Abstract
Suppression of red blood cell production is a common complication of chemotherapy, causing anemia in a significant number of cancer patients. We have evaluated the sensitivity of human hematopoietic progenitors and erythroid precursor cells to chemotherapeutic drugs and found that probasophilic erythroblasts represent the stage of erythroid differentiation more vulnerable to the cytotoxic effects of myelosuppressive agents. Stem cell factor (SCF) supports proliferation and survival of early hematopoietic cells by binding to the c-kit receptor. In unilineage erythropoietic culture of CD34 + progenitors, short-term pretreatment of immature erythroid precursors with SCF results in protection from apoptosis induced by chemotherapeutic agents and restores normal proliferation and differentiation after removal of the cytotoxic stimulus. The levels of drug-induced caspase processing are significantly reduced in erythroblasts treated with SCF, indicating that activation of the c-kit receptor generates antiapoptotic signals acting before amplification of the caspase cascade. Accordingly, we found that SCF up-regulates Bcl-2 and Bcl-X L in erythroid precursors and that exogenous expression of these proteins protects erythroblasts from caspase activation and death induced by chemotherapeutic agents. These results suggest a possible mechanism for SCF-mediated protection of erythroid precursor cells from apoptosis and may contribute to devise new strategies for prevention and treatment of chemotherapy-induced anemia. (Blood. 2003; 102:87-93)
Introduction
Anemia is a common complication of cancer, often resulting in a significant decrease of quality of life and influencing the outcomes of patient care. The myelosuppressive effects of chemotherapy are a major cause of anemia in cancer patients.1 While inducing apoptosis in malignant cells, anticancer drugs interfere with the normal production of red blood cells by mechanisms that are not completely understood. Specifically, it is unknown if chemotherapy-induced anemia is the result of a generalized suppression of hematopoiesis occurring at the level of bone marrow progenitor/stem cell compartment or if differentiated erythroid precursors are directly affected by the toxic effects of antineoplastic drugs.
Erythroid progenitor cells originate from the stem cell pool in the bone marrow and enter a differentiation/maturation process that is primed by early-acting cytokines such as stem cell factor (SCF; also known as kit ligand or steel factor) and interleukin-3 (IL-3) and lately orchestrated by erythropoietin (Epo).2,3 Expansion of the erythroid compartment is controlled by positive and negative signals operating on immature erythroblasts that are Epo-dependent and highly susceptible to apoptosis.4-6 We proposed that, inside the erythroblastic islands of the bone marrow, the interaction between death receptors expressed on the surface of immature erythroblasts with their ligands produced by mature erythroblasts induces a controlled activation of caspases responsible for the degradation of the major erythroid transcription factor GATA-1.6 Depending on the levels of circulating Epo, caspase activation in immature erythroblasts results in either apoptosis or temporary inhibition of the maturation process.5,6
Due to their high proliferative activity, it is likely that immature hematopoietic cells are particularly vulnerable to the toxic effects of chemotherapeutic agents. Most antineoplastic drugs are nonselective in their mechanism of action, interfering with DNA replication or transcription or directly targeting the proliferative process. Cytotoxic drug damage leads to the activation of cell death pathways that converge in 2 central events: activation of the caspase cascade and release from mitochondria of apoptogenic factors such as cytochrome c, Apaf-1, AIF (apoptosis-inducing factor), and Smac/DIABLO (second mitochondria-derived activator of caspases/direct IAP binding protein with low pI) release.7 Release of cytochrome c into the cytosol results in the formation of a holoenzyme complex called apoptosome, which triggers activation of downstream caspases and completion of the death program. A network of cellular proteins influences the ultimate outcome of drug-induced damage. The relative levels and competing dimerization between Bcl-2 family members regulate cytochrome c release through the outer mitochondrial membrane, thus determining cell susceptibility to apoptotic signals.7
The proliferation and survival of early hematopoietic cells of the bone marrow is supported by cytokines produced mainly by marrow stromal cells. SCF is a cytokine that promotes growth and survival of hematopoietic progenitors and lineage-restricted precursors.8 Absence of SCF protein (the Steel mutation) or c-kit receptor (the White spotting mutation) in mice results in prenatal or perinatal death due to severe macrocytic anemia, indicating an essential role for SCF and c-kit in erythropoiesis.9,10 SCF has been reported to promote the survival of erythroid colony-forming cells through the phosphatidylinositol-3 kinase (PI-3K)/AKT pathway and to synergize with Epo in apoptosis inhibition of primary erythroblasts.11,12
To identify the preferential target of chemotherapy-induced erythroid suppression, we investigated the effects of antineoplastic drugs on highly purified populations of hematopoietic progenitors and erythroid precursors. We show that immature erythroblasts are an extremely sensitive target of cytotoxic drugs and that SCF protects these cells from chemotherapy-induced apoptosis. We also demonstrate that SCF inhibits drug-induced caspase activation in erythroid precursors and up-regulates Bcl-2 and Bcl-XL proteins, which protect erythroblasts from chemotherapeutic agents. The results presented in this study identify SCF as a protective factor for erythroid cells during chemotherapy and may promote a future use of this cytokine in the supportive care of anemic cancer patients.
Materials and methods
Cytokines, antibodies, and chemicals
Human recombinant SCF, IL-3, granulocyte-macrophage colony-stimulating factor (GM-CSF), and Flt3 ligand were purchased from Peprotech (Rocky Hill, NJ). Recombinant human Epo was supplied by Amgen (Thousand Oaks, CA). Polyclonal antibody against caspase 3 (65906E) was from BD Pharmingen (San Diego, CA), anti—caspase 8 (clone 5F7) was from Upstate Biotechnology (Lake Placid, NY), and anti—caspase 7/Mch3 was from BD Transduction Laboratories (San Diego, CA). Anti—Bcl-2 antibody was from BD Pharmingen, and anti—Bcl-XL (H5) was from Santa Cruz Biotechnology (Santa Cruz, CA). zVAD-fmk was purchased from Bachem (Bubendorf, Switzerland). Anti-tubulin antibody and chemotherapeutic drugs were purchased from Sigma-Aldrich (St Louis, MO). Anti—glycophorin A fluorescein isothiocyanate (559943) was from BD Pharmingen.
Adult peripheral blood human progenitor cell (HPC) purification and culture
Adult peripheral blood was obtained from male donors after their informed consent and the approval by the Committee for Human Studies.13 Human CD34+ precursor cells were purified from peripheral blood by positive selection using the midi-MACS (magnetic cell separation) immunomagnetic separation system (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer's instructions. CD34+ precursors were cultured in serum-free medium supplemented with 0.01 U/mL interleukin-3 (IL-3), 0.001 ng/mL GM-CSF, and 3 U/mL Epo to induce unilineage erythroid differentiation. In these culture conditions a progeny of cells 98% ± 2% glycophorin A+ is generated.14 Alternatively, CD34+ cells were kept for 2 days in serum-free medium supplemented with cycling growth factors (100 U/mL IL-3, 100 ng/mL Flt3 ligand, 100 ng/mL SCF) for subsequent retroviral infection. Cell viability was evaluated by ethidium bromide—acridine orange staining and fluorescence microscopy analysis.15 The differentiation stage of erythroid precursor cells was evaluated by May-Grünwald-Giemsa staining and cytologic analysis.14 For clonogenetic assay, 102 CD34+ cells and 2 × 102 cells from day 5 or day 7 unilineage erythroid cultures were plated in duplicate in 0.9% methylcellulose containing 40% fetal calf serum and saturating concentrations of erythropoietin (3 U/mL), thrombopoietin (50 ng/mL), granulocyte macrophage-colony stimulating factor (GM-CSF; 10 ng/mL), macrophage-colony stimulating factor (M-CSF; 250 U/mL), granulocyte-colony stimulating factor (G-CSF; 500 U/mL), IL-6 (10 ng/mL), stem cell factor (100 ng/mL), Flt3 ligand (100 ng/mL), and IL-3 (100 U/mL). Colonies were scored after 14 days of culture at 37°C in a 5%CO2/5% O2/90%N2 humidified atmosphere.
Western blotting
For detection of caspases, protein extracts were prepared by resuspending cell pellets in 1% NP40 lysis buffer (20 mM Tris [tris(hydroxymethyl)aminomethane]/HCl pH 7.2, 200 mM NaCl,1% NP40) in the presence of 1 mM phenylmethylsulphonyl fluoride (PMSF) and 2 μg/mL each of leupeptin, aprotinin, and pepstatin. Concentration of lysates was determined by the Bradford assay (Bio-Rad Laboratories, Richmond, CA), and equal amounts of proteins were used for sodium dodecyl sulfate—polyacrylamide gel electrophoresis. Samples were analyzed by standard immunoblot procedure and visualized by chemiluminescence (Super Signal West Pico Pierce, Rockford, IL). Quantification of protein expression levels was performed with the National Institutes of Health IMAGE software version 1.62 (by Wayne Rasband, National Institutes of Health, Research Services Branch, National Institute of Mental Health, Bethesda, MD).
Production of retroviral particles and transduction of hematopoietic progenitor cells
Bcl-2 and Bcl-XL cDNAs were cloned into the PINCO retroviral vector carrying the green fluorescent protein (GFP) as a reporter gene.16 The amphotropic packaging cell line Phoenix was transfected by standard calcium-phosphate/chloroquine method, and culture supernatants containing retroviral particles were collected after 48 hours. Human progenitor cell (HPC) infection was performed by suspending the cells at 5 × 104/mL in the viral supernatant supplemented with cycling growth factors. For one cycle of infection, cells were centrifuged at 1800 rpm for 45 minutes at 32°C and placed back in the incubator for 1 hour. Cells were subjected to 3 infection cycles each day for 2 consecutive days and then placed in growth medium supplemented with cycling growth factors for 48 hours before sorting. GFP-positive cells were separated by flow cytometry using a FACS-Vantage (Becton Dickinson, Omaha, CA). Immediately after sorting, hematopoietic progenitor cells were placed in serum-free medium supplemented with erythroid growth factors to obtain virtually pure erythroblast populations.
Results
Immature erythroblasts are the preferential target of chemotherapeutic drugs in unilineage erythropoietic culture
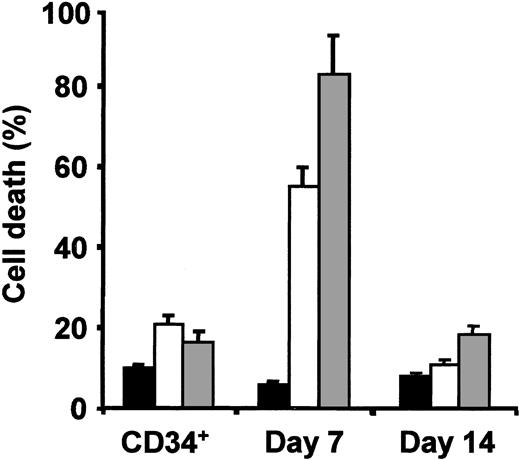
To identify the cellular target of erythroid suppression induced by antineoplastic agents, we analyzed the chemosensitivity of hematopoietic CD34+ progenitors, as compared with highly purified populations of erythroblasts at different maturation stages generated from CD34+ cells in erythroid specific medium.5,6,14 Cytotoxic drugs were used at doses compatible with their in vivo levels during cancer treatment. CD34+ progenitors, day 7 (> 70% pro/basophilic) and day 14 (> 80% orthochromatic) erythroblasts were incubated for 24 hours with cisplatin or camptothecin in standard erythroid medium. Evaluation of cell death revealed that CD34+ cells exhibited a modest sensitivity to drug-induced apoptosis (Figure 1). Despite the presence of saturating concentrations of Epo, immature erythroblasts were extremely sensitive to the cytotoxic effect of chemotherapeutic agents, while becoming almost completely resistant at the advanced (orthochromatic) stage of maturation (Figure 1). Thus, pro/basophilic erythroblasts likely represent the primary target of chemotherapy-induced anemia.
Chemosensitivity of CD34 progenitors+ and erythroid precursors. Highly purified populations of erythroid precursors were obtained by unilineage culture of peripheral blood CD34+ cells and analyzed at day 7 and day 14 of differentiation. Cells were incubated with erythroid medium alone (▪), with 1.5 μg/mL cisplatin (□) or 50 ng/mL camptothecin (▦) for 24 hours. The percentage of cell death was evaluated by ethidium bromide—acridine orange staining. The results shown are the mean ± SD of 4 independent experiments performed with cells from different donors.
Chemosensitivity of CD34 progenitors+ and erythroid precursors. Highly purified populations of erythroid precursors were obtained by unilineage culture of peripheral blood CD34+ cells and analyzed at day 7 and day 14 of differentiation. Cells were incubated with erythroid medium alone (▪), with 1.5 μg/mL cisplatin (□) or 50 ng/mL camptothecin (▦) for 24 hours. The percentage of cell death was evaluated by ethidium bromide—acridine orange staining. The results shown are the mean ± SD of 4 independent experiments performed with cells from different donors.
Chemotherapy-induced erythroblast apoptosis is mediated by caspase activation
Apoptosis induced by cytotoxic drugs has been shown to depend critically on activation of caspases in many cellular systems.7,17 Therefore, we analyzed caspase activation by immunoblot in immature erythroid precursors treated with cisplatin, camptothecin, or etoposide. All the 3 drugs activated caspase 3, 7, and 8, as deduced by detection of procaspases and their active fragments (Figure 2A). The pan-caspase inhibitor zVAD abolished caspase activation and abrogated completely chemotherapy-induced erythroblast death (Figure 2B), indicating that caspases are key effectors of drug-induced apoptosis in erythroid precursors.
Drug-induced apoptosis of erythroid precursor cells is mediated by caspase activation. (A) Chemotherapeutic agents activate caspases in immature erythroblasts. Erythroblasts at day 7 of differentiation were stimulated with 200 ng/mL camptothecin (CPT), 3 μg/mL cisplatin (CDDP), or 5 μM etoposide (VP-16) in the presence or absence of zVAD 40 μM, lysed after 8 hours, and analyzed by immunoblot with antibodies against caspase 3, caspase 7, and caspase 8. (B) zVAD inhibits apoptosis induced by chemotherapeutic agents. Day-7 erythroblasts were stimulated with 50 ng/mL camptothecin (CPT), 3 μg/mL cisplatin (CDDP), or 2 μM etoposide (VP-16) in the presence (□) or in the absence (▪) of zVAD 40 μM. After 24 hours the percentage of cell death was determined by ethidium bromide—acridine orange staining. A typical experiment of 5 performed with cells from different donors is shown.
Drug-induced apoptosis of erythroid precursor cells is mediated by caspase activation. (A) Chemotherapeutic agents activate caspases in immature erythroblasts. Erythroblasts at day 7 of differentiation were stimulated with 200 ng/mL camptothecin (CPT), 3 μg/mL cisplatin (CDDP), or 5 μM etoposide (VP-16) in the presence or absence of zVAD 40 μM, lysed after 8 hours, and analyzed by immunoblot with antibodies against caspase 3, caspase 7, and caspase 8. (B) zVAD inhibits apoptosis induced by chemotherapeutic agents. Day-7 erythroblasts were stimulated with 50 ng/mL camptothecin (CPT), 3 μg/mL cisplatin (CDDP), or 2 μM etoposide (VP-16) in the presence (□) or in the absence (▪) of zVAD 40 μM. After 24 hours the percentage of cell death was determined by ethidium bromide—acridine orange staining. A typical experiment of 5 performed with cells from different donors is shown.
Stem cell factor protects erythroblasts from chemotherapy-induced apoptosis
SCF promotes survival of primary erythroid precursors and inhibits apoptosis induced by cytokine deprivation.11,12,18 To determine whether SCF is capable of protecting erythroblasts from the effects of cytotoxic drugs, we pretreated immature erythroblasts at day 5 of differentiation with SCF for 48 hours before exposure to chemotherapeutic agents. The concentration of SCF used in all the experiments was 100 ng/mL, as this dose has been shown to support maximal erythroid proliferation in vitro.19 Cells pretreated with SCF and exposed to camptothecin, cisplatin, or etoposide were significantly protected from the toxic effects of antineoplastic drugs (Figure 3), suggesting that SCF interferes with the apoptotic program activated by chemotherapeutic agents in erythroid precursor cells.
SCF protects erythroid precursors from drug-induced apoptosis. Day-7 erythroblasts were incubated with 50 ng/mL camptothecin (CPT), 2 μM etoposide 7 erythroblasts were incubated with 50 ng/mL camptothecin (CPT), 2 μM etoposide percentage of cell death was determined after 12, 24, and 48 hours. Where indicated (+SCF) cells were preincubated for 2 days with 100 ng/mL SCF and kept in the presence of SCF during incubation with chemotherapeutic drugs. A typical experiment of 6 performed with cells from different donors is shown.
SCF protects erythroid precursors from drug-induced apoptosis. Day-7 erythroblasts were incubated with 50 ng/mL camptothecin (CPT), 2 μM etoposide 7 erythroblasts were incubated with 50 ng/mL camptothecin (CPT), 2 μM etoposide percentage of cell death was determined after 12, 24, and 48 hours. Where indicated (+SCF) cells were preincubated for 2 days with 100 ng/mL SCF and kept in the presence of SCF during incubation with chemotherapeutic drugs. A typical experiment of 6 performed with cells from different donors is shown.
SCF promotes erythroblast recovery and expansion after removal of chemotherapeutic drugs
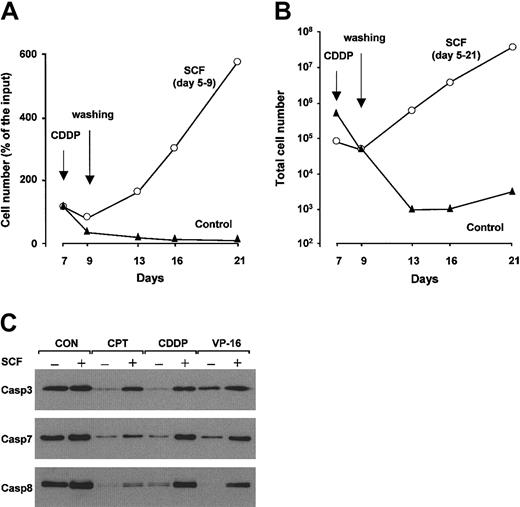
To analyze the long-term effects of SCF-mediated inhibition of erythroblast apoptosis, immature erythroid precursors untreated or pretreated for 2 days with SCF were exposed to cisplatin, washed after 48 hours, and placed in fresh medium. Whereas in standard erythroid medium control cells were not able to recover from the toxic effects of chemotherapy, erythroblasts preincubated with SCF showed an enhanced survival to cisplatin treatment and regained full proliferative activity after removal of the cytotoxic agent (Figure 4A). SCF enhances proliferation and delays differentiation of erythroid cells in culture.19,20 During the 2-day exposure to chemotherapeutic drugs, SCF-treated erythroblasts are significantly protected from apoptosis, but do not proliferate. However, the maintenance of SCF in the culture medium after removal of the chemotherapeutic agent results in a high proliferation rate of survived erythroid cells, which behaved similarly to drug-untreated cells in terms of expansion and maturation (Figure 4B and data not shown). In contrast, erythroblasts kept in standard erythroid medium underwent massive apoptosis during cisplatin treatment and continued to die for a few days after removal of the cytotoxic stimulus (Figure 4B), indicating that a small portion of erythroid cells undergo a slower apoptotic process. These results suggest that SCF activates an apoptosis inhibitory mechanism that allows efficient recovery of erythroid progenitors from the toxic effects of chemotherapy.
Erythroblasts treated with SCF are able to expand after removal of chemotherapeutic drugs and have lower levels of drug-induced caspase activation. (A) Day-7 erythroblasts, untreated (control) or pretreated for 2 days with 100 ng/mL SCF (SCF days 5-9), were exposed to 3 μg/mL cisplatin (CDDP) for 48 hours, washed, and placed in fresh medium with or without SCF, which was maintained in the culture medium until day 9. (B) Day-7 erythroblasts, untreated (control), or pretreated for 2 days with SCF (SCF days 5-21) were exposed to cisplatin as in panel A. After 48 hours, 4 × 104 cells were washed and replated in standard erythroid medium with or without 100 ng/mL SCF. In order to obtain equal numbers of erythroblasts after the cytotoxic treatment, a higher number of control cells were plated at the beginning of the experiment. The total number of cells obtained from the 4 × 104 cells plated at day 9 is shown. (C) Western blot analysis of caspases in immature erythroblasts untreated (Con) or pretreated for 2 days with SCF and exposed for 8 hours to camptothecin (CPT), cisplatin (CDDP), and etoposide (VP-16), showing the proforms of caspase 3 (top panel), caspase 7 (middle), and caspase 8 (bottom). One representative experiment of 4 performed with cells from different donors is shown.
Erythroblasts treated with SCF are able to expand after removal of chemotherapeutic drugs and have lower levels of drug-induced caspase activation. (A) Day-7 erythroblasts, untreated (control) or pretreated for 2 days with 100 ng/mL SCF (SCF days 5-9), were exposed to 3 μg/mL cisplatin (CDDP) for 48 hours, washed, and placed in fresh medium with or without SCF, which was maintained in the culture medium until day 9. (B) Day-7 erythroblasts, untreated (control), or pretreated for 2 days with SCF (SCF days 5-21) were exposed to cisplatin as in panel A. After 48 hours, 4 × 104 cells were washed and replated in standard erythroid medium with or without 100 ng/mL SCF. In order to obtain equal numbers of erythroblasts after the cytotoxic treatment, a higher number of control cells were plated at the beginning of the experiment. The total number of cells obtained from the 4 × 104 cells plated at day 9 is shown. (C) Western blot analysis of caspases in immature erythroblasts untreated (Con) or pretreated for 2 days with SCF and exposed for 8 hours to camptothecin (CPT), cisplatin (CDDP), and etoposide (VP-16), showing the proforms of caspase 3 (top panel), caspase 7 (middle), and caspase 8 (bottom). One representative experiment of 4 performed with cells from different donors is shown.
Western blot analysis of erythroid precursors exposed to chemotherapeutic agents revealed that the amount of inactive procaspase 3, 7, and 8 was significantly higher in cells pretreated with SCF compared to control erythroblasts exposed to the same cytotoxic stimuli (Figure 4C). These results suggest that signals generated by the activated c-kit receptor are able to inhibit drug-induced caspase activation in erythroid precursor cells, allowing the accomplishment of the erythropoietic process.
SCF supports the selective expansion of erythroid cells in unilineage culture conditions after treatment with chemotherapeutic agents
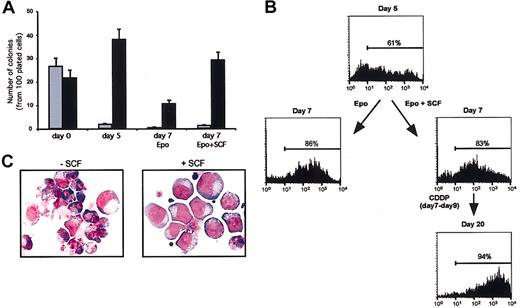
Theoretically, SCF may promote the growth of nonerythroid cells present in small numbers in erythroblastic cultures and even specifically protect these contaminants from cytotoxic drugs, thus generating a postchemotherapy population of nonerythroid cells. To rule out this possibility, we compared the number of erythroid colonies generated by SCF-treated or control cells at day 7 of erythroid culture in a semisolid medium supplied with multilineage growth factors. Two days' treatment with SCF did not significantly increase the percentage of granulomonocytic colonies present in the culture (Figure 5A), while exerting a significant stimulatory effect on the growth of erythroid colonies. Accordingly, cytofluorimetric analysis of glycophorin A expression on cells at day 7 of erythroid liquid culture revealed a similar percentage of glycophorin-positive cells regardless of 2 days' SCF pretreatment (Figure 5B). Moreover, SCF-pretreated erythroblasts that survived cisplatin treatment showed glycophorin positivity and clear erythroid phenotype (Figure 5B-C), indicating that SCF does not promote growth and survival of nonerythroid cells in the experimental conditions used.
SCF does not promote the growth of nonerythroid cells in erythroid unilineage culture. (A) Clonogenetic assay of CD34+ cells (day 0) or cells grown in liquid culture and subsequently plated in semisolid medium at day 5 or day 7 of erythroid differentiation. Control cells (day 5 and day 7 Epo) were cultivated in standard erythroid medium before the colony assay, whereas SCF-treated cells (day 7 Epo + SCF) received 100 ng/mL SCF from day 5 to day 7 of liquid culture. The results are expressed as number of granulomonocytic (▦) or erythroid (▪) colonies per 100 plated cells. Mixed colonies were rare in day 0 cultures and not present thereafter. (B) Cytofluorimetric analysis of glycophorin A-positive cells in erythroid unilineage culture. Cells were analyzed after 5 (day 5) or 7 days (day 7) of erythroid differentiation in standard erythroid medium (Epo) or in the same medium supplemented with 100 ng/mL SCF starting from day 5 (Epo + SCF). At day 7, cells were treated with cisplatin (CDDP days 7-9), and SCF-treated cells that survived the treatment were analyzed at day 20 of culture in standard erythroid medium supplemented with SCF. (C) May-Grünwald-Giemsa staining of day-7 erythroid cells treated with cisplatin for 48 hours in standard erythroid medium alone (-SCF) or with 100 ng/mL SCF (+ SCF), which was supplied 2 days before and during the cytotoxic treatment. Original magnification, × 400.
SCF does not promote the growth of nonerythroid cells in erythroid unilineage culture. (A) Clonogenetic assay of CD34+ cells (day 0) or cells grown in liquid culture and subsequently plated in semisolid medium at day 5 or day 7 of erythroid differentiation. Control cells (day 5 and day 7 Epo) were cultivated in standard erythroid medium before the colony assay, whereas SCF-treated cells (day 7 Epo + SCF) received 100 ng/mL SCF from day 5 to day 7 of liquid culture. The results are expressed as number of granulomonocytic (▦) or erythroid (▪) colonies per 100 plated cells. Mixed colonies were rare in day 0 cultures and not present thereafter. (B) Cytofluorimetric analysis of glycophorin A-positive cells in erythroid unilineage culture. Cells were analyzed after 5 (day 5) or 7 days (day 7) of erythroid differentiation in standard erythroid medium (Epo) or in the same medium supplemented with 100 ng/mL SCF starting from day 5 (Epo + SCF). At day 7, cells were treated with cisplatin (CDDP days 7-9), and SCF-treated cells that survived the treatment were analyzed at day 20 of culture in standard erythroid medium supplemented with SCF. (C) May-Grünwald-Giemsa staining of day-7 erythroid cells treated with cisplatin for 48 hours in standard erythroid medium alone (-SCF) or with 100 ng/mL SCF (+ SCF), which was supplied 2 days before and during the cytotoxic treatment. Original magnification, × 400.
SCF-mediated up-regulation of Bcl-2 and Bcl-XL protects immature erythroblasts from drug-induced apoptosis
The observation that drug-induced caspase activation is inhibited in cells pretreated with SCF prompted us to investigate whether this cytokine modulates the levels of proteins that influence caspase activity. Extensive Western blot analysis of apoptosis-related proteins was performed on erythroblasts treated for 2 days with SCF as compared with cells at the same differentiation stage kept in standard erythroid medium. Among the proteins examined, we found that Bcl-2 and Bcl-XL are up-regulated in SCF-treated cells, while levels of other Bcl-2 family members remain unchanged (Figure 6A and data not shown). Since antiapoptotic Bcl-2 family proteins can indirectly prevent the activation of executioner caspases by controlling cytochrome c efflux from mitochondria,21 it is likely that the increased levels of Bcl-2 and Bcl-XL may explain the lower caspase activity observed in erythroblasts treated with cytotoxic drugs.
SCF up-regulates Bcl-2 and Bcl-XL, which are able to protect erythroblasts from apoptosis induced by chemotherapeutic drugs. (A) Western blot analysis of Bcl-2 and Bcl-XL levels in day-7 erythroblasts cultured in standard erythroid medium (- SCF) or preincubated from day 5 to day 7 in erythroid medium supplemented with 100 ng/mL SCF (+ SCF).(B) Western bolt analysis of erythroblasts transduced with empty vector, Bcl-2, or Bcl-XL. Cycling CD34+ cells were transduced with a retroviral vector containing cDNAs for Bcl-2 or Bcl-XL and GFP as a reporter gene. Cells were sorted for GFP expression, placed in standard erythroid medium, and analyzed at day 4 of differentiation. One representative experiment of 5 performed with cells from different donors is shown. (C) Comparison of Bcl-2 or Bcl-XL expression in SCF-treated erythroblasts (Epo + SCF) versus erythroblasts grown in standard medium and transduced with Bcl-2 or Bcl-XL. The analysis was performed most cells reached the basophilic stage of differentiation, which corresponded to day 7 of erythroid unilineage culture for untransduced erythroblasts (Epo + SCF vs Epo) and to day 4 of erythroid unilineage culture for transduced cells (Bcl-2 vs empty vector and Bcl-XL vs empty vector). The latter were previously cultivated with cycling growth factors for 5 days (including the transduction and sorting procedures) as described in “Materials and methods.” Counting of unilineage erythroid culture days started when sorted GFP-positive cells were placed in erythroid specific medium. Data show the increase in gene-transduced erythroblasts as compared to empty vector-transduced erythroblasts (▪) or in SCF-treated versus untreated erythroblasts (□). (D) Cells at day 4 of erythroid unilineage culture, previously transduced with empty vector (▪), Bcl-2 (□), or Bcl-XL (▦) as in C, were incubated with medium alone (Con), 50 ng/mL camptothecin (CPT), 2 μM etoposide (VP-16), or 3 μg/mL cisplatin (CDDP). The percentage of cell death was evaluated after 24 hours by staining with ethidium bromide—acridine orange. The results shown are the mean ± SD of 3 independent experiments performed with cells from different donors.
SCF up-regulates Bcl-2 and Bcl-XL, which are able to protect erythroblasts from apoptosis induced by chemotherapeutic drugs. (A) Western blot analysis of Bcl-2 and Bcl-XL levels in day-7 erythroblasts cultured in standard erythroid medium (- SCF) or preincubated from day 5 to day 7 in erythroid medium supplemented with 100 ng/mL SCF (+ SCF).(B) Western bolt analysis of erythroblasts transduced with empty vector, Bcl-2, or Bcl-XL. Cycling CD34+ cells were transduced with a retroviral vector containing cDNAs for Bcl-2 or Bcl-XL and GFP as a reporter gene. Cells were sorted for GFP expression, placed in standard erythroid medium, and analyzed at day 4 of differentiation. One representative experiment of 5 performed with cells from different donors is shown. (C) Comparison of Bcl-2 or Bcl-XL expression in SCF-treated erythroblasts (Epo + SCF) versus erythroblasts grown in standard medium and transduced with Bcl-2 or Bcl-XL. The analysis was performed most cells reached the basophilic stage of differentiation, which corresponded to day 7 of erythroid unilineage culture for untransduced erythroblasts (Epo + SCF vs Epo) and to day 4 of erythroid unilineage culture for transduced cells (Bcl-2 vs empty vector and Bcl-XL vs empty vector). The latter were previously cultivated with cycling growth factors for 5 days (including the transduction and sorting procedures) as described in “Materials and methods.” Counting of unilineage erythroid culture days started when sorted GFP-positive cells were placed in erythroid specific medium. Data show the increase in gene-transduced erythroblasts as compared to empty vector-transduced erythroblasts (▪) or in SCF-treated versus untreated erythroblasts (□). (D) Cells at day 4 of erythroid unilineage culture, previously transduced with empty vector (▪), Bcl-2 (□), or Bcl-XL (▦) as in C, were incubated with medium alone (Con), 50 ng/mL camptothecin (CPT), 2 μM etoposide (VP-16), or 3 μg/mL cisplatin (CDDP). The percentage of cell death was evaluated after 24 hours by staining with ethidium bromide—acridine orange. The results shown are the mean ± SD of 3 independent experiments performed with cells from different donors.
Antiapoptotic Bcl-2 family members have been implicated in modulation of apoptosis induced by chemotherapeutic drugs in several cellular systems.22-24 Therefore, we speculated that the up-regulation of Bcl-2 and Bcl-XL induced by SCF in erythroid precursor cells may contribute to the cytoprotective effect of this cytokine. To determine if increased levels of Bcl-2 and Bcl-XL are sufficient to protect erythroblasts from the effects of chemotherapeutic drugs, CD34+ hematopoietic progenitors were transduced with a retroviral vector containing the cDNA for Bcl-2 or Bcl-XL with the GFP as a reporter gene and subsequently sorted on the basis of GFP expression. After sorting, CD34+ cells were cultured in unilineage erythroid medium until they reached the probasophilic stage of differentiation and then exposed to chemotherapeutic agents. Quantitative immunoblot analysis showed that the levels of Bcl-2 and Bcl-XL obtained after retroviral transduction were comparable with those observed in SCF-stimulated erythroblasts (Figure 6A-C) and sufficient to significantly reduce chemotherapy-induced cell death (Figure 6D). Thus, SCF-induced Bcl-2 and Bcl-XL up-regulation likely contribute to protect immature erythroid cells from the cytotoxic effects of chemotherapeutic agents.
Discussion
Myelosuppression is a major side effect of chemotherapy, often resulting in dose modification and treatment delay. Different approaches have been used with the intent of recovering blood cell production, including the use of recombinant cytokines and transplantation of normal or genetically modified hematopoietic progenitor/stem cells.25,26
Chemotherapy-induced anemia is very common in patients with solid tumors and reaches the highest incidence in patients with lung cancer and gynecologic or genitourinary tumors, where blood transfusions are required in 50% to 60% of cases.1 Suppression of red blood cell production caused by chemotherapeutic agents appears to involve both inhibition of erythropoiesis and reduction of serum Epo levels.27 Recombinant human Epo is used in several clinical settings such as the treatment of anemia associated with chronic renal failure or zidovudine therapy in HIV patients.28-30 In patients undergoing anticancer therapy, Epo administration has been shown to alleviate anemia and reduce transfusion requirements and is currently used to treat one third of patients with substantial anemia (hemoglobin [Hb] level < 10 g/dL) induced by chemotherapy.28 However, Epo treatment does not result in improvement of anemia in a consistent fraction of patients, and no reliable predictors of its therapeutic response have been found to date.31,32
The data presented in this work suggest that SCF may be a promising candidate for the treatment of chemotherapy-induced anemia in cancer patients. The use of SCF to prevent and treat chemotherapy-induced anemia could be particularly indicated in (1) adult patients not responsive to epoetin treatment, that is, when causes of anemia are not related to inadequate Epo response, (2) children with cancer, where anemia is associated with a decreased bone marrow erythropoietic activity and seems unrelated to defective Epo production,33 and (3) patients who already receive epoetin alfa, because the synergy between Epo and SCF could achieve optimal precursor cell proliferation with reduced doses of growth factors.
The myelotoxic effects of chemotherapeutic agents involve activation of apoptotic pathways in hematopoietic cells. Our study identifies apoptosis of immature erythroblasts as a likely cause of drug-induced erythroid suppression. Therefore, strategies aimed at the prevention and treatment of chemotherapy-induced anemia should focus on this cell population whose integrity is crucial for homeostasis of the erythroid compartment. Our work demonstrates that SCF protects immature erythroid precursors from drug-induced apoptosis. It is noteworthy that in the presence of chemotherapeutic drugs, Epo alone does not effectively protect early erythroid cells from apoptosis, whereas combined treatment with Epo and SCF induces a striking rescue of erythroblast survival and optimal expansion after drug removal. Furthermore, our studies indicate that this effect may be mediated by the up-regulation of Bcl-2 and Bcl-XL. This observation is in line with previous studies showing that susceptibility to drug-induced apoptosis correlates with levels of Bcl-2 and Bcl-XL,22,24,34,35 which regulate the release of cytochrome c from mitochondria, thus controlling apoptosome formation and activation of executioner caspases.
The capacity of SCF to modulate Bcl-2 and Bcl-XL expression has been reported in human and murine hematopoietic cells.36-38 In human erythroid colony-forming cells, SCF and Epo cooperate in maintaining basal Bcl-XL levels.11 Inhibition of SCF-mediated mitogen-activated protein kinase (MAPK) activation results in a decrease of Bcl-XL that is not sufficient per se to induce apoptosis in unstimulated cells.11 However, it is likely that SCF-induced Bcl-2 and Bcl-XL up-regulation turns out to be essential for the survival of erythroid precursors when cells are exposed to apoptotic stimuli.
SCF-mediated up-regulation of Bcl-2 and Bcl-XL proteins in erythroblasts does not seem to correlate with increased production of the corresponding RNA (data not shown). A similar finding has been reported recently for fibroblast growth factor-2, which prevents etoposide-induced apoptosis by up-regulating Bcl-2 and Bcl-XL proteins through a translational mechanism dependent on activation of mitogen-induced extracellular kinase/extracellular signal-related kinase (MEK/ERK) kinases.39 Because SCF binding to the c-kit receptor activates the MEK/ERK signaling pathway in erythroid precursor cells,11 it is conceivable that a similar mechanism may explain SCF-induced up-regulation of Bcl-2 and Bcl-XL in the absence of mRNA increase.
The c-kit receptor promotes cell survival through activation of multiple signaling pathways.40 One of these pathways involves activation of PI 3-kinase and Akt and subsequent phosphorylation of Bad, which results in its cytosolic sequestration and dissociation from Bcl-XL and Bcl-2.41 Although this pathway is not specifically involved in the antiapoptotic effect of SCF in human erythroid colony-forming cells,11 it is possible that other signals produced by the activated c-kit receptor and acting downstream of MAPK or PI-3K may be involved in SCF-mediated protection of erythroblasts from drug-induced apoptosis.
Cisplatin-based chemotherapy regimens cause progressive and persistent anemia, which is commonly attributed to renal tubular damage and subsequent decrease in serum Epo.42 However, some studies report a lack of correlation between serum Epo levels in patients treated with cisplatin or with other chemotherapeutics,43-45 raising the possibility that other mechanisms may contribute to cisplatin-induced erythroid suppression. We have observed a direct cytotoxic effect of cisplatin on erythroid precursors, which undergo caspase-mediated apoptosis following exposure to this drug. Because SCF seems particularly effective in preventing cisplatin-induced erythroblast apoptosis and promoting postchemotherapy erythroblast recovery in vitro, it would be interesting to evaluate a possible use of this cytokine to ameliorate anemia caused by platinum-based therapies.
SCF is used in clinical practice in conjunction with G-CSF to mobilize CD34+ cells into the peripheral blood of patients with lymphoma, multiple myeloma, and breast or ovarian cancer.46-51 Promising results also have been obtained with the use of SCF alone or combined with G-CSF in the treatment of aplastic anemia.52 Because CD34+ cells of aplastic anemia patients appear to have a greater apoptotic tendency than normal hematopoietic progenitors,53 it may be speculated that the positive effect of SCF on marrow repopulation may be due to the antiapoptotic properties of this cytokine.
A major concern for the use of SCF in the treatment of cancer-related anemia is the possibility that SCF might stimulate the proliferation of c-kit—expressing malignant cells. Indeed, c-kit expression has been detected in most acute myeloid leukemia cells54-56 and in a variety of human solid tumors, including testicular germ cell tumors,57 glioblastoma,58 melanoma,59 breast cancer,59 and small-cell lung carcinoma.60 However, binding of SCF to c-kit expressed on the surface of tumor cells does not necessarily result in proliferation but can elicit an extreme variety of biologic responses. While enhanced proliferation in response to SCF has been reported for colon carcinoma61 and for cells from a portion of acute myeloid leukemia patients,62,63 SCF fails to induce growth of acute lymphoid leukemia blasts64 or cells derived from breast carcinoma, gastric carcinoma, and small-cell lung carcinoma.59 Interestingly, SCF has been reported to induce apoptosis of melanoma cells and has been suggested as a possible treatment for human melanomas in early stages.65,66 To offset the potential adverse effects of SCF therapy in clinical oncology, a predictive in vitro assay of proliferative response of neoplastic cells to SCF may be developed for cases at risk.
In conclusion, we provide evidence that SCF protects immature erythroid precursors from chemotherapy-induced apoptosis via up-regulation of Bcl-2 and Bcl-XL. While the clinical use of SCF for the prevention of chemotherapy-induced anemia in cancer patients may represent an interesting future possibility, further studies are required to understand the effects of SCF on the biology of neoplastic cells and to rule out the possibility of enhanced tumor cell growth after cytokine treatment.
Prepublished online as Blood First Edition Paper, March 13, 2003; DOI 10.1182/blood-2002-08-2369.
Supported by funding from the Italian Association for Cancer Research (A.I.R.C.) and the Italian Health Ministry. A.Z. is a recipient of a Fondazione Italiana per la Ricerca sul Cancro (F.I.R.C.) fellowship.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal