Abstract
Many surface receptors and signaling molecules are thought to associate with unique membrane microdomains termed lipid rafts. We examined the involvement of lipid rafts in the activation of leukocyte function–associated antigen-1 (LFA-1). Depletion or sequestration of cholesterol with methyl-β-cyclodextrin (MCD) or filipin, respectively, strongly inhibited LFA-1–mediated adhesion of T-cell lines and primary T cells. This inhibition was reversed by cholesterol reconstitution. LFA-1 on T-cell lines was detected in cold Triton X-100–insoluble lipid rafts, which were disrupted by MCD or filipin treatment. However, no LFA-1 on primary T cells was detected in lipid rafts isolated by the same procedures, and these rafts were resistant to cholesterol depletion or sequestration. Association of LFA-1 with lipid rafts of primary T cells could be detected only when they were isolated with another nonionic detergent, Brij 35. Upon treatment with MCD, LFA-1 in Brij 35–insoluble lipid rafts partially shifted to nonraft fractions. T-cell lines were found to have a high level of cholesterol and a low level of ganglioside GM1, a common marker for lipid rafts, whereas primary T cells have a much lower level of cholesterol and a very high amount of GM1. Cross-linking of LFA-1 on primary T cells induced cocapping of cholesterol but not GM1. These results suggest that lipid rafts of T cells are heterogenous, and LFA-1 associates with a subset of lipid rafts containing a high level of cholesterol. This association seems to regulate LFA-1 functions, possibly by facilitating LFA-1 clustering. (Blood. 2003;102: 215-222)
Introduction
Leukocyte function–associated antigen-1 (LFA-1) (αLβ2; CD11a/CD18) is a member of the leukocyte integrin family and consists of a 180-kDa α-chain (CD11a or αL) and a 95-kDa β-chain (CD18 or β2).1 It plays a critical role in the inflammation process. Chemokines released during inflammation activate LFA-1 to mediate firm adhesion of leukocytes to endothelial cells and induce their migration and extravasation from the blood vessel.2 Binding of LFA-1 on T cells to its ligand intercellular adhesion molecule-1 (ICAM-1) has been shown to provide a second signal for T-cell activation.3 LFA-1 also participates in immune responses by forming an immunologic synapse together with T-cell receptors (TCRs) and other costimulatory molecules when T cells interact with antigen-presenting cells.4-6
Although resting leukocytes constitutively express LFA-1, they do not readily adhere to cells expressing its ligands, as the adhesive functions of LFA-1 are regulated by cell activation. Through the process of inside-out signaling, intracellular activation signals convert low-avidity LFA-1 on resting leukocytes into an active form capable of mediating cell adhesion. This conversion does not require an increase in the cell-surface expression of LFA-1.1,7 The prevailing theories to explain this phenomenon are that LFA-1 undergoes a conformational change upon cell activation or that LFA-1 is redistributed at the cell surface.8,9 Evidence for the former comes from the existence of antibodies that recognize epitopes on activated LFA-1.10-13 Other studies suggest that clustering of LFA-1 leading to multivalent interaction between LFA-1 and ICAM-1 may be important.14,15
Lipid rafts are highly organized microdomains of the plasma membrane. They have a high content of cholesterol, gangliosides, sphingolipids, and phospholipids with long saturated fatty acyl chains, and they are resistant to extraction with cold nonionic detergents. Lipid rafts are isolated as detergent-insoluble glycolipid-enriched membranes in low-density fractions of sucrose gradient centrifugation.16 Molecules such as glycosylphosphatidylinositol (GPI)–anchored proteins and acylated proteins are known to partition into lipid rafts. Cholesterol plays an important role in maintaining lipid rafts in a liquid-ordered phase, whereas the rest of the membrane that contains phosphatidyl ethanolamine and phosphatidyl choline combined with lesser amounts of cholesterol exists in a liquid-disordered phase. Depletion of cholesterol by methyl-β-cyclodextrin (MCD) or sequestering cholesterol out of lipid rafts by filipin is commonly used to disrupt lipid rafts.17 Many cell-surface receptors and intracellular signaling proteins are thought to localize in lipid rafts. However, direct visualization of these molecules in lipid rafts is difficult because they are thought to be only 50 to 100 nm in diameter, which is below the resolution of optical microscopes.18,19 Therefore, the detection of receptors and signaling molecules in the low-density fractions of sucrose gradient centrifugation combined with raft-disrupting reagents such as MCD and filipin is often used to determine the localization of receptors in lipid rafts. Cross-linking of various cell-surface receptors can induce fusion of lipid rafts to form clusters large enough to be visible by fluorescence microscope upon staining with fluorescence-conjugated cholera toxin subunit B (CTxB), which binds to ganglioside GM1. Colocalization of molecules with GM1 may be used as an indication that the molecules associate with lipid rafts. However, recent studies have suggested heterogeneity among lipid rafts. In human peripheral T lymphoblasts, cholesterol extraction disrupts lipid rafts that contain Lck, CD4, and TCRζ but not those containing LAT.20,21 Upon TCR cross-linking, these different rafts are thought to coalesce to facilitate T-cell activation signaling.
The involvement of lipid rafts in LFA-1 regulation is still controversial. LFA-1 on mouse thymocytes was shown to associate with lipid rafts isolated with 1% Triton X-100, and cross-linking of ganglioside GM1 with CTx induced activation of LFA-1.22 In contrast, confocal microscopic analysis of LFA-1 transfected into the human T-cell line Jurkat showed that LFA-1 does not associate with rafts unless it is activated by Mn2+ or the I-domain is deleted from LFA-1.23 Shamri et al also reported that cholesterol extraction by MCD does not inhibit LFA-1–mediated adhesion of human peripheral blood T cells to ICAM-1.24 Thus, studies with different cells and techniques have resulted in conflicting results regarding the association of LFA-1 with lipid rafts and its functional significance. Here we show that cholesterol depletion or sequestration strongly inhibits LFA-1–mediated adhesion of murine T cells. Although cholesterol is thought to be an essential component of lipid rafts in general, our results suggest that lipid rafts of primary T cells are heterogeneous and some lipid rafts seem to contain little cholesterol. LFA-1 is detected in a subset of lipid rafts that is cholesterol rich and sensitive to MCD treatment.
Materials and methods
Cells, antibodies, and reagents
The murine T-cell leukemia line EL4 was obtained from the American Type Culture Collection (ATCC, Rockville, MD). The murine T-cell hybridoma line T28 has been described.25 They were maintained in Dulbecco modified Eagle medium plus 5% fetal calf serum (FCS) and penicillin/streptomycin (StemCell Technologies, Vancouver, BC, Canada). Splenocytes were harvested from 6- to 8-week-old C57BL/6 mice. Splenic T cells were isolated by murine T-cell enrichment kit, Spin Sep (StemCell Technologies), which resulted in 98% to 99% pure T cells as assessed by fluorescence-activated cell sorter (FACS) analysis using fluorescein isothiocyanate (FITC)–conjugated anti-CD3 monoclonal antibody (mAb; BD Pharmingen, Mississauga, ON, Canada). Rat anti-CD18 hybridomas (TIB213 and TIB218), hamster anti-CD18 (2E6) hybridoma, and mouse anti–rat immunoglobulin Gκ (IgGκ) hybridoma (TIB169) were from ATCC. TIB218 was used in Western blots to detect LFA-1 since T cells do not express other β2 integrins. Hamster antimouse CD3ϵ antibody was purchased from BD Pharmingen. Rat anti–Thy1 monoclonal antibody (clone 3.2) was generated in our laboratory. Its specificity for Thy1 was confirmed by the binding to the murine lymphoma cell line S49.1 (ATCC TIB28) but not its Thy1–negative mutant S49 (Thy1-a) (ATCC TIB36). Rat anti-CD45 (YE 1/21.2.1) was described previously.25 Purification of these mAbs has been described.26 Mouse anti-Lck (3A5) was from Santa Cruz Biotechnology (Santa Cruz, CA) and anti–extracellular signal regulated kinase 1/2 (Erk-1/2) and anti–phospho-Erk-1/2 were from Cell Signalling Technology (Beverly, MA). Horseradish peroxidase–conjugated CTxB, FITC-conjugated CTxB, MCD, filipin III, water-soluble cholesterol (MCD-cholesterol complex), bovine serum albumin (BSA) fragment V, Triton X-100, Brij 99, Brij 58, Brij 56, Brij 35, CHAPS (3-[(3-cholamidopropyl)dimethylammonio]-1-propane-sulfonic acid), and protease inhibitors (leupeptin, phenylmethyl-succinyl fluoride, aprotinin, and pepstatin A) were from Sigma (St Louis, MO). Murine recombinant soluble ICAM-1 has been described.27 Calcein-AM and Alexa Fluor 568–conjugated goat anti–rat Ig were from Molecular Probes (Eugene, OR).
Cholesterol depletion, sequestration, and reconstitution
For cholesterol depletion, cells were treated with various concentrations of MCD in Hanks balanced salt solution (HBSS) containing 50 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) for 30 minutes at 37°C. For cholesterol sequestration, cells were treated with various concentrations of filipin III in HBSS containing 0.2% BSA and incubated at 37°C for one hour. To reconstitute cholesterol of MCD-treated cells, 60 μg/mL water-soluble cholesterol in HBSS containing 0.2% BSA was added to the cells and incubated for 30 minutes at 37°C.
Cell adhesion assay
LFA-1–mediated cell adhesion to immobilized soluble ICAM-1 was assayed as described.27 Briefly, cells labeled with Calcein-AM were incubated in microwells coated with soluble ICAM-1 for 30 minutes at 37°C. Nonadherent cells were washed away, and the fluorescence intensities of the cells before and after the wash were measured by CytoFluor 2300 (Millipore, Bedford, MA). The percentages of cell adhesion were determined by the ratio of the postwash over prewash fluorescence values after subtracting the background fluorescence values. For stimulation of LFA-1, cells were preincubated with 50 ng/mL phorbol myristate acetate (PMA) for 30 minutes at 37°C. For specificity control, anti–LFA-1 mAb (TIB213) was also added. For the cholesterol depletion or sequestration experiments, MCD or filipin was added to cells as they were treated with PMA.
Sucrose gradient centrifugation and Western blotting
Cells (5 × 107) were washed twice with phosphate-buffered saline and lysed in 1 mL ice-cold lysis buffer containing 20 mM Tris (tris(hydroxymethyl)aminomethane)–HCl (pH 7.2), 150 mM KCl, various concentrations of nonionic detergents, and protease inhibitors. The cell lysates were sheared by 5 successive passages through no. 26 gauge hypodermic needles, then mixed with an equal volume of 80% sucrose (wt/vol) in ice-cold lysis buffer without detergent, and transferred to SW41 centrifuge tubes. The samples were then overlaid with 6 mL of 30% sucrose and 3.5 mL of 5% sucrose and centrifuged (Beckman, Palo Alto, CA) at 200 000g for 18 hours. All of the procedures were done at 4°C. Following centrifugation, 8 fractions of 1.5 mL each were collected, starting at the top of the gradient. Fractions 2 and 3 corresponding to the 5% to 30% sucrose interface were referred to as low-density fractions. Fractions 7 and 8 were referred to as high-density fractions. The materials at the bottom of the tube were referred to as pellet. Aliquots of each fraction were boiled in sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer (nonreducing condition), loaded onto SDS-PAGE, and transferred to polyvinylidene fluoride transfer membrane (Pall Gelman Lab, Ann Arbor, MI). Proteins on the blots were detected by specific antibodies and visualized by an enhanced chemiluminescence system (Amersham Pharmacia Biotech, Piscataway, NJ) according to the manufacturer's protocols. For mitogen-activated protein (MAP) kinase analysis, 106 cells were treated with or without 50 ng/mL PMA and with or without 10 mM MCD for 30 minutes at 37°C, and then subjected to SDS-PAGE and Western blotting.
Flow cytometry
Cells were directly stained with the FITC-conjugated anti-CD18 mAb 2E6 (10 μg/mL), FITC-CTxB (15 μg/mL), or FITC-conjugated anti-CD3ϵ (5 μg/mL) for 30 minutes on ice. For the staining of CD45 and Thy1, cells were incubated with the appropriate hybridoma supernatants for 30 minutes on ice, washed twice, and then stained with 5 μg/mL FITC-conjugated anti–rat Igκ (TIB169) mAb. The stained cells were washed with HBSS containing 2% FCS and 0.1% sodium azide and analyzed by a FACScan flow cytometer (Becton Dickinson, San Jose, CA). For filipin staining,28 cells were treated with 12.5 μg/mL fresh filipin in HBSS at 4°C in the dark for 2 hours and analyzed by a FACStar Plus (Becton Dickinson) equipped with a 360-nm Coherent Enterprise Argon laser (Santa Clara, CA). Emissions were collected via a 640-nm dichroic long-pass filter with 424/44-nm band-pass filter.29,30
Confocal microscopy
T28 cell line and mouse primary T cells were stained at 4°C with anti–LFA-1 antibody (TIB213), anti–Thy1 antibody, 15 μg/mL FITCCTxB, and 12.5 μg/mL filipin where indicated. Secondary staining for LFA-1, Thy1, and CD45 were with Alexa Fluor 568–conjugated goat anti–rat Ig. Capping was induced by incubation at 37°C for 30 minutes, followed by fixation with 4% formaldehyde. Cells were cytospun onto poly-D-lysine–coated glass coverslips. Then samples were analyzed by confocal microscope (BioRad Radiance 2000 Multiphoton; Hercules, CA) with × 60 objective lens. Lasers used were Kr and Mai Tai Ti Sapphire (SpectroPhysics, Mountainview, CA). Filipin III was excited with a multiphoton laser at 779 nm and the emission filter was HQ 450/80 with a BGG 22 blocking filter. FITC was excited by 488 nm and the emission filter was HQ 515/30. Alexa Fluor 568 was excited by 568 nm and the emission filter was HQ 600/50. Signals were collected sequentially to avoid bleed through.
Results
MCD disrupts lipid rafts and inhibits LFA-1–mediated adhesion of T-cell lines
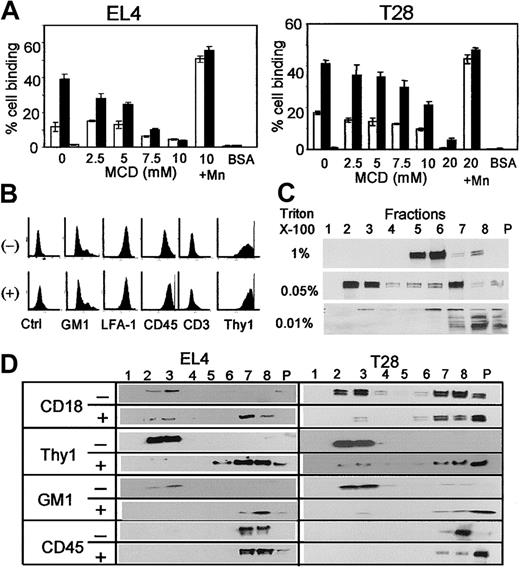
To determine whether lipid rafts have any role in LFA-1 activation, the murine T-cell leukemia line EL4 and the T-cell hybridoma line T28 were treated with MCD to remove membrane cholesterol, and its effect on LFA-1–mediated cell adhesion to ICAM-1 (CD54) was tested. EL4 cells showed a low level of adhesion to ICAM-1, but upon stimulation with PMA they readily adhered to ICAM-1 immobilized on a plastic surface (Figure 1A, left panel). This adhesion was mediated by LFA-1 as indicated by almost complete inhibition by anti–LFA-1 mAb. MCD inhibited LFA-1–mediated T-cell adhesion in a dose-dependent manner. MCD at these concentrations did not cause cell death as determined by trypan blue staining. MCD-treated cells strongly adhered to ICAM-1 when treated with 2 mM MnCl2, which binds to and directly activates LFA-1, indicating that LFA-1 on MCD-treated cells is potentially functional, but MCD treatment inhibits inside-out activation of LFA-1. Similar results were obtained with T28 cells (Figure 1A, right panel). Flow cytometric analysis of MCD-treated cells showed that MCD had no effects on the expression levels of LFA-1 (Figure 1B). The levels of the ganglioside GM1 and the GPI-anchored protein Thy1, which together with cholesterol have been considered to be components of lipid rafts, were also unaffected by MCD treatment.
Disruption of lipid rafts and inhibition of LFA-1 activation by MCD treatment. (A) EL4 cells (left panel) or T28 cells (right panel) were incubated with (▪) or without (□) 50 ng/mL PMA in the presence of the indicated concentrations of MCD in serum-free HBSS and incubated at 37°C for 30 minutes, and their adhesion to immobilized soluble ICAM-1 was analyzed. Cells treated with or without PMA, 10 mM or 20 mM MCD, and 2 mM MnCl2 were also tested (shown as 10 + Mn and 20 + Mn). PMA-activated cells blocked with anti–LFA-1 were tested as specificity control (▦). For control cell adhesion, BSA was immobilized in place of ICAM-1. The results are representative of 5 independent experiments, each done in triplicate. Error bars indicate SD. (B) Flow cytometric analysis of control (-) and 10 mM MCD-treated (+) EL4 cells. Ganglioside GM1 was stained with FITC-conjugated CTxB. All other molecules were stained with the appropriate mAb with secondary FITC-conjugated antibodies. Ctrl shows unstained control. (C) EL4 cells were solubilized with indicated concentrations of Triton X-100 and subjected to sucrose gradient centrifugation. Proteins in the sucrose gradient fractions were separated by SDS-PAGE, and CD18 was detected by Western blotting. Fractions 2 and 3 are low-density fractions and contain lipid rafts, whereas fractions 5 to 8 are high-density fractions, and P indicates pellet. (D) EL4 cells were either treated (+) or not (-) with 10 mM MCD as in panel A, solubilized with 0.05% Triton X-100, subjected to sucrose gradient centrifugation, and analyzed by Western blotting for the indicated molecules. The numbers indicate sucrose gradient fractions. Fractions 2 and 3 are low-density fractions containing lipid rafts. CD18, Thy1, and CD45 were detected by specific mAb and horseradish peroxidase–conjugated secondary anti–rat Ig antibody. GM1 was detected by horseradish peroxidase–conjugated CTxB.
Disruption of lipid rafts and inhibition of LFA-1 activation by MCD treatment. (A) EL4 cells (left panel) or T28 cells (right panel) were incubated with (▪) or without (□) 50 ng/mL PMA in the presence of the indicated concentrations of MCD in serum-free HBSS and incubated at 37°C for 30 minutes, and their adhesion to immobilized soluble ICAM-1 was analyzed. Cells treated with or without PMA, 10 mM or 20 mM MCD, and 2 mM MnCl2 were also tested (shown as 10 + Mn and 20 + Mn). PMA-activated cells blocked with anti–LFA-1 were tested as specificity control (▦). For control cell adhesion, BSA was immobilized in place of ICAM-1. The results are representative of 5 independent experiments, each done in triplicate. Error bars indicate SD. (B) Flow cytometric analysis of control (-) and 10 mM MCD-treated (+) EL4 cells. Ganglioside GM1 was stained with FITC-conjugated CTxB. All other molecules were stained with the appropriate mAb with secondary FITC-conjugated antibodies. Ctrl shows unstained control. (C) EL4 cells were solubilized with indicated concentrations of Triton X-100 and subjected to sucrose gradient centrifugation. Proteins in the sucrose gradient fractions were separated by SDS-PAGE, and CD18 was detected by Western blotting. Fractions 2 and 3 are low-density fractions and contain lipid rafts, whereas fractions 5 to 8 are high-density fractions, and P indicates pellet. (D) EL4 cells were either treated (+) or not (-) with 10 mM MCD as in panel A, solubilized with 0.05% Triton X-100, subjected to sucrose gradient centrifugation, and analyzed by Western blotting for the indicated molecules. The numbers indicate sucrose gradient fractions. Fractions 2 and 3 are low-density fractions containing lipid rafts. CD18, Thy1, and CD45 were detected by specific mAb and horseradish peroxidase–conjugated secondary anti–rat Ig antibody. GM1 was detected by horseradish peroxidase–conjugated CTxB.
To determine whether LFA-1 on T-cell lines associates with lipid rafts, EL4 cells were solubilized with Triton X-100 on ice and fractionated by sucrose gradient centrifugation at 4°C. Under these conditions, lipid rafts are detergent-insoluble and recovered in low-density fractions (fractions 2 and 3 in Figure 1C). As expected, GM1 and Thy1 are detected in the low-density fractions (Figure 1D). It should be noted that LFA-1 is the only β2 integrin on these cells, and no other leukocyte integrins are detected by anti-CD18 mAb on T cells. Western blot analysis of each fraction using anti-CD18 mAb detected no LFA-1 in the low-density fractions when 1% Triton X-100 was used. However, when the concentration of Triton X-100 was lowered to 0.05%, which is thought to allow better preservation of lipid rafts,31 the majority of CD18 was recovered in the low-density fraction (Figure 1C). Similar results were also obtained with T28 cells (results not shown). Triton X-100 at less than 0.05% caused incomplete cell lysis and most CD18 was found in the pellet. These results suggest that LFA-1 on these T-cell lines associates with lipid rafts, but the association is disrupted by a high concentration (> 0.05%) of Triton X-100.
To confirm that MCD treatment disrupts lipid rafts of T-cell lines, EL4 cells treated with MCD were analyzed by sucrose gradient centrifugation and Western analysis. As shown in Figure 1D, all the molecules in the low-density fractions, including Thy1, GM1, and LFA-1, were shifted to detergent-soluble high-density fractions by MCD treatment, whereas CD45 remained in the high-density fractions regardless of MCD treatment. The same results were obtained with T28 cells (Figure 1D, right). Therefore, MCD treatment indeed disrupts lipid rafts of the murine T-cell lines EL4 and T28, and the association of LFA-1 with lipid rafts appeared to be important for its functions.
MCD inhibits LFA-1 on primary T cells but does not disrupt Triton X-100–insoluble lipid rafts
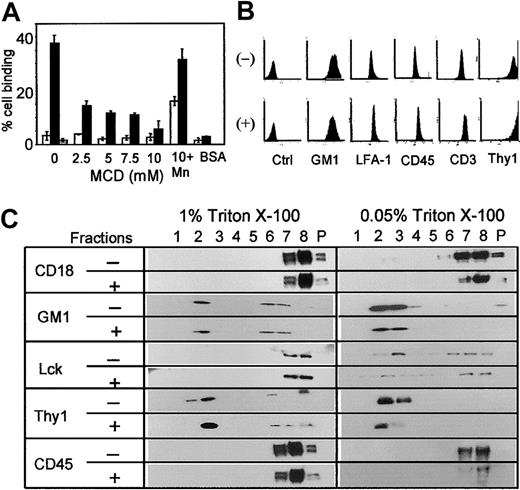
To extend the above findings to freshly isolated primary T cells, the effect of MCD on LFA-1 of splenic T cells was tested. Similar to the above results (Figure 1A) with T-cell lines, MCD inhibited LFA-1–mediated adhesion of PMA-activated splenic T cells in a dose-dependent manner, and the inhibition was overcome by manganese treatment (Figure 2A). Flow cytometric analysis confirmed that MCD treatment did not alter the expression levels of LFA-1 or other molecules tested (Figure 2B). However, sucrose gradient centrifugation analysis showed that LFA-1 on splenic T cells differed from that on T-cell lines, as no LFA-1 on splenic T cells was detected in the detergent-insoluble low-density fractions isolated with 1% or 0.05% Triton X-100 (Figure 2C). Activation of LFA-1 on primary T cells with PMA or Mn2+ treatment for 30 minutes or by CD3 cross-linking for 3 days or 1 week failed to induce association of LFA-1 with rafts (results not shown). Triton X-100 at concentrations lower than 0.05% failed to solubilize splenic T cells. Furthermore, quite unexpectedly, sucrose gradient centrifugation analysis showed that the treatment of splenic T cells with MCD did not change the distribution of any of the molecules tested, including GM1 (83%-99% in raft fractions as measured by densitometry) and Thy1 (100%). The Src family of protein tyrosine kinase p56lck, which was found in rafts isolated with 0.05%, but not with 1%, Triton X-100, remained in raft fractions with MCD treatment. CD45 was found to be outside of rafts regardless of Triton X-100 concentrations or MCD treatment.
Effects of MCD treatment on primary T cells. (A) Purified splenic T cells (> 98% CD3+) were treated with the indicated concentrations of MCD, and their adhesion to immobilized ICAM-1 was analyzed as in Figure 1A. Open bars represent no PMA stimulation, solid bars represent PMA-stimulated (50 ng/mL) cells, and gray bar represents PMA stimulation with anti–LFA-1 blocking antibody (TIB213). Cells treated with 10 mM MCD and 2 mM MnCl2 (10 + Mn) were included. BSA instead of ICAM-1 was used as a control. The results are representative of 4 independent experiments, each done in triplicate. Error bars indicate SD. (B) Purified splenic T cells treated (+) or not (-) with 10 mM MCD were stained for the indicated cell-surface molecules and analyzed by flow cytometer as in Figure 1B. (C) Splenic T cells were treated with (+) or without (-) 10 mM MCD, lysed with 1% or 0.05% Triton X-100, subjected to sucrose gradient centrifugation, and indicated molecules in each fraction were detected by Western blotting as in Figure 1D.
Effects of MCD treatment on primary T cells. (A) Purified splenic T cells (> 98% CD3+) were treated with the indicated concentrations of MCD, and their adhesion to immobilized ICAM-1 was analyzed as in Figure 1A. Open bars represent no PMA stimulation, solid bars represent PMA-stimulated (50 ng/mL) cells, and gray bar represents PMA stimulation with anti–LFA-1 blocking antibody (TIB213). Cells treated with 10 mM MCD and 2 mM MnCl2 (10 + Mn) were included. BSA instead of ICAM-1 was used as a control. The results are representative of 4 independent experiments, each done in triplicate. Error bars indicate SD. (B) Purified splenic T cells treated (+) or not (-) with 10 mM MCD were stained for the indicated cell-surface molecules and analyzed by flow cytometer as in Figure 1B. (C) Splenic T cells were treated with (+) or without (-) 10 mM MCD, lysed with 1% or 0.05% Triton X-100, subjected to sucrose gradient centrifugation, and indicated molecules in each fraction were detected by Western blotting as in Figure 1D.
Specificity of MCD treatment
The above results showed that LFA-1 on splenic T cells does not seem to associate with lipid rafts isolated with Triton X-100, and MCD does not seem to disrupt lipid rafts of splenic T cells. Nevertheless, MCD treatment profoundly inhibited LFA-1–mediated T-cell adhesion to ICAM-1. To determine whether the effects of MCD treatment were solely due to the removal of cholesterol from the membrane or are due to unrelated effects of MCD, we first confirmed that MCD indeed removed cholesterol from the membrane of T cells. Filipin III was used to stain cholesterol of splenic T cells. Filipin is a fluorescent polyene antibiotic from Saccharomyces filipinensis, and it forms a multimeric globular complex with cholesterol in the cell membrane.29 Flow cytometric analysis showed that treatment of primary T cells with MCD reduced the staining with filipin to approximately 50% of control cells (Figure 3A), indicating that MCD indeed removed cholesterol from the membrane of primary T cells.
Specificity of MCD treatment. (A) Splenic T cells were treated with 10 mM MCD (filled histogram) or not (open histogram with solid line), and cholesterol in the plasma membrane was stained with 12.5 μg/mL filipin and analyzed by flow cytometer. The open histogram with broken line shows autofluorescence of unstained splenic T cells. (B) Splenic T cells were treated with 10 mM MCD, and after washing away MCD, they were incubated with water-soluble cholesterol (60 μg/mL) at 37°C for 30 minutes for cholesterol reconstitution. The treated cells were analyzed for LFA-1–mediated adhesion to ICAM-1 as in Figure 1A. Open bar (□) indicates unstimulated cells; solid bar (▪), PMA stimulation; gray bar (▦), PMA stimulation and anti–LFA-1 antibody blocking; horizontally striped bar (▤), 10 mM MCD treatment after PMA stimulation; and diagonally striped bar (▨), PMA stimulation and MCD treatment followed by cholesterol reconstitution. Error bars indicate SD. (C) Splenic T cells were stimulated with (+) or without (-) PMA in the presence (+) or absence (-) of MCD and the phosphorylation of MAP kinase was analyzed by Western blotting using anti–phospho-ERK-1/2 antibody (top panel). The same blot was stripped and probed with anti–ERK-1/2 antibody to confirm equal loading of the samples (bottom panel).
Specificity of MCD treatment. (A) Splenic T cells were treated with 10 mM MCD (filled histogram) or not (open histogram with solid line), and cholesterol in the plasma membrane was stained with 12.5 μg/mL filipin and analyzed by flow cytometer. The open histogram with broken line shows autofluorescence of unstained splenic T cells. (B) Splenic T cells were treated with 10 mM MCD, and after washing away MCD, they were incubated with water-soluble cholesterol (60 μg/mL) at 37°C for 30 minutes for cholesterol reconstitution. The treated cells were analyzed for LFA-1–mediated adhesion to ICAM-1 as in Figure 1A. Open bar (□) indicates unstimulated cells; solid bar (▪), PMA stimulation; gray bar (▦), PMA stimulation and anti–LFA-1 antibody blocking; horizontally striped bar (▤), 10 mM MCD treatment after PMA stimulation; and diagonally striped bar (▨), PMA stimulation and MCD treatment followed by cholesterol reconstitution. Error bars indicate SD. (C) Splenic T cells were stimulated with (+) or without (-) PMA in the presence (+) or absence (-) of MCD and the phosphorylation of MAP kinase was analyzed by Western blotting using anti–phospho-ERK-1/2 antibody (top panel). The same blot was stripped and probed with anti–ERK-1/2 antibody to confirm equal loading of the samples (bottom panel).
If the inhibition of LFA-1 by MCD treatment was solely due to cholesterol depletion, it should be reversed by restoring the cholesterol level of the cell membrane.21 To test this, water-soluble cholesterol was added to MCD-treated splenic T cells, and whether the inhibition of LFA-1 by MCD could be reversed by cholesterol reconstitution was examined. As seen in Figure 3B, LFA-1–dependent adhesion of splenic T cells to ICAM-1, induced by PMA treatment, was strongly inhibited by 10 mM MCD. Addition of water-soluble cholesterol to MCD-treated splenic T cells effectively reversed the inhibitory effects of MCD. Similar results were also obtained with T-cell lines (data not shown).
To confirm that MCD does not inhibit the intracellular signaling induced by PMA, the effects of MCD on PMA-induced activation of the MAP kinase pathway were tested. Phosphorylation of ERK-1/2 was strongly induced by PMA treatment of splenic T cells and it was not affected by MCD treatment (Figure 3B), indicating that the inhibitory effects of MCD on PMA-induced LFA-1 activation was not due to inhibition of PMA-induced signaling pathways. Taken together, these results indicate that the inhibition of LFA-1–mediated T-cell adhesion by MCD is indeed due to depletion of cholesterol, not due to some other unrelated effects.
Filipin inhibits LFA-1 activation
Filipin, through its ability to form complexes with cholesterol, has been used to sequester cholesterol in the plasma membrane.16 We examined whether sequestering cholesterol in the plasma membrane by filipin treatment has similar effects on LFA-1 as those of cholesterol depletion by MCD treatment. Cell adhesion assays showed that PMA-induced LFA-1 activation on EL4 cells and splenic T cells was inhibited by filipin in a dose-dependent manner (Figure 4A). Inhibition of LFA-1 on EL4 cells required a 4-fold higher concentration of filipin (0.4 mg/mL versus 0.1 mg/mL) than that for primary T cells, presumably due to a higher cholesterol content of EL4 cells than primary T cells (Figure 5). In the range of 0.2 mg/mL to 0.4 mg/mL of filipin, the inhibition of LFA-1 was dose dependent (data not shown). Sucrose gradient centrifugation analysis of Triton X-100–solubilized EL4 cells showed that cholesterol sequestration by filipin disrupted lipid rafts, and LFA-1 and Thy1 in the low-density detergent-insoluble fractions were shifted to the high-density fractions (Figure 4B, left panel). With primary T cells, similar to MCD treatment, filipin treatment did not significantly change the distribution of any of the molecules tested (Figure 4B, right panel). These results indicate that the effects of cholesterol sequestering by filipin are similar to those of cholesterol depletion by MCD. In both cases, lipid rafts are disrupted only in T-cell lines and not in primary T cells, and yet LFA-1 activation is inhibited in both cell types.
Effects of filipin on LFA-1. (A) EL4 cells and primary T cells were incubated with (+) or without (-) PMA in the presence of the indicated amount of filipin, and their adhesion to immobilized ICAM-1 in the presence (+) or absence (-) of blocking anti–LFA-1 antibody was assessed. Error bars indicate SD. (B) EL4 cells (left panel) and splenic T cells (right panel) were treated with (+) or without (-) 0.4 mg/mL or 0.1 mg/mL filipin, respectively, lysed with 0.05% Triton X-100, subjected to sucrose gradient centrifugation, and indicated molecules in the sucrose gradient were detected by Western blotting. The numbers indicate fractions starting from the low-density fraction.
Effects of filipin on LFA-1. (A) EL4 cells and primary T cells were incubated with (+) or without (-) PMA in the presence of the indicated amount of filipin, and their adhesion to immobilized ICAM-1 in the presence (+) or absence (-) of blocking anti–LFA-1 antibody was assessed. Error bars indicate SD. (B) EL4 cells (left panel) and splenic T cells (right panel) were treated with (+) or without (-) 0.4 mg/mL or 0.1 mg/mL filipin, respectively, lysed with 0.05% Triton X-100, subjected to sucrose gradient centrifugation, and indicated molecules in the sucrose gradient were detected by Western blotting. The numbers indicate fractions starting from the low-density fraction.
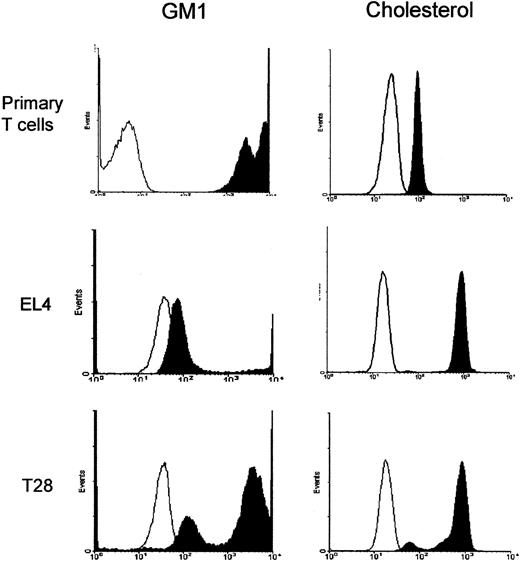
Comparison of levels of GM1 and cholesterol in the plasma membrane of splenic T cells and T-cell lines. Splenic T cells, EL4, and T28 cell lines were stained with FITC-CTxB that binds to GM1 or with filipin (12.5 μg/mL) that binds to cholesterol and analyzed by flow cytometer. The machine setting for the fluorescence detection was the same for all the cell types to allow direct comparison of the expression levels. Open histograms show fluorescence of unstained cells and filled histograms show those of stained cells. The results are representative of 3 independent experiments.
Comparison of levels of GM1 and cholesterol in the plasma membrane of splenic T cells and T-cell lines. Splenic T cells, EL4, and T28 cell lines were stained with FITC-CTxB that binds to GM1 or with filipin (12.5 μg/mL) that binds to cholesterol and analyzed by flow cytometer. The machine setting for the fluorescence detection was the same for all the cell types to allow direct comparison of the expression levels. Open histograms show fluorescence of unstained cells and filled histograms show those of stained cells. The results are representative of 3 independent experiments.
Lipid rafts of T-cell lines and primary T cells are different
The levels of ganglioside GM1 and cholesterol of the plasma membrane of primary T cells and T-cell lines were directly compared. GM1 was stained with FITC-CTxB, whereas cholesterol was stained with filipin. Flow cytometry analysis of the stained cells showed that splenic T cells are very rich in GM1 and have an approximately 10- to 100-fold higher level of GM1 than T28 or EL4 cells (Figure 5). On the other hand, the level of cholesterol in the plasma membrane of splenic T cells is nearly 10-fold lower than those of the T-cell lines.
LFA-1 in primary T cells is found in Brij 35–insoluble and MCD-sensitive lipid rafts
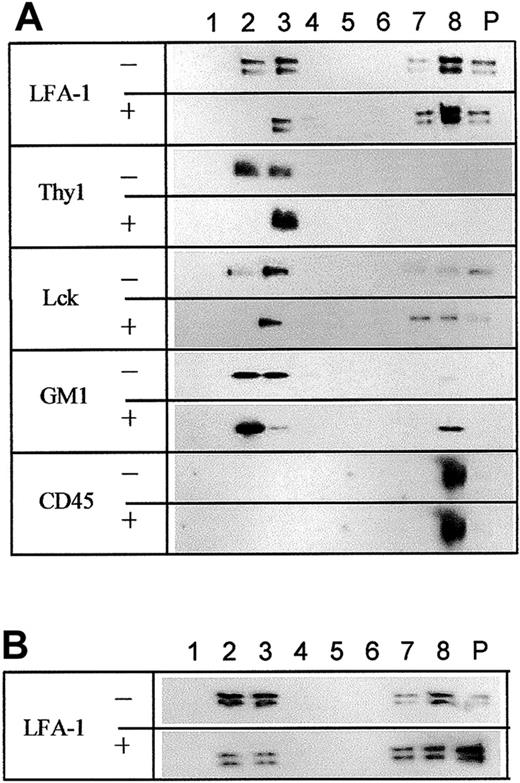
The above results suggest that lipid rafts of primary T cells may significantly differ from those of T-cell lines, and it is possible that LFA-1 on primary T cells may localize in lipid rafts that are soluble in cold 0.05% Triton X-100, whereas lipid rafts of T-cell lines containing LFA-1 are rich in cholesterol and insoluble in Triton X-100. Therefore, we tested other nonionic detergents. Results with CHAPS, Brij 99, Brij 58, and Brij 56 were the same as that with Triton X-100 (data not shown). In contrast, a significant amount (48%-65% as measured by densitometer) of LFA-1 was detected in the low-density fractions of sucrose gradient centrifugation when 1% Brij 35 was used to solubilize primary T cells (Figure 6A, row 1), as confirmed in 6 independent experiments. The low-density fractions also contained GM1, Thy1, and Lck but not CD45. Furthermore, upon treatment with 10 mM MCD to extract cholesterol, almost 50% of LFA-1 in the low-density fractions was shifted to the high-density fractions (Figure 6A, row 2). This result was confirmed in 3 independent experiments. Similarly, MCD treatment reduced GM1 in the low-density fractions by approximately 10% with a corresponding increase in the high-density fractions (Figure 6A, rows 7-8). However, the distribution of Thy1 and Lck was not significantly disrupted by MCD treatment (Figure 6A, rows 3-6). CD45 was found outside rafts regardless of MCD treatment (Figure 6A, rows 9-10). Filipin treatment shifted LFA-1 not only to soluble fractions but also to the pellet (Figure 6B), possibly because of the formation of a multimeric complex of filipin–cholesterol–LFA-1. These results suggest that some LFA-1 on primary T cells indeed localizes in lipid rafts, and cholesterol depletion with MCD or sequestration by filipin disrupts those containing LFA-1. However, lipid rafts containing LFA-1 seem to be different from those containing Thy1 or Lck. The former are disrupted by MCD treatment, whereas the latter are resistant. Experiments using Brij 35 at a concentration of 0.05% resulted in incomplete lysis of cells, making results hard to interpret.
Detection of LFA-1 in Brij 35–insoluble, MCD- and filipin-sensitive lipid rafts of primary T cells. (A) Primary T cells, untreated (-) or treated (+) with 10 mM MCD, were solubilized with 1% Brij 35 and subjected to sucrose gradient centrifugation. Fractions were blotted with corresponding antibodies. (B) Primary T cells were either untreated (-) or treated (+) with 0.1 mg/mL filipin, lysed with 1% Brij 35, subjected to sucrose gradient centrifugation, and fractions were probed with anti-CD18 (LFA-1). The results are representative of 3 independent experiments.
Detection of LFA-1 in Brij 35–insoluble, MCD- and filipin-sensitive lipid rafts of primary T cells. (A) Primary T cells, untreated (-) or treated (+) with 10 mM MCD, were solubilized with 1% Brij 35 and subjected to sucrose gradient centrifugation. Fractions were blotted with corresponding antibodies. (B) Primary T cells were either untreated (-) or treated (+) with 0.1 mg/mL filipin, lysed with 1% Brij 35, subjected to sucrose gradient centrifugation, and fractions were probed with anti-CD18 (LFA-1). The results are representative of 3 independent experiments.
LFA-1 and cholesterol on primary T cells cocap
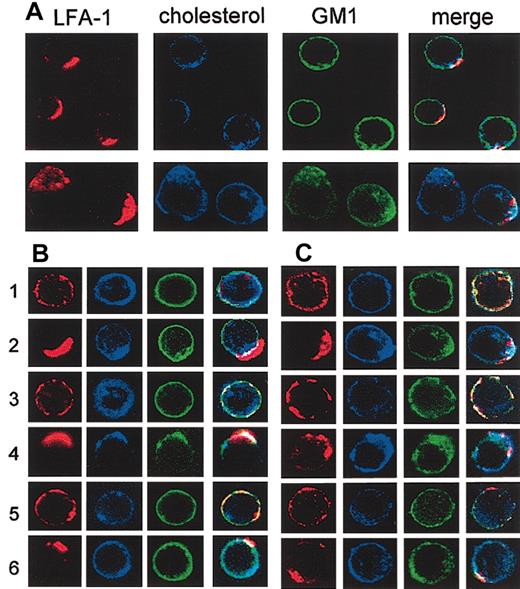
To further compare lipid rafts of T-cell lines and primary T cells, we examined the codistribution of LFA-1, cholesterol, and GM1 by confocal microscopy. LFA-1 on primary T cells and T28 cells was cross-linked to induce capping of LFA-1, and the cells were triple stained for LFA-1 (red), cholesterol (blue), and ganglioside GM1 (green). When capping of LFA-1 on primary T cells was induced (Figure 7A, upper row; and 7B, row 2), cholesterol cocapped with LFA-1, whereas GM1 was more evenly distributed on the cell surface, suggesting that LFA-1 localizes in cholesterol-rich, but GM1-poor, rafts. However, cholesterol-rich lipid rafts containing LFA-1 were not totally devoid of GM1 as some GM1 colocalized with LFA-1, resulting in yellow-white caps on the merged images. The results shown are representative of 3 independent experiments, in which 92 ± 7% (n = 26) of capped cells demonstrated similar features. When the capping of the GPI-anchored protein Thy1 on primary T cells and T28 cells was induced, both GM1 and most, but not all, cholesterol cocapped with Thy1 (Figure 7B-C, row 4), suggesting that Thy1 localizes in lipid rafts that are different from those containing LFA-1 (“Discussion”). The results were representative of 3 independent experiments in which all of the cells have similar features (n = 9). CD45 was used as a control protein that does not localize in lipid rafts. As expected, capping of CD45 did not induce changes in the distribution of cholesterol or GM1 (Figure 7B, row 6) in any (n = 9) of the cells examined.
Confocal microscopic analysis of cocapping of LFA-1 and cholesterol. (A) Splenic T cells (upper row) and T28 cells (lower row) were stained with anti–LFA-1 and Alexa Fluor 568–conjugated goat anti-rat secondary antibody (red) and incubated at 37°C for 30 minutes to induce capping of LFA-1. The cells were then stained at 4°C for cholesterol with filipin (blue) and for GM1 with FITC-CTxB (green), fixed with formaldehyde, and analyzed by confocal microscopy. Images of midlevel sections of the cells are shown. Merge images are shown in the right-most columns. Results are representatives of multiple cells in 3 independent experiments. (B) Primary T cells were either untreated (rows 1, 3, and 5) or subjected to antibody cross-linking to induce capping of LFA-1 (row 2), Thy1 (row 4), or CD45 (row 6). The cells were then stained in 3 colors for cholesterol (blue), GM1 (green), and either LFA-1, Thy1, or CD45 (red) and analyzed by confocal microscopy as in panel A. (C) The T28 cell line was analyzed as in panel B. The images were captured with a × 60 objective lens, and the final magnifications in the figure are approximately × 1000 for primary T cells and × 500 for T28.
Confocal microscopic analysis of cocapping of LFA-1 and cholesterol. (A) Splenic T cells (upper row) and T28 cells (lower row) were stained with anti–LFA-1 and Alexa Fluor 568–conjugated goat anti-rat secondary antibody (red) and incubated at 37°C for 30 minutes to induce capping of LFA-1. The cells were then stained at 4°C for cholesterol with filipin (blue) and for GM1 with FITC-CTxB (green), fixed with formaldehyde, and analyzed by confocal microscopy. Images of midlevel sections of the cells are shown. Merge images are shown in the right-most columns. Results are representatives of multiple cells in 3 independent experiments. (B) Primary T cells were either untreated (rows 1, 3, and 5) or subjected to antibody cross-linking to induce capping of LFA-1 (row 2), Thy1 (row 4), or CD45 (row 6). The cells were then stained in 3 colors for cholesterol (blue), GM1 (green), and either LFA-1, Thy1, or CD45 (red) and analyzed by confocal microscopy as in panel A. (C) The T28 cell line was analyzed as in panel B. The images were captured with a × 60 objective lens, and the final magnifications in the figure are approximately × 1000 for primary T cells and × 500 for T28.
With T28 cells, capping of LFA-1 resulted in cocapping of both GM1 and cholesterol (Figure 7C, row 2), indicating that lipid rafts of T28 cells containing LFA-1 are rich in both. The results are representatives of 3 independent experiments, in which 94 ± 9% (n = 17) of the cells showed similar features. Thy1 similarly cocapped with both GM1 and cholesterol (Figure 7C, row 4), whereas no such cocapping was seen with CD45 (row 6). Confocal microscopic analysis of EL4 cells was difficult because of a very low level of GM1 on the surface.
Discussion
We have shown here that cholesterol is important for the activation of leukocyte integrin LFA-1. Cholesterol depletion or sequestration by MCD or filipin treatment, respectively, results in profound inhibition of LFA-1–mediated adhesion of T-cell lines and primary T cells to ICAM-1. The inhibition of LFA-1 by MCD and filipin does not seem to be due to their nonspecific effects. Pizzo et al32 reported that MCD treatment of the human T-cell line Jurkat inhibits Ca2+ release from the intracellular storage. However, the inhibitory effects of MCD treatment in our study were reversed by cholesterol reconstitution, and MCD treatment did not inhibit PMA-induced MAP kinase phosphorylation. Therefore, the inhibition of LFA-1 by MCD is most probably due to depletion of cholesterol, not due to unrelated nonspecific effects.
It is generally believed that cholesterol depletion/sequestration disrupts lipid rafts in general.16 However, our current results showed that lipid rafts of T cells are heterogeneous and not all of them are disrupted by cholesterol depletion. Those isolated from primary T cells with Triton X-100 are not disrupted by MCD or filipin treatment. In contrast, lipid rafts isolated from T-cell lines by the same procedure are sensitive to MCD or filipin. Furthermore, LFA-1 is detected in lipid rafts isolated with 0.05% Triton X-100 for T-cell lines or with 1% Brij 35 for primary T cells. Importantly, cholesterol depletion with MCD disrupted lipid rafts that contain LFA-1 in both cases. Thus, it seems likely that the inhibition of LFA-1–mediated T-cell adhesion to ICAM-1 by MCD or filipin treatment is due to disruption of lipid rafts containing LFA-1. However, it should be noted that a substantial portion of LFA-1 on primary T cells is found in nonraft fractions, and MCD treatment reduces the amount of LFA-1 in lipid rafts and cholesterol in the plasma membrane only by about 50%. Nevertheless, the same MCD treatment almost completely inhibits LFA-1–mediated T-cell adhesion. It is possible that the density of LFA-1 or cholesterol in lipid rafts may have to exceed a critical threshold for the activation of LFA-1, and the treatment with MCD may lower the levels of LFA-1 and/or cholesterol in lipid rafts below the threshold. The precise role of lipid rafts in the regulation of LFA-1 remains to be determined. It is possible that lipid rafts may fuse to form larger rafts upon cell activation and induce clustering of LFA-1, which enhances avidity of LFA-1. Cholesterol may mediate lipid raft association of LFA-1, and dissociating LFA-1 from rafts may impair its ability to cluster and hence disturb its avidity.
Our results have demonstrated significant differences between primary T cells and T-cell lines. Primary T cells are rich in GM1 but have only a small amount of cholesterol, whereas T-cell lines have high levels of cholesterol and a relatively low amount of GM1. Tuosto et al have reported that GM1 in resting human peripheral blood T cells is stored in the intracellular compartment and mobilized to the cell surface upon cell activation.33 Therefore, we tested the level of GM1 in murine splenic and peripheral blood T cells by cell-surface staining of intact cells as well as intracellular staining of permeabilized and fixed cells with FITC-CTxB. Confocal microscopy of the unfixed cells showed that both splenic and peripheral blood T cells expressed a high level of GM1 on the cell surface, and the expression level did not change upon activation with concanavalin A. When the cells were fixed with paraformaldehyde and permeabilized with Triton X as described by Tuosto et al,33 all of the cell-surface staining was lost, leaving only weak intracellular staining (results not shown). Therefore, we were unable to properly examine intracellular GM1. Lipid rafts of primary T cells seem to be rather heterogeneous with respect to their lipid and protein compositions. Most of them seem to consist mainly of GM1 and perhaps only a small amount of cholesterol. They are insoluble in Triton X-100 or Brij 35 and are resistant to cholesterol depletion because of low cholesterol content, and they contain Thy1 and Lck but not LFA-1. However, some lipid rafts of primary T cells seem to contain a higher amount of cholesterol and only a small amount of GM1. These cholesterol-rich lipid rafts contain LFA-1 but not Thy1 or Lck, and they are sensitive to cholesterol depletion. They are also insoluble in Brij 35 but not other detergents tested in our study, including Triton X-100, CHAPS, Brij 99, Brij 98, Brij 58, and Brij 56. On the other hand, lipid rafts of T-cell lines seem to have a higher amount of cholesterol and substantially less GM1 and they contain LFA-1, Thy1, and Lck. They are mostly insoluble in Triton X-100 and sensitive to MCD or filipin. Sphingolipids have long saturated acyl chains and are thought to be critical for the formation of a liquid-ordered phase of lipid rafts.31,34,35 Ganglioside-rich membrane microdomains are not disrupted by MCD treatment.36 The existence of a glycosphingolipid core rich in ganglioside GM1 and GPI-anchored proteins and resistant to Triton X-100 treatment has also been suggested.37,38 Our finding that the membranes of primary T cells are rich in GM1 and resistant to cholesterol depletion is consistent with this idea. In contrast to primary T cells, T-cell lines have high levels of cholesterol, and their lipid rafts are susceptible to disruption by MCD or filipin treatment. Results from confocal microscopy also support the idea of the existence of heterogeneous lipid rafts in primary T cells. LFA-1 cocaps with cholesterol but not with GM1 suggesting that LFA-1 is associated with cholesterol-rich, but low in GM1, lipid rafts. GPI-anchored Thy1 cocaps with both GM-1 and cholesterol, suggesting that Thy1 localizes in lipid rafts containing both.
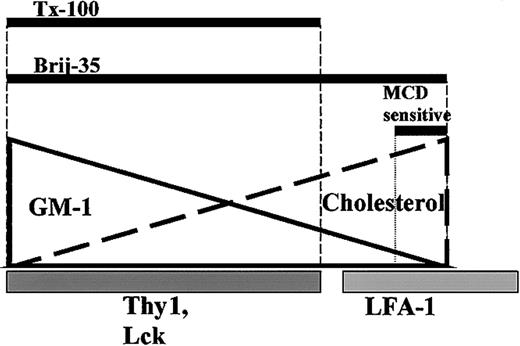
Based on our current results, we propose a model for lipid raft heterogeneity in primary T cells (Figure 8), where different rafts are not distinctly separated, but rather exist as a continuous spectrum, ranging from those consisting of mostly GM1 to those of mostly cholesterol. The range of lipid rafts that remains cold detergent–insoluble depends on the detergent. A much wider range of lipid rafts seems to be insoluble in Brij 35 than in Triton X-100 or other detergents. This model explains why cholesterol, but not most GM1, cocaps with LFA-1, whereas some, but not all, cholesterol and GM1 cocaps with Thy1. The model predicts that the sensitivity of lipid rafts to MCD treatment depends on the cholesterol content of the rafts. In primary T cells, which have a relatively low level of cholesterol, only those with the highest level of cholesterol are MCD sensitive, and LFA-1 seems to be associated with these lipid rafts.
A model for lipid raft heterogeneity in primary T cells. The solid line represents GM1 content of lipid rafts, and the thick dashed line represents cholesterol content. Thy1 and Lck localize in GM1-rich lipid rafts (darker gray bar), while LFA-1 partially localizes in cholesterol-rich rafts (lighter gray bar). Thick black bars represent lipid raft insolubility in Triton X-100 (1% and 0.05%) and Brij 35 (1%). MCD sensitive bar shows the portion of lipid rafts that is insoluble in 1% Brij 35 and sensitive to MCD treatment.
A model for lipid raft heterogeneity in primary T cells. The solid line represents GM1 content of lipid rafts, and the thick dashed line represents cholesterol content. Thy1 and Lck localize in GM1-rich lipid rafts (darker gray bar), while LFA-1 partially localizes in cholesterol-rich rafts (lighter gray bar). Thick black bars represent lipid raft insolubility in Triton X-100 (1% and 0.05%) and Brij 35 (1%). MCD sensitive bar shows the portion of lipid rafts that is insoluble in 1% Brij 35 and sensitive to MCD treatment.
The role of lipid rafts in LFA-1 regulation has been controversial. Krauss and Altevogt reported that LFA-1 on mouse thymocytes is found in lipid rafts isolated with 1% Triton X-100.22 However, we failed to detect a significant amount of LFA-1 in lipid rafts isolated from murine thymocytes with 1% or 0.05% Triton X-100 (data not shown). It is possible that only a very small proportion of total LFA-1 on thymocytes is in lipid rafts. Leitinger and Hogg reported that LFA-1 transfected into the human T-cell line Jurkat did not localize in lipid rafts unless it was activated by Mn2+ or its I-domain was deleted.23 As shown in our current study, T-cell lines and primary T cells significantly differ in lipid rafts, and findings with T-cell lines do not necessarily apply to primary T cells. Shamri et al reported that MCD did not inhibit LFA-1–mediated adhesion of chemokine-stimulated human peripheral blood T cells to ICAM-1,24 whereas MCD strongly inhibited LFA-1 activation with PMA in our study. This may be due to differences in the inside-out signaling pathways involved in PMA- and chemokine-induced activation of LFA-1.
Redistribution of LFA-1 on the surface of T cells is thought to be important for the formation of immunologic synapse between T cells and antigen-presenting cells.4 LFA-1 on T cells has been shown to initially form a cluster in the center of a T-cell/antigen-presenting cell contact point and subsequently move to the periphery, forming a ring around the central core of the TCR cluster.5,6 Since many intracellular signaling molecules involved in TCR-mediated signaling are thought to associate with lipid rafts, it is speculated that lipid rafts may play an active role in the formation of immunologic synapse. Our results suggest that in primary T cells, LFA-1 and Lck may associate with different subsets of lipid rafts. This may explain why in immunologic synapse formation LFA-1 is found in the periphery supramolecular activation complexes (SMAC), while TCRs and Lck are in the center SMAC.5,6 Recently it has been demonstrated that GM1 accumulates in the center SMAC and it is resistant to MCD treatment, whereas peripheral SMAC, which contains LFA-1, seems to be sensitive to MCD treatment.39 These results further supported the idea of lipid raft heterogeneity and its involvement in immunologic synapse. Taken together with our data, we predict that cholesterol accumulates in peripheral SMAC. The relationship between distributions of LFA-1, cholesterol, lipid rafts, and immunologic synapse requires further investigation. The results presented in this report have important implications to the studies on lipid rafts of T cells in general. We have demonstrated that lipid rafts of primary T cells are quite different from those of T-cell lines. Therefore, many studies on lipid rafts using T-cell lines will have to be re-examined with primary T cells.
Prepublished online as Blood First Edition Paper, March 13, 2003; DOI 10.1182/blood-2002-10-3195.
Supported by a grant from the Canadian Institute of Health Research.
M.R.M. and J.R.-L. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank StemCell Technologies (STI) for providing us with the T-cell purification kit, Spin Sep, and technical advice; Dr Motoi Maeda for helpful discussion; and Ryan Russell for computer work.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal