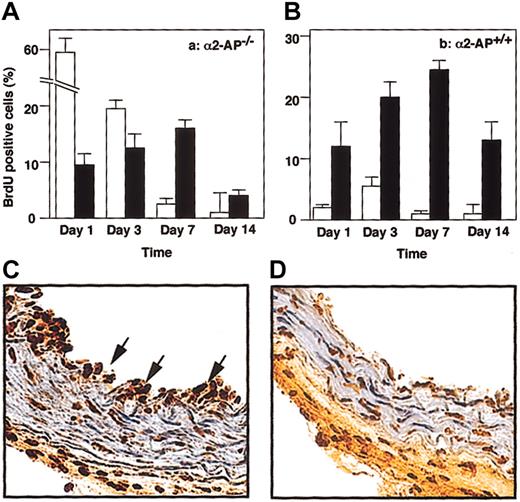
Proliferation index in vivo
Figure 4 shows the percentage of BrdU-positive cells in regenerating endothelial cells or in newly formed intima on day 1, 3, 7, or 14 after vascular injury. The lack of α2-AP caused a significant increase of BrdU-positive cells in recovered endothelial cells. Typical observations of BrdU-positive cells 1 day after injury are shown in Figure 4C-D.
Proliferative index in vivo. Cell proliferation (n = 4, for each time point) measured as the BrdU index (percentage) of recovered endothelial cells (□) and SMCs in media (•) following vascular injury in α2-AP+/+ (A) or α2-AP-/- mice (B). Data represents mean ± SEM. Typical observations of BrdU-positive cells in the injured carotid artery of α2-AP-/- (C) and in α2-AP+/+ mice (D). Twenty-four hours after injury, BrdU-positive cells (arrows) were clearly observed in the recovered endothelial layer in α2-AP-/- mice (C). However, BrdU-positive cells were present mainly in media (SMCs) in α2-AP+/+ mice (D). Original magnification, × 400.
Proliferative index in vivo. Cell proliferation (n = 4, for each time point) measured as the BrdU index (percentage) of recovered endothelial cells (□) and SMCs in media (•) following vascular injury in α2-AP+/+ (A) or α2-AP-/- mice (B). Data represents mean ± SEM. Typical observations of BrdU-positive cells in the injured carotid artery of α2-AP-/- (C) and in α2-AP+/+ mice (D). Twenty-four hours after injury, BrdU-positive cells (arrows) were clearly observed in the recovered endothelial layer in α2-AP-/- mice (C). However, BrdU-positive cells were present mainly in media (SMCs) in α2-AP+/+ mice (D). Original magnification, × 400.
Spontaneous secretion of VEGF in cultured cells of α2-AP-/- and α2-AP+/+ mice
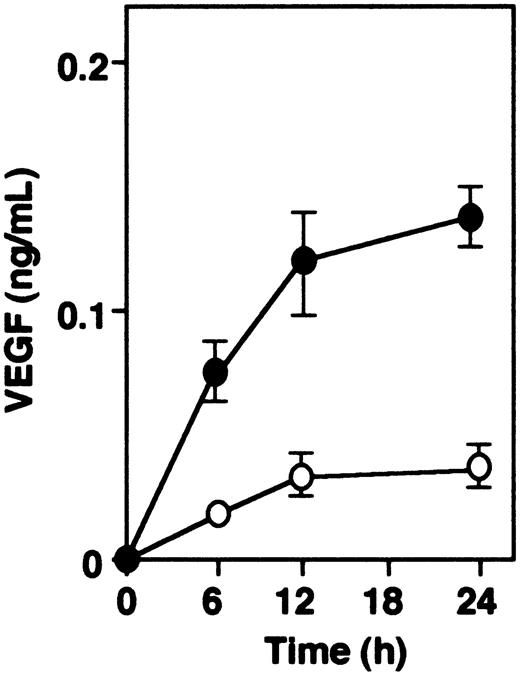
Spontaneous release of VEGF was observed in vascular SMCs from both mice types. VEGF in the conditioned medium, measured by ELISA in function of time (Figure 5), showed that the levels of VEGF from vascular smooth muscle cells of α2-AP-/- mice were about 2.5 times higher than those of α2-AP+/+ mice.
VEGF secretion in vitro. Spontaneous secretion of VEGF is increased in vascular SMCs from α2-AP-/- (•) as compared with those from α2-AP+/+ mice (○). Each point represents the mean of duplicate cultures. Data represents mean ± SEM.
VEGF secretion in vitro. Spontaneous secretion of VEGF is increased in vascular SMCs from α2-AP-/- (•) as compared with those from α2-AP+/+ mice (○). Each point represents the mean of duplicate cultures. Data represents mean ± SEM.
Binding and competitive inhibition assay of α2-AP to endothelial cells
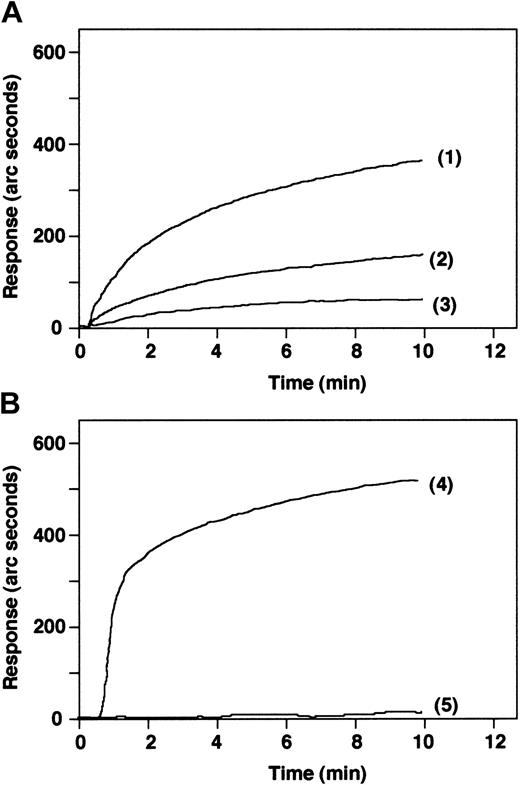
Immobilized plasminogen binds to endothelial cells, and the intensity of the signal was dependent on the cell number (Figure 6A). However, immobilized α2-AP binds to plasminogen but not to endothelial cells (Figure 6B).
Binding of endothelial cells to immobilized plasminogen or α2-AP. (A) Binding of 5 × 105 (1), 2.5 × 105 (2), or 1 × 105 endothelial cells/mL (3) to immobilized plasminogen was measured by biosensor. Immobilized α2-AP (B) readily binds to plasminogen (4) but fails to bind endothelial cells when 5 × 105 cells/mL are added (5).
Binding of endothelial cells to immobilized plasminogen or α2-AP. (A) Binding of 5 × 105 (1), 2.5 × 105 (2), or 1 × 105 endothelial cells/mL (3) to immobilized plasminogen was measured by biosensor. Immobilized α2-AP (B) readily binds to plasminogen (4) but fails to bind endothelial cells when 5 × 105 cells/mL are added (5).
Effect of injection of an anti-VEGF antibody in α2-AP-/- mice
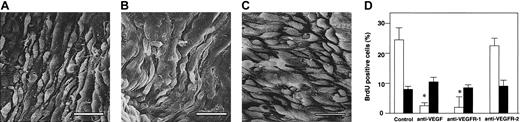
Intravenous injection of an anti-VEGF antibody 5 minutes before the initiation of endothelial injury in α2-AP-/- mice markedly changed the recovering endothelial surface in α2-AP-/- mice, which now was almost the same as in the wild-type mice (Figure 7A). Moreover, the action of VEGF is mediated by a particular family of receptor tyrosine kinases, VEGFR-1 (Flt-1) and VEGFR-2 (KDR), which are expressed almost exclusively on endothelial cells.27 The regenerating endothelial surface of α2-AP-/- mice treated with an inhibitory anti-VEGFR-1 antibody was not different from the one of the wild-type mice (Figure 7B), whereas all 4 α2-AP-/- mice treated with the anti-VEGFR-2 antibody showed a prompt endothelial healing after injury (Figure 7C). BrdU-positive cells were also counted (Figure 7D). The number of positive cells in α2-AP-/- mice treated with either VEGF antibody or anti-VEGFR-1 antibody did not significantly differ as compared with that of wild-type mice. However, the treatment with an anti-VEGFR-2 antibody in α2-AP-/- mice did not affect BrdU intake in vivo. Four weeks after the vascular injury, a neointima had developed in both types of mice, and the stenosis area of both types of mice was not significantly different (data not shown).
Effects of VEGF antibodies in α2-AP-/- mice. Scanning electron photomicrographs of the regenerated endothelial surface of a carotid artery 48 hours after injury in α2-AP-/- mice. When either VEGF antibody or anti-EGFR-1 antibody was administered to α2-AP-/- mice, the regeneration of the endothelium was normalized (A-B, respectively). α2-AP-/- mice treated with an anti-VEGFR-2 antibody showed a prompt and dense re-endothelialization (C). Cell proliferation (n = 4) measured as the BrdU index (percentage) of recovered endothelial cell layer (□) and SMCs in media (•) following vascular injury in α2-AP-/- mice (D). An anti-VEGF antibody and antibodies against VEGFR-1 (Flt-1) or VEGFR-2 (KDR), (25 μg per body) were injected as a bolus 5 minutes before the initiation of endothelial injury in α2-AP-/- mice (n = 3, each group). BrdU (50 mg/kg) was injected subcutaneously 1, 8, 16, and 24 hours prior to removal of the carotid arteries. Two days after the injury, the injured artery was removed and stained for the measurement of BrdU-positive cells. * indicates P < .05 control. Scale bars represent 10 μm.
Effects of VEGF antibodies in α2-AP-/- mice. Scanning electron photomicrographs of the regenerated endothelial surface of a carotid artery 48 hours after injury in α2-AP-/- mice. When either VEGF antibody or anti-EGFR-1 antibody was administered to α2-AP-/- mice, the regeneration of the endothelium was normalized (A-B, respectively). α2-AP-/- mice treated with an anti-VEGFR-2 antibody showed a prompt and dense re-endothelialization (C). Cell proliferation (n = 4) measured as the BrdU index (percentage) of recovered endothelial cell layer (□) and SMCs in media (•) following vascular injury in α2-AP-/- mice (D). An anti-VEGF antibody and antibodies against VEGFR-1 (Flt-1) or VEGFR-2 (KDR), (25 μg per body) were injected as a bolus 5 minutes before the initiation of endothelial injury in α2-AP-/- mice (n = 3, each group). BrdU (50 mg/kg) was injected subcutaneously 1, 8, 16, and 24 hours prior to removal of the carotid arteries. Two days after the injury, the injured artery was removed and stained for the measurement of BrdU-positive cells. * indicates P < .05 control. Scale bars represent 10 μm.
Effect of injection of VEGF in α2-AP+/+ mice and of α2-AP in α2-AP-/- mice
When the highest dose of VEGF was injected in wild-type mice with endothelial injury, the endothelial layer of all mice was slightly thickened (Figure 8A). BrdU-positive cells in regenerating endothelial cells increased in a dose-dependent manner (Figure 8B). However, the development of the neointima 4 weeks after injury was not decreased (data not shown). However, intravenous injection of α2-AP 5 minutes before the initiation of endothelial injury in α2-AP-/- mice markedly changed, and the recovering endothelial surface (48 hours after injury) in α2-AP-/- mice now was almost the same as the one in the wild-type mice (Figure 8C). When α2-AP was continuously administered for more than 1 week, development of neointimal area was almost the same as that of wild-type mice (Figure 8D). On the contrary, when α2-AP was treated for 1 day or 3 days, development of neointimal area 4 weeks after injury was still reduced compared with that of wild-type mice.
Effect of VEGF in α2-AP+/+ mice and of α2-AP in α2-AP-/- mice. Scanning electron photomicrographs of the regenerated endothelial surface of a carotid artery 48 hours after injury in α2-AP+/+ mice treated with VEGF at a dose of 8 ng per body (A) and treated with α2-AP at a dose of 75 mg per body (C). Cell proliferation (n = 4) measured as the BrdU index (percentage) of recovered endothelial cell layer (□) and SMCs in media (•) following vascular injury in α2-AP+/+ mice (B). VEGF (2, 4, 8 ng/body) was injected as a bolus before the initiation of endothelial injury. BrdU (50 mg/kg) was injected subcutaneously 1, 8, 16, and 24 hours prior to removal of the carotid arteries. Two days after the injury, the injured artery was removed and stained for the measurement of BrdU-positive cells. The development of neointima in α2-AP-/- mice is shown as the percentage of luminal stenosis by newly formed intima (D). α2-AP was injected as a bolus via tail vein for 1 day, 3 days, 1 week, 2 weeks, or 4 weeks after endothelial injury, and the injured carotid artery was removed 4 weeks after injury (n = 4 each). α2-AP+/+ mice (wild type [WT]) were treated with α2-AP for 4 weeks. * indicates P < .05 versus control (without α2-AP). Scale bar represents 10 μm.
Effect of VEGF in α2-AP+/+ mice and of α2-AP in α2-AP-/- mice. Scanning electron photomicrographs of the regenerated endothelial surface of a carotid artery 48 hours after injury in α2-AP+/+ mice treated with VEGF at a dose of 8 ng per body (A) and treated with α2-AP at a dose of 75 mg per body (C). Cell proliferation (n = 4) measured as the BrdU index (percentage) of recovered endothelial cell layer (□) and SMCs in media (•) following vascular injury in α2-AP+/+ mice (B). VEGF (2, 4, 8 ng/body) was injected as a bolus before the initiation of endothelial injury. BrdU (50 mg/kg) was injected subcutaneously 1, 8, 16, and 24 hours prior to removal of the carotid arteries. Two days after the injury, the injured artery was removed and stained for the measurement of BrdU-positive cells. The development of neointima in α2-AP-/- mice is shown as the percentage of luminal stenosis by newly formed intima (D). α2-AP was injected as a bolus via tail vein for 1 day, 3 days, 1 week, 2 weeks, or 4 weeks after endothelial injury, and the injured carotid artery was removed 4 weeks after injury (n = 4 each). α2-AP+/+ mice (wild type [WT]) were treated with α2-AP for 4 weeks. * indicates P < .05 versus control (without α2-AP). Scale bar represents 10 μm.
Discussion
The present study demonstrated that lack of α2-AP, a physiologic inhibitor of plasmin, resulted in the improvement of vascular patency after experimental endothelial injury in mice and indicates that α2-AP is essential for the preservation of the regenerating endothelial cells via regulation of local VEGF secretion. This effect could be explained mainly by the prompt re-endothelialization because of the over-release of VEGF by continuous local activation of plasmin.
Mice deficient in α2-AP were described, but no major physiologic dysfunction could be observed when they were not challenged.28 In our first experiment, we could demonstrate that the arterial blood flow after spontaneous reperfusion was well maintained in mice deficient in α2-AP, even though the time to occlusion was slightly prolonged, albeit not statistically significant, compared with that of wild-type mice. Moreover, the area of the neointima was significantly decreased in α2-AP-/- mice compared with that of wild-type mice. Time-dependent profiles of the BrdU index showed that proliferating cells were densely located in the newly formed intima 24 hours after injury in α2-AP-/- mice. These results made us speculate that the lack of α2-AP may play a key role in the regeneration of endothelial cells following endothelial injury. Immunohistochemical stain of endothelial cells using anti-VWF antibodies clearly indicated that regeneration of endothelial cells in α2-AP-/- mice was more rapid and dense than in α2-AP+/+ mice. Additionally, electron microscopic observations showed that the vascular surface after endothelial injury in α2-AP-/- mice was markedly different from that of wild-type mice in which repairing endothelial cells did not yet completely cover the injured area 24 to 48 hours after injury. We previously reported that local adhering microthrombi, including activated platelets, were observed in wild-type mice until a few days after injury.22 However, in α2-AP-/- mice, a large number of endothelial cells overcrowded the injured area, whereas no thrombi were observed. These results indicate that a significant endothelial regeneration is rapidly induced in α2-AP-/- mice following experimental endothelial denudation.
It is well known that VEGF is a major regulator of endothelial cell production under both physiologic and pathologic conditions.29 Previous studies carried out in a variety of animal species have repeatedly shown that extensive endothelial denudation of the arterial wall leads to neointimal thicking.30,31 Local delivery of VEGF to the site of vascular injury resulted in expeditious reendothlialization30 and reduced the development of a neointima.31,32 In the present study, the plasma concentrations of VEGF in both types of mice were almost the same before and after the initiation of endothelial injury when blood samples were taken from the jugular vein (data not shown). However, we confirmed that spontaneous release of VEGF by cultured vascular SMCs from α2-AP-/- mice was significantly higher than that by wild-type mice cells. Plasmin plays a role in the cleavage of VEGF from SMCs.9 α2-AP and plasminogen, but not plasmin, are secreted mainly by the liver into the circulation,13 indicating that lack of α2-AP is expected to have a local effect but not a systemic effect. Our binding studies on the one hand showed that plasminogen binds readily to endothelial cells and on the other hand indicated that α2-AP prevents the binding between plasminogen and endothelial cells dose dependently. Therefore, we speculated that lack of α2-AP could induce conditions that allow for easy binding of plasminogen to endothelial cells. This plasminogen then can be activated by plasminogen activators, such as tissue-type plasminogen activator secreted by endothelial cells, resulting in local production of plasmin, as in the above situation, in the absence of α2-AP, as in α2-AP-/- mice. This phenomenon might continuously stimulate the secretion of VEGF.
To further define the physiologic relation between α2-AP and VEGF in vascular remodeling, we performed several additional experiments using α2-AP-/- and α2-AP+/+ mice. When α2-AP-/- mice were supplemented with α2-AP for 1 day or 3 days, the vascular surface was not completely covered with regenerating endothelial cells until 2 days after the injury. Under those conditions, the vascular patency and the development of neointimal area were different from those of wild-type mice. However, when α2-AP-/- mice were continuously supplemented with α2-AP for over 1 week after injury, the development of neointimal area was almost the same as that of wild-type mice. These results directly prove that lack of α2-AP, especially in the early phase of the recovery process from vascular injury, results in the maintenance of vascular patency and in the reduction of neointima formation after injury in mice. Second, when a high dose of VEGF (8 ng per body) was injected as a bolus in wild-type mice before the injury, the number of endothelial cells in the injured area was only slightly elevated, and the stenosis area at 4 weeks after injury was not significantly diminished even if BrdU-positive cells in the recovering endothelial layer increased in a dose-dependent manner. The half-life of recombinant VEGF in the circulation is only minutes, and, indeed, administration of recombinant VEGF, also in humans, was shown to be ineffective.34 These findings clearly indicate that only local release of a high concentration of VEGF can promote a prompt and intense regenerating of endothelial cells after vascular injury. Additionally, when administration of an anti-VEGF antibody to α2-AP-/- mice resulted in normalization of the repair process with the condition of the vascular surface not different from that of wild-type mice, the proliferating endothelial cell number was decreased. This rescue was also detected when α2-AP-/- mice were treated with an anti-VEGF receptor-1 antibody, but not with an anti-VEGF receptor-2 antibody. This result supports the previous observation that the effect of VEGF is mediated mainly via VEGFR-1 (Flt-1). Indeed, our data show that also proliferation of endothelial cells in mice in vivo is regulated mainly by VEGFR-1, but not by VEGFR-2.
To the best of our knowledge, the present report is the first to describe an essential role of α2-AP in vivo in the recovery after vascular injury. The physiologic scenario of such a response might be as follows: first, VEGF is released mainly from vascular SMCs after endothelial injury. VEGF physiologically stimulates the production of plasminogen activators, and then plasminogen is converted into the active plasmin on endothelial cells. Plasmin further stimulates the release of VEGF.10 During this process, α2-AP plays an inhibitory action on the mutual induction of VEGF and plasmin, and α2-AP prevents plasminogen to bind to endothelial cells. Therefore, lack of α2-AP locally induces over-release of VEGF after vascular injury, which extremely changes the rate of endothelial healing mainly via activation of VEGFR-1 (Flt-1). Additionally, α2-AP deficiency enhances activation of plasmin leading to fibrinolysis.21 Both the prompt healing of endothelial cells by over-release of VEGF and the enhancement of the fibrinolytic action are beneficial for vascular repairing. VEGF is also known as a vascular permeability factor on the basis of its ability to induce vascular leakage.9 Therefore, local highly elevated VEGF levels in α2-AP-/- mice may in addition induce vascular permeability and subsequent VEGF oversecretion from SMCs stimulated by plasmin. Indeed, our previous findings showed that oversecretion of VEGF in experimental acute myocardial infarction in α2-AP-/- mice increased the vascular permeability.5 This observation may indicate a link between plasmin generation and SMCs.
In conclusion, lack of α2-AP improves the vascular patency after endothelial injury which is mainly because of the enhancement of endothelial cell healing via an over-release of VEGF as a result of the exaggerated activity of plasmin no longer tempered by α2-AP. Moreover, the increased fibrinolytic potential in addition would reduce thrombotic vessel reocclusion. This dual effect might regulate the neointimal thickening after endothelial injury. We, therefore, concluded that α2-AP has an important local physiologic role in vascular remodeling. The findings in this report have identified a new target for the development of new therapeutics for the clinical therapy of cardiovascular diseases.
Prepublished online as Blood First Edition Paper,July 31, 2003; DOI 10.1182/blood-2003-03-0700.
Supported by a Grant for Scientific Research (no. 15590223) from Ministry of Education, Science, Sports and Culture of Japan and by Hitech research grant at Kinki University, Graduate School of Medicine from the Ministry of Education, Culture, Sports, Science and Technology.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Prof Hans Deckmyn (K. U. Leuven, Campus Kortrijk, Belgium) for his help.


![Figure 8. Effect of VEGF in α2-AP+/+ mice and of α2-AP in α2-AP-/- mice. Scanning electron photomicrographs of the regenerated endothelial surface of a carotid artery 48 hours after injury in α2-AP+/+ mice treated with VEGF at a dose of 8 ng per body (A) and treated with α2-AP at a dose of 75 mg per body (C). Cell proliferation (n = 4) measured as the BrdU index (percentage) of recovered endothelial cell layer (□) and SMCs in media (•) following vascular injury in α2-AP+/+ mice (B). VEGF (2, 4, 8 ng/body) was injected as a bolus before the initiation of endothelial injury. BrdU (50 mg/kg) was injected subcutaneously 1, 8, 16, and 24 hours prior to removal of the carotid arteries. Two days after the injury, the injured artery was removed and stained for the measurement of BrdU-positive cells. The development of neointima in α2-AP-/- mice is shown as the percentage of luminal stenosis by newly formed intima (D). α2-AP was injected as a bolus via tail vein for 1 day, 3 days, 1 week, 2 weeks, or 4 weeks after endothelial injury, and the injured carotid artery was removed 4 weeks after injury (n = 4 each). α2-AP+/+ mice (wild type [WT]) were treated with α2-AP for 4 weeks. * indicates P < .05 versus control (without α2-AP). Scale bar represents 10 μm.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/10/10.1182_blood-2003-03-0700/6/m_h82235228008.jpeg?Expires=1769089075&Signature=uA3vvKcBeyExYYzyUw91SGISooVkeZxghjeK8ShCOmHvyiEtIBrf34lH67RqSWKtoWOa1QwOcTXj-duQEiUroXutC7RtgoVsQCEyae5Z-KpXEFsW-JoAaVfhwyXooCoQJ~A8xZJMJBd~-O9T5rMlng9f9pmmy~vyFl~Ip2BhpCpZsUSmPNY5gaIeaiVYs0I0-S4Yf2orwY8xsgs98fyxHXmRiOqKq0qZMiZ50h4cCl0cMlqqm4BFqAiTuPZWpqRWTc1vO0KKP2d0znoxz-FVpAjayi8-3T44MTpWFv03wX9voSEwxZr5n5YCaD~~HjsZ46B2xo2vk44C~KFOvwJr8Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal