High-dose therapy in multiple myeloma
The outcome of patients with multiple myeloma (MM) is unsatisfactory, with a median survival of less than 3 years.1,2 The prospects for survival at 10 years are poor with conventional chemotherapy.3,4 The limited efficacy of conventional treatment prompted the introduction of high-dose therapy (HDT) followed by stem cell support in an attempt to achieve a greater tumor reduction with longer disease-free and overall survival (OS). Not all patients in whom HDT is feasible obtain a significant benefit from the procedure.
Autologous transplantation
During the last 15 years a large number of reports on autologous transplantation have been published.5-11 These studies have demonstrated that the procedure is feasible, with a transplant-related mortality (TRM) less than 3%. The roles of autologous transplantation for resistant disease, intensification as part of up-front therapy, advanced age, and renal failure are reviewed.
Resistant disease
The first studies of autotransplantation in MM were performed in patients with advanced disease.12-14 Patients with refractory relapsed myeloma generally do not benefit from HDT.15,16
Patients with primary resistant disease can benefit from early myeloablative therapy. The median event-free survival (EFS) and OS of 27 patients with primary resistant disease who received a transplant during the first year following the initiation of therapy were 3.5 and 6 years, respectively.17 In 72 patients with primary unresponsive disease, the median EFS and OS were 21 and 47 months, respectively.16 However, for a meaningful interpretation of the data, it is crucial that the 2 categories of patients generally considered as primary refractory (ie, primary unresponsive with progressive disease versus minimal response or no change without clinical progression) are analyzed separately. It is likely that the results reported in the above 2 studies would not have been as good if only patients with primary unresponsive progressive disease had been included.
Front-line therapy
A randomized trial from the French Intergroup (Intergroupe Francophone du Myélome [IFM]) showed that HDT increased the complete remission/response (CR) rate, EFS, and OS.18 One case-control19 and 2 population-based studies20,21 demonstrated the superiority of HDT over conventional chemotherapy (CC). Three studies have shown that MM patients responding to initial chemotherapy and who were eligible for HDT/intensification, but who did not receive such treatment, had a similar survival to those whose therapy intensified with HDT.17,22,23 Median survival from initiation of treatment was 60 months and was 52 months following the time when stem cell transplantation (SCT) would be performed. This is similar to patients offered HDT. Furthermore, Fermand et al24 showed, in a prospective randomized trial, that HDT was not superior to conventional therapy in patients aged 55 to 65 years. The preliminary results of the PETHEMA (Programa para el Estudio y Tratamiento de las Hemopatias Malignas) trial showed a significant increase in the CR rate (30% versus 11%) with no significant prolongation of EFS (median, 42 versus 34 months) or OS (median, 61 versus 56 months) with HDT intensification in patients who had responded to initial chemotherapy.25 The Medical Research Council (MRC) VII trial demonstrated that progression-free survival (PFS; 32 versus 20 months; P < .01) and OS (55 versus 42 months; P = .04) were superior in the HDT arm.26
There is increasing evidence that CR is necessary for a durable response after HDT.18 Moreover, in 2 single-institution series, patients who achieved CR by stringent criteria27 (ie, negative immunofixation) after receiving early HDT had an EFS and OS significantly longer than those who achieved a partial response. Patients who did not achieve CR with HDT had similar EFS and OS to patients who met the eligibility criteria for autotransplantation but who received standard chemotherapy.22,28 CR should be the goal in patients with myeloma undergoing HDT. The benefit from HDT is in those patients attaining CR after transplantation. A small group of patients from the Alexanian series who achieved CR with CC had the same EFS and OS as patients attaining CR after transplantation.28 The difference between conventional and HDT is that CR is achieved in 10% and 30% of patients, respectively. Identification of factors that can predict CR after HDT is important to select those patients most likely to benefit. The sensitivity to initial treatment accurately predicts the probability of CR.28,29 CD34+ selection results in a significant reduction in myeloma cell contamination of the apheresis product and in rapid and sustained engraftment30-34 without improvement in EFS and OS.
The introduction of tandem transplantation9 prompted the design of several prospective trials in which a single versus double transplant was compared. The French Intergroup found that double autotransplantation improved EFS and OS.35 The median survival of the 2-transplant arm (58 months) was the same as the survival in their single-transplant arm in IFM 90 (57 months). The preliminary analysis of the Italian Bologna 96 study reported a significant prolongation in EFS in favor of the 2-transplant arm. However, no significant differences in CR rate and OS were found.36 In the HOVON (Stichting Haemato-Oncologie voor Volwassen Nederland) group a significant increase in CR rate from 13% to 29% was observed; however, the second intensification did not result in EFS or OS prolongation.37 Finally, another cooperative French trial failed to show any significant difference between single and tandem transplants.33 The failure to achieve a higher CR rate following the second transplantation is the most likely explanation for the lack of benefit.
Advanced age
Siegel et al38 compared the outcome of 49 patients older than 65 years with that of 49 pair mates younger than 65 years. The TRM was higher (8% versus 2%) and both the EFS (1.5 versus 2.8 years) and OS (3.3 versus 4.8 years) were shorter in older patients. The CR rate was significantly lower (20% versus 43%) in the elderly population. Badros et al39 reported the outcome of 70 patients aged 70 years or older. A TRM of 16% was noted with MEL-200 (melphalan 200 mg/m2), leading to a dose reduction to MEL-140 (melphalan 140 mg/m2). The CR rate was 20% with a median response duration of 8 months after a single transplantation. The EFS and OS at 3 years were only 20% and 31%, respectively. Autologous transplantation cannot be enthusiastically recommended for elderly patients with myeloma.
Renal failure
About 20% of patients with MM have renal insufficiency at diagnosis.40,41 Should patients with persistent renal failure be excluded from HDT programs? The Spanish Registry reported data on 14 patients with renal failure; the TRM was 29%.42 Badros et al43 reported the results of HDT with MEL-200 or MEL-140 in 81 patients with renal failure. The TRM was 6% and 13% after single and double transplantation, respectively. Nonhematologic toxicity, particularly in dialysis-dependent patients receiving MEL-200, was high.44 Patients given MEL-140 had a similar outcome in terms of CR, EFS, and OS, with significantly lower toxicity. In patients with renal failure, HDT should be performed in younger patients (< 50 years) with chemosensitive disease and good general condition.
Long-term outcome
When considering the results of HDT, one important aspect is whether or not a significant proportion of patients achieve prolonged EFS. Tricot et al45 reported that one fourth of 515 consecutive patients entering a tandem transplant program before 1997 had an EFS of 5 years or more. However, only 31 (6%) were in continuous CR 7 years following HDT. Moreau et al46 reported a 10-year PFS of 3% in 127 “de novo” MM patients treated with at least one course of HDT.47
Allogeneic transplantation
Allogeneic transplantation with “conventional intensity” conditioning
The main advantages of the allogeneic approach are the absence of tumor cells in the graft and the existence of a graft-versus-myeloma (GVM) effect, resulting in a proportion of long-term survivors in molecular remission.44,48,49 The results in 25 patients who received a syngeneic graft demonstrate a low TRM with high CR and a median EFS and OS of about 6 years.50
The TRM, mainly due to graft-versus-host disease (GVHD) and infectious complications, ranges from 30% to 50%.51-53 Using less intensive conditioning regimens and including patients with chemosensitive disease, TRM remains 30%.54,55 The European Blood and Marrow Transplantation (EBMT) study reported a decrease in TRM from 46% in patients undergoing transplantation from 1983 to 1993 to 33% in patients receiving allografts from 1994 to 1998.56 The relapse-free survival at 3 years in patients who achieved CR is less than 50%.51-53,56
Only 10% to 20% of patients receiving allografts were in continued CR 5 or more years after transplantation.51-53 The potential for cure in a small proportion of patients should be weighed against the shortening of survival due to the high TRM. The efficacy of allogeneic versus autologous transplantation was investigated by the EBMT group in a retrospective case-matched study.57 The median survival was significantly longer in the group receiving autografts. The HOVON group, in a case-control study, has also reported shorter survival with allogeneic transplantation than with autologous transplantation.58
T-cell depletion has not improved the results of allogeneic transplantation.51,58 A TRM of 30% and a CR rate of 81% with allogeneic transplantation of peripheral blood progenitor cells was reported in 30 patients.59 The EBMT group reported a 33% TRM at 1 year in 224 patients receiving allografts from 1994 to 2001 with peripheral blood progenitor cells and found no significant differences in outcome when compared with 297 patients receiving allografts of bone marrow progenitor cells during the same period.60
Donor lymphocyte infusions
The well-documented GVM effect of donor lymphocyte infusions (DLIs) has led to the use of DLIs in the treatment of either persistent disease or relapse after allogeneic transplantation.61 Although there are clear responses to DLI, its overall benefit is uncertain.62 The largest reported experience with DLI is 27 patients.63 Five of 14 responders had a response duration exceeding 30 months, with 2 in sustained molecular remission. Many of the patients responding to DLI had severe GVHD. The overall benefit of DLI is tempered by severe GVHD.62
Dose-reduced intensity conditioning
The efficacy of allogeneic transplantation with dose-reduced intensity conditioning regimens has been investigated. The reported studies include heavily pretreated patients, and the median follow-up is 1 year or less.64-66 These studies showed the feasibility of the procedure with stable engraftment and a CR rate from 22% to 44%. The TRM was below 20%. The Seattle group has reported data on patients given intensive therapy with MEL-200 with autologous rescue followed 40 to 120 days later by conditioning with 200 cGy total body irradiation (TBI) and a compatible sibling allograft.67 Among 32 patients the CR rate was 53%. However, 45% and 55% of the patients developed acute or chronic GVHD, respectively, with a TRM of 16%.67 The use of a nonmyeloablative allograft after autologous transplantation in patients with less advanced disease is under way.65
Patient selection and goals
Autologous transplantation
Consolidation of initial therapy
HDT with AHSCT improved response rates (RRs), EFS, and OS compared to CC (IFM 90).18 CR rates (5% versus 22%), EFS (18 months versus 28 months; P = .01), and OS (44 months versus 57 months; P = .03) were all statistically superior in the HDT group. Survival was related to the β2-microglobulin level. Fermand et al showed no survival advantage for HDT in patients aged 55 to 65 years.24 The MRC analysis showed 12 months longer OS in the HDT group.26 The preliminary results of the Spanish PETHEMA study25 are given by Bladé.
The Arkansas group performed a pair-mate study comparing tandem HDT to Southwest Oncology Group (SWOG) patients treated with VAD (vincristine, doxorubicin, dexamethasone) chemotherapy19 ; the Italian group compared 2 to 3 cycles of melphalan 100 mg/m2 supported with AHSCT to conventional melphalan and prednisone21 ; the Swedish group reported a population-based study of HDT in newly diagnosed patients younger than 60 years old to CC historic controls.20 All favored HDT.
The goal of therapy: CR
Because CR is associated with improved outcome, attempts to increase CR with tandem autologous transplantation,9-11,19,35-37 posttransplantation immunotherapy,69 and autologous/nonablative allogeneic tandem transplantation67,70 have been pursued. CR with a single course of HDT is reported in the 25% to 35% range.9-11,18,19 Reece et al71 reported a pilot trial of 40 patients treated with higher doses of melphalan, 280 mg/m2, with amifostine as a cytoprotectant; they observed a 60% CR and 22% partial response rate at 100 days. There were no transplant-related deaths.
Tandem transplants
The “Total Therapy” protocol consisted of a series of non-cross-resistant chemotherapy regimens followed by tandem HDT.9 CR was achieved in 26% and 41%; TRM was 1% and 7%, respectively, following the first and second transplantations. The median EFS and OS were 43 and 68 months, respectively. In multivariate analysis, patients with no chromosome 13 abnormalities, low β2-microglobulin levels, low C-reactive protein levels, achievement of CR, and 2 transplantations were all favorable prognostic factors. A small subset of patients (n = 46) with good prognostic variables remained progression-free over 7 years. A coined term was an “operational cure.”72
The IFM 94 and Myeloma Autogreffe (MAG)33 from France, the Italian Bologna 96, and the Dutch-Belgian HOVON studies all attempt to address the value of tandem transplants. The most mature study is the IFM 94 trial. The 7-year probabilities of EFS (20% versus 10%) and OS (42% versus 21%) were superior in the tandem HDT group.35 In the Bologna 96 trial, only the subgroup that actually received a tandem transplant showed a prolongation in response duration but no difference in CR and OS. The HOVON trial compared 2 cycles of intermediate-dose melphalan (70 mg/m2) without AHSCT versus the same therapy followed by cyclophosphamide/TBI with AHSCT. Although the CR rate increased from 13% to 29% in the double-dose intensive arm, there was no difference in EFS or OS. The quality-of-life assessment favored the nontransplant group.73 The French MAG study showed no differences in EFS or OS between single and tandem HDT.33
Patients with very good biologic risk factors (no cytogenetic abnormalities, normal β2-microglobulin level, normal albumin level, normal platelet count) would be predicted to have improved outcomes without tandem transplants, whereas the poorest risk patients clearly do poorly despite tandem HDT (eg, chromosome 13 abnormalities).11,28 There is a significant selection bias inherent in these clinical trials—both physician and patient generated.74 Thus, interpretation of these studies may not be applicable to the MM patient seen by the community oncologist.
Primary refractory disease
The outcome of patients with primary refractory disease (PRD) treated with HDT compared to CC has not been evaluated in randomized trials. Patients with PRD undergoing transplantation within the first year of diagnosis had a superior outcome compared to those patients who underwent HDT beyond 1 year.75 In the latter group, there was no advantage in survival compared to historical controls treated with conventional therapy. Vesole et al reported the Arkansas experience in patients with PRD; the EFS and OS were 23 and 39 months, respectively.10,15 Singhal et al reported the Royal Marsden experience showing that patients with PRD had similar outcomes to chemosensitive patients.76 Therefore, in the absence of disease progression, PRD patients should be treated aggressively with HDT and AHSCT.
Older patients
Palumbo et al21 did a pair-mate analysis of conventional melphalan plus prednisone versus 2 to 3 cycles of melphalan 100 mg/m2 supported by AHSCT (3 cycles administered if CR not achieved following second cycle) in patients older than 60 years (n = 71). CR, EFS, and OS were all superior in the HDT group. Siegel et al38 (as summarized by Bladé) showed no significant differences in CR, EFS, and OS in older patients. Using melphalan 200 mg/m2, the Arkansas study showed a 16% TRM in the first 25 patients.39 The melphalan dose was lowered to 140 mg/m2 with a 2% TRM. The CR rates were 20% and 27% after single and tandem HDT, respectively. The median CR duration, EFS, and OS were 18 months, 15 months, and 24 months, respectively. Finally, Reece et al reported the Autologous Blood and Marrow Transplant Registry (ABMTR) experience in HDT with AHSCT in 110 patients older than 60 years and 382 patients younger than 60 years. There were no differences in CR, TRM (at day 100 or at 1 year), EFS, or OS between the 2 groups.77
Renal insufficiency
The outcome of patients with persistent renal insufficiency is extremely poor, whereas those whose renal function improves have outcomes comparable to patients with normal renal function.41 There is limited literature describing the use of HDT with AHSCT in MM patients with renal insufficiency.43,78 The Arkansas group78 reported the largest trial of 81 patients with nonreversible renal insufficiency, including 38 on chronic hemodialysis, who underwent HDT. The first 60 patients received melphalan 200 mg/m2; the TRM was 7%. Subsequently, the next 21 patients received melphalan 140 mg/m2; the TRM decreased to 5%. Thirty-one patients (38%) completed tandem transplants; TRM following the second transplant was 13%. The CR rates were 26% and 38% after the first and second transplantation, respectively; EFS and OS were 23 months and 53+ months, respectively. There was no improvement in EFS and OS in those patients who completed tandem transplantation. Patients on dialysis had similar outcomes compared to patients with less impaired renal function.
Allogeneic transplantation
Even with tandem HDT, a plateau in survival curves has not been appreciated.9-11 The most likely explanation for disease relapse is the persistence of resistant residual minimal disease. In an attempt to reduce tumor cell contamination, various CD34+ selection methodologies have been reported.29-31,79 None of these trials have demonstrated an improvement in PFS or OS. Allotransplantation provides 2 distinct advantages: absence of contaminating tumor cells in the autograft and the benefit of alloreactive donor T lymphocytes producing a GVM effect.61,80 Fewer than 10% of patients with MM are candidates for allotransplantation.
Allotransplantation may result in higher CR compared with autotransplantation (25%-60%); true molecular remissions have been observed.48,49,81 Allotransplantation, however, is associated with excessively high TRM, ranging from 20% to 57%.56,82,83 Late relapses continue to occur.56 Following disease relapse, as many as 50% of patients respond to DLIs, which provide a GVM effect.62,63,84
Nonablative “mini-transplants”
The TRM of conventional myeloablative allotransplants has constrained this option. Pilot trials of nonablative allogeneic stem cell transplantation (NST), which relies almost exclusively on the GVM effect, have been preliminarily reported.
The Arkansas group66,85 reported data on a total of 31 patients undergoing NST; 14 had single HDT, 14 had 2 prior HDTs, and 3 had 3 prior HDTs. At the time of NST, 17 had progressive disease and 14 had chemosensitive disease. Overall response rate was 61%: 12 CR, 7 near CR, 3 PR; 58% developed grades II to IV GVHD; 16 had chronic GVHD (10 limited, 6 extensive); TRM was 29% (3 early and 6 late TRM). At a median follow-up of 6 months, the EFS and OS were 15 months, with 71% alive at 1 year and 31% alive at 2 years.
Giralt et al86 reported the M.D. Anderson experience in NST in 22 MM patients from either an HLA-matched sibling (n = 13) or matched unrelated donor (n = 9). Pretransplantation disease status included PRD (n = 2), refractory relapse (n = 11), chemosensitive relapse (n = 8), and initial remission consolidation (n = 1). Seven patients achieved a CR; 6 were alive at a median follow-up of 15 months. The actuarial PFS and OS at 2 years are 19% and 30%, respectively. TRM at 100 days was 19% and 40% at 1 year.
The long-term outcome in heavily pretreated and chemotherapy-resistant patients was poor. These patients should not be considered for NST.
Autologous/nonablative allogeneic tandem transplants
HDT with AHSCT followed by NST uses the upfront autograft for maximal tumor cytoreduction and the alloreactive donor T cells to eradicate residual disease through the GVM effect. The Seattle group67 results have been reviewed by Bladé. With a median follow-up of 13 months, 85% of patients were alive and 80% were progression free.
Kröger et al70 reported the German-Israeli experience in 17 patients undergoing unrelated NST; 9 of these were planned autologous/NST sequential transplants. The day-100 TRM was 11%. The CR rate increased from 18% after AHSCT to 73% after NST. With a median follow-up of 13 months after NST, 76% of patients were alive and 71% progression free. Chronic GVHD developed in 40% of the patients, but was extensive in only 1 patient.
Pertinent questions
What are the facts?
To date, there have been only 2 published prospective randomized studies comparing high-dose with conventional-dose therapy for MM.18,26 The first study used bone marrow rather than peripheral blood stem cells. As pointed out by both Bladé and Vesole on an intention-to-treat basis, there was a statistically significant improvement in OS for patients who received HDT. The benefit over standard therapy was 15 months. Of the 100 patients randomized to HDT, only 74 actually proceeded to transplantation. One anticipates that the morbidity associated with peripheral blood stem cells would be less, given the faster engraftment when peripheral blood stem cells are used.
This protocol was activated before the routine measurement of CD34+ cells by flow cytometry. HDT patients received conditioning with TBI, which is used infrequently today. Radiation does not lengthen relapse-free survival or OS and adds significantly to the mucositis and need for supportive parenteral nutrition and narcotics.
The results, however, are not applicable to all patients with MM. Patients older than age 65 years were not eligible. The serum creatinine concentration had to be less than 150.28 μM (1.7 mg/dL).18 This study has also been criticized because the median survival of the conventionally treated patients was 44 months (Table 1), with a 5-year survival rate of 12%, which has been considered a poor outcome for patients younger than age 65 years with adequate renal and cardiac function.
CC versus HDT
Study . | No. of patients . | EFS, mo CC/HDT . | OS, mo CC/HDT . | P . |
|---|---|---|---|---|
| IFM 9018 | 200 | 18/28 | 44/57 | .03 |
| MRC VII26 | 407 | 20/32 | 42/54 | .04 |
| Little Rock SWOG case control19 | 229 | 22/49 | 49/62+ | .01 |
| Nordic20 | 548 | 27/32 | 44/NR | .01 |
| Torino21 | 142 | 18/34 | 48/56+ | .01 |
| PETHEMA25 | 164 | 34/43 | 56/62 | NS |
| MAG 9124 | 190 | 19/24 | 50/55 | NS |
Study . | No. of patients . | EFS, mo CC/HDT . | OS, mo CC/HDT . | P . |
|---|---|---|---|---|
| IFM 9018 | 200 | 18/28 | 44/57 | .03 |
| MRC VII26 | 407 | 20/32 | 42/54 | .04 |
| Little Rock SWOG case control19 | 229 | 22/49 | 49/62+ | .01 |
| Nordic20 | 548 | 27/32 | 44/NR | .01 |
| Torino21 | 142 | 18/34 | 48/56+ | .01 |
| PETHEMA25 | 164 | 34/43 | 56/62 | NS |
| MAG 9124 | 190 | 19/24 | 50/55 | NS |
NR indicates not reached; NS, not significant.
What does the survival curve mean?
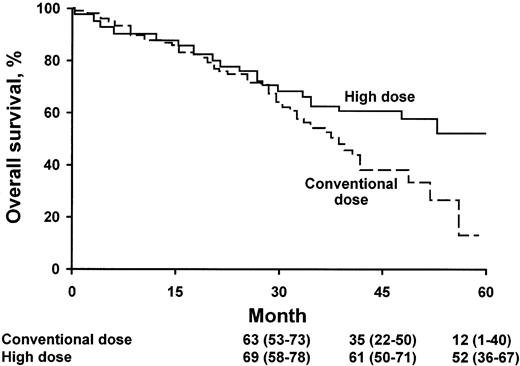
The analysis of the survival curve18 is quite different from the usual Kaplan-Meier curve seen in other treatment studies (Figure 1). When there is divergence of 2 survival curves, the trend is usually seen immediately after therapy is initiated. In the high-dose versus conventional-dose study, the 2 curves show no evidence of divergence for the first 2 years of follow-up. What does this mean? There are many possible explanations, but one emphasized by Vesole is that there are certain subsets of myeloma patients for whom transplantation provides little or no benefit. Which subsets are these? It appears that patients “destined” to die of the disease before 2 years show little benefit. Who are these patients? Several prognostic systems can predict those patients who have an anticipated survival less than 2 years; typically this includes patients with an increased concentration of β2-microglobulin in the serum and an increased bone marrow labeling index. Patients with multiple cytogenetic abnormalities, particularly those with deletions of the long arm of chromosome 13 or complete monosomy 13, may not benefit from HDT.11
OS according to treatment group. The numbers shown below the time points are probabilities of OS (the percentages of patients surviving) and 95% confidence intervals. (From Attal M et al18 by permission of the Massachusetts Medical Society.)
OS according to treatment group. The numbers shown below the time points are probabilities of OS (the percentages of patients surviving) and 95% confidence intervals. (From Attal M et al18 by permission of the Massachusetts Medical Society.)
The patients who appear to benefit the most are those with the most indolent disease. Theoretically, the time to progression with slow-growing myeloma is lengthened. For patients with a creatinine value less than 150.28 μM (1.7 mg/dL) and an age younger than 65 years, autologous transplantation results in definite improvement in OS even though the median survival prolongation was only 15 months. Bladé and Vesole demonstrate consensus on this issue.
What other evidence is there?
The experience of investigators at the University of Arkansas using Total Therapy I was compared with the outcome of patients receiving standard therapy in the SWOG trials. These patients were matched for the major prognostic features. In the standard therapy group, the median OS was 48 months. The OS in the standard therapy arm in the French randomized study was only 37 months.19 Moreover, the control groups were not contemporaneous. In the Total Therapy study, registration ended in September 1994. The standard therapy controls were recruited in 2 studies; one recruited patients between October 1982 and March 1987,87 and the second recruited patients between February 1985 and October 1990.88 Clearly, the fact that these controls are historical makes it a bit more difficult to draw conclusions, given the improvements in supportive care seen over a 10-year period. If the 48-month median survival observed in the SWOG study had been seen in the French standard therapy group, no statistical difference in survival would have been recorded. The issues surrounding the use of historical controls for patients treated with HDT are familiar to all those involved in stem cell transplantation for metastatic breast cancer. Historical control groups have led to misleading conclusions, which have been subsequently overturned by randomized trials.89
The Nordic Myeloma Study Group published outcomes comparing recipients of HDT and standard therapy. Patients had to be younger than age 60 years for inclusion. They used historical controls but published their experience in conventionally treated patients, demonstrating that from 1970 to 1983, 1984 to 1989, and 1990 to 1992 there was no improvement in survival. This historical control group would likely make a more valid comparison.90 Median survival for patients receiving standard therapy was 44 months, relatively close to the outcomes reported by the SWOG study. Unlike the other 2 transplant studies, this protocol did not require transplantation in patients not achieving at least a partial response.
Failure to respond to initial induction therapy such as vincristine, doxorubicin, and dexamethasone or vincristine, doxorubicin, and methylprednisolone does not preclude an excellent outcome.76 In this study, only 9% of the patients had a creatinine value more than 203.32 μM (2.3 mg/dL), so the outcome in patients who had significant renal insufficiency cannot be determined. Once again, inspection of the survival curve shows no divergence for the first year, reflecting minimal impact of transplantation in those patients with aggressive prognostic features. HDT is an appropriate part of the initial treatment for patients up to age 60 years.20 No published studies indicate that conventional therapy provides a survival similar to HDT. All 3 authors agree on this point.
The quality-of-life indicators reflect the fact that during the first 6 months after diagnosis, patients receiving HDT spend more time in the hospital than conventionally treated patients. A better quality of life was not seen after HDT. There was a trend to higher functioning scores and less pain and fatigue at 36 months, but this trend did not reach statistical significance. There was also a modest reduction of the functional aspects of health-related quality of life during the first 6 months. No quality-of-life benefit was apparent during the first 2 years, and after 2.5 years, there was a trend only.31
The MRC trial has just been published, comparing standard therapy consisting of ABCM (doxorubicin, BCNU, cyclophosphamide, melphalan) every 6 weeks or C-VAMP (cyclophosphamide, vincristine, doxorubicin, methylprednisolone) every 3 weeks followed by mobilization with cyclophosphamide 2 to 4 g/m2 followed by stem cell transplantation using melphalan 200 mg/m2 in patients younger than 65 years. The CR rate was 44% in the high-dose arm compared to 8% in the standard-dose arm, and median survivals for standard- and high-dose treatment were 42.3 and 54.1 months, respectively (P = .04). The treatment effect varied depending on the level of β2-microglobulin. The survival benefit was greatest among those whose β2-microglobulin concentration was more than 8 mg/L (41.9 versus 13.1 months).
What about patients who are older than age 60 to 65 years or who have renal impairment?
The University of Arkansas group reported the impact of age on outcome in patients receiving “Total Therapy” with tandem transplantation.38,39 Both Bladé and Vesole have summarized the data. In a Cox model, age could not be demonstrated to have an impact on outcome. In a multivariate model, many critical prognostic factors can overpower the statistical impact of age. Given the number of patients reported older than age 65 years,49 one cannot determine with certainty what the degree of survival prolongation is for patients older than age 65 years compared to those who receive standard therapy. TRM in the older patients was 8% (Table 2).
Transplantation in older patients
Study . | No. of patients . | Age, y . | EFS, mo . | OS, mo . |
|---|---|---|---|---|
| MAG24 | 190 | 55-65 | NS | NS |
| Little Rock38 | 49 | >65 | 18 | 40 |
| Little Rock39 | 70 | >70 | 15 | 24 |
| Torino21 | 53 | >60 | 34 | 56+ |
| ABMTR77 | 110 | >60 | NS | NS |
Study . | No. of patients . | Age, y . | EFS, mo . | OS, mo . |
|---|---|---|---|---|
| MAG24 | 190 | 55-65 | NS | NS |
| Little Rock38 | 49 | >65 | 18 | 40 |
| Little Rock39 | 70 | >70 | 15 | 24 |
| Torino21 | 53 | >60 | 34 | 56+ |
| ABMTR77 | 110 | >60 | NS | NS |
NS indicates not significant.
The Arkansas group subsequently updated their results. Older patients received melphalan 140 mg/m2. Although it is unclear what the relationship between dose and outcome is, one could infer that a lower dose of chemotherapy might produce less cytoreduction and fewer CRs. At this lower dose, the mortality rate was reduced to 2%. The OS for all 70 patients receiving an autotransplant was a median of 24 months. The question, “How much longer do patients older than age 65 years live after receiving a transplant compared to conventional therapy?” cannot be answered. Concurrence among all 3 authors exists on this point.
What are the outcomes when transplantation is performed with renal failure (Table 3) present? The largest published experience comes from the University of Arkansas. Renal failure was defined as a creatinine value more than 176.8 μM (2 mg/dL). Because of excessive toxicity, the dose was decreased to 140 mg/m2. TRM for all 81 patients was 6%. The 3-year survival for the renal failure group was 55%. Patients with renal failure can receive transplants, but the extent of survival prolongation with reduced-dose melphalan (140 mg/m2) makes it impossible to answer the question, “Doctor, how much longer would I live if I had a stem cell transplant, given my kidney function?”43
Transplantation with renal failure
Study . | No. of patients . | TRM, % . | EFS . | OS . |
|---|---|---|---|---|
| Little Rock78 | 81 | 6 (single); 13 (tandem) | 23 mo | 53 + mo |
| Salamanca42 | 14 | 29 | — | 56% at 3 y |
Study . | No. of patients . | TRM, % . | EFS . | OS . |
|---|---|---|---|---|
| Little Rock78 | 81 | 6 (single); 13 (tandem) | 23 mo | 53 + mo |
| Salamanca42 | 14 | 29 | — | 56% at 3 y |
—indicates not reported.
There is variability in outcome from trial to trial. In the French IFM 90 trial, the median EFS for the patients receiving melphalan and TBI was 28 months. Patients conditioned with melphalan and TBI in the IFM 94 trial had a median EFS of only 21 months. In both studies, the serum creatinine concentration had to be less than or equal to 150.28 μM (1.7 mg/dL) to proceed with stem cell collection; patients with persistent renal insufficiency were excluded from randomization. Whether the difference between a 28- and 21-month EFS for the same conditioning regimen was due to the use of stem cells rather than bone marrow or patient population variation cannot be determined. This patient heterogeneity has been emphasized by Vesole. A 21-month EFS is disturbing, given the large size of the population studied.91
When?
Only one randomized trial was designed to assess the optimal timing of HDT. This study limited enrollment to patients up to age 56 years, with half being randomized to early transplantation and half to late transplantation. The median OS was 5 years in both groups, with no survival advantage for early transplantation. Quality-of-life measures favored patients in the early treatment group.92
In a nonrandomized setting, we have also had extensive experience with early stem cell collection, cryopreservation, and transplantation at progression.93,94 We do not believe that the time of transplantation is critical to OS in patients with MM. Over the past 36 months, we have tended to recommend early transplantation because occasional patients have aggressive relapse after conventional therapy, and there is insufficient time to begin the treatment process and insurance approval process for stem cell transplantation. In addition, insurance issues in the United States can change over a 3- to 4-year observation period, so that patients previously approved to proceed with stem cell transplantation can subsequently be denied the procedure, even though stem cells have already been procured and cryopreserved. There is a subset of patients who wish to have stem cells collected and cryopreserved and not proceed immediately with transplantation, and in this patient population, our policy is to honor the preference.
How many?
With total therapy, the median OS was 68 months, with a 5-year OS of 58%.9 Are these results better than for single transplantation followed by subsequent salvage chemotherapies? Table 4 presents some data.
Single versus double HSCT
. | No. of patients . | Follow-up, mo . | EFS (y follow-up) . | . | . | OS (y follow-up) . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study . | . | . | Single . | Double . | P . | Single . | Double . | P . | ||||
| IFM 9435 | 399 | 70 | 10% (7) | 20% (7) | .03 | 20% (7) | 42% (7) | .01 | ||||
| HOVON MM37 | 255 | 40 | 15% (4) | 29% (4) | .03 | 50% (4) | 55% (4) | .31 | ||||
| Bologna 9636 | 220 | 38 | 25 mo | 34 mo | .05 | 56 mo | 60 mo | NS | ||||
| MAG 9533 | 193 | 40 | 31 mo | 33 mo | .55 | 49 mo | 73 mo | .14 | ||||
. | No. of patients . | Follow-up, mo . | EFS (y follow-up) . | . | . | OS (y follow-up) . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study . | . | . | Single . | Double . | P . | Single . | Double . | P . | ||||
| IFM 9435 | 399 | 70 | 10% (7) | 20% (7) | .03 | 20% (7) | 42% (7) | .01 | ||||
| HOVON MM37 | 255 | 40 | 15% (4) | 29% (4) | .03 | 50% (4) | 55% (4) | .31 | ||||
| Bologna 9636 | 220 | 38 | 25 mo | 34 mo | .05 | 56 mo | 60 mo | NS | ||||
| MAG 9533 | 193 | 40 | 31 mo | 33 mo | .55 | 49 mo | 73 mo | .14 | ||||
NS indicates not significant.
Fermand et al33 reported data on 230 patients randomized between single and tandem HDT. This study did not show any survival benefit of tandem HDT. The Dutch Belgium group randomized 255 patients to intensive versus double-intensive therapy. The double-intensive treatment did not result in a better OS when applied as first-line treatment.
Although IFM 94 showed superior survival for tandem HDT, the median OS for tandem transplantation was 58 months but was 57 months for the single transplant arm of IFM 90 and was 54 months in the single transplant MRC7 arm (J. L. Harousseau, personal communication, January 24, 2003). The use of stem cells as opposed to bone marrow, the lack of interferon in IFM 94, and supportive care differences do not adequately explain why the single-transplant arm in IFM 94 achieved a median survival of only 50 months.
An interim analysis of the first 178 patients accrued to the Bologna 96 trial of single versus double transplant showed no significant difference in OS, with a median follow-up of 2.5 years.
What is the take-home message?
Each year 14 400 patients are diagnosed with MM in the US. The year 2000 Bone Marrow Transplant Registry data showed 3200 transplantations performed for MM. Registry data show that in patients who receive transplants within 18 months of diagnosis, the median OS is 46 months. Despite the fact that transplantation is resource intensive and significantly reduces quality of life for the first 24 months after the procedure, it provides a clear and unequivocal, albeit minor, survival benefit of 12 to 15 months for patients younger than age 60 to 65 years with reasonable renal function; it remains uncertain what benefit is derived in older patients, those with impaired renal function, and patients with biologically aggressive disease.
What is the future for transplantation in MM?
Given the biology of MM, it is unlikely that the philosophy of ever-increasing intensity of therapy in an effort to produce CRs (which are never curative) is a correct strategy. Rather than treating patients who have MM along the paradigm of acute leukemia, it is more likely that long-term control will be achieved following the theme of our infectious disease colleagues, who have converted active HIV infection from a disease with a median survival of 1 year to one for which 10-year survivors are common, using combinations of suppressive agents given sequentially.
Reports at the American Society of Hematology meeting in 2002 on the efficacy of proteasome inhibitors and the new immunomodulatory agent CC-5013 are encouraging. Dendritic cell immunotherapy is being developed using myeloma antigens as the sensitizing agent. DNA vaccines against myeloma are being developed. The potential for an in vitro antitumor effect of bisphosphonates and natural killer cells has been recognized, and the use of cytokines to induce a T-cell response against MM continues. With the use of strategies directed at cell signaling, patients' lives can be prolonged, and the quality of their lives can be improved compared with the current approach that transplantation provides, despite its clear survival benefit.
From the Department of Hematology, Institute of Hematology and Oncology, Postgraduate School of Hematology “Farreras Valentí,” Institut d'Investigacions Biomèdiques “August Pi i Sunyer,” Hospital Clínic, University of Barcelona, Barcelona, Spain; Blood and Marrow Transplant Program, Division of Neoplastic Diseases and Related Disorders, Medical College of Wisconsin, Milwaukee, WI; and Division of Hematology and Internal Medicine, Mayo Clinic, Rochester, MN.
Prepublished online as Blood First Edition Paper, July 31, 2003; DOI 10.1182/blood-2003-01-0073.
Supported by a grant from the Fondo de Investigationes Sanitarias de la Seguridad Social (FIS 00/642) (J.B.).
Reprints: Morie Gertz, Mayo Clinic, Division of Hematology and Internal Medicine, 200 First St SW, Rochester, MN 55905; e-mail: gertm@mayo.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal