Abstract
There is increasing evidence that γδ T cells have potent innate antitumor activity. We described previously that synthetic aminobisphosphonates are potent γδ T cell stimulatory compounds that induce cytokine secretion (ie, interferon γ [IFN-γ]) and cell-mediated cytotoxicity against lymphoma and myeloma cell lines in vitro. To evaluate the antitumor activity of γδ T cells in vivo, we initiated a pilot study of low-dose interleukin 2 (IL-2) in combination with pamidronate in 19 patients with relapsed/refractory low-grade non-Hodgkin lymphoma (NHL) or multiple myeloma (MM). The objectives of this trial were to determine toxicity, the most effective dose for in vivo activation/proliferation of γδ T cells, and antilymphoma efficacy of the combination of pamidronate and IL-2. The first 10 patients (cohort A) who entered the study received 90 mg pamidronate intravenously on day 1 followed by increasing dose levels of continuous 24-hour intravenous (IV) infusions of IL-2 (0.25 to 3 × 106 IU/m2) from day 3 to day 8. Even at the highest IL-2 dose level in vivo, γδ T-cell activation/proliferation and response to treatment were disappointing with only 1 patient achieving stable disease. Therefore, the next 9 patients were selected by positive in vitro proliferation of γδ T cells in response to pamidronate/IL-2 and received a modified treatment schedule (6-hour bolus IV IL-2 infusions from day 1-6). In this patient group (cohort B), significant in vivo activation/proliferation of γδ T cells was observed in 5 patients (55%), and objective responses (PR) were achieved in 3 patients (33%). Only patients with significant in vivo proliferation of γδ T cells responded to treatment, indicating that γδ T cells might contribute to this antilymphoma effect. Overall, administration of pamidronate and low-dose IL-2 was well tolerated. In conclusion, this clinical trial demonstrates, for the first time, that γδ T-cell–mediated immunotherapy is feasible and can induce objective tumor responses. (Blood. 2003;102:200-206)
Introduction
Despite significant improvement in the treatment of low-grade non-Hodgkin lymphoma (NHL) and multiple myeloma (MM), most patients relapse or become resistant to conventional treatment strategies such as chemotherapy or radiation. Therefore, there is need for alternative tumor therapies. One possibility is manipulating the immune system to target and eliminate neoplastic cells.
Most current immunotherapeutic approaches aim at inducing antitumor response via stimulation of the adaptive immune system, which is dependent on major histocompatibility complex (MHC)–restricted αβ T cells. Despite major advances in our understanding of the adaptive immunity toward tumors and the introduction of vaccine-based strategies, durable responses are rare, and active immunotherapy is still not an established treatment modality. Adaptive immunotherapeutic approaches have several disadvantages: αβ T cells need specific tumor-associated antigens (TAAs) and appropriate costimulatory molecules for activation. Failure or loss of TAAs, MHC molecules, and/or costimulatory molecules renders tumor cells resistant to αβ T-cell–mediated cytotoxicity or induces anergy of specific T cells.1
Mice deficient in innate effector cells such as natural killer (NK) cells, NK T cells, or γδ T cells show a significantly increased incidence of tumors and provide clear evidence for an immune surveillance function of the innate immune system.2-4 Recognition of transformed cells by the innate immune system seems to be dependent on expression of stress-induced ligands and/or loss of MHC class I molecules on tumor cells.5 Several studies have demonstrated a role for human γδ T cells in recognition of transformed cells.6,7 γδ T cells exhibit a potent MHC-unrestricted lytic activity against different tumor cells in vitro.8-10 In addition, γδ T cells have been found with increased frequency in disease-free survivors of acute leukemia following allogeneic bone marrow transplantation.11 Adoptive transfer of ex vivo–expanded human γδ T cells in a mouse tumor model further supports the in vivo antitumor effects of γδ T cells.12
Vγ9Vδ2 T cells, which represent most of the human circulating γδ T cells, recognize small nonpeptide compounds with an essential phosphate residue (ie, microbial metabolites) or alkylamines.13-17 As we have shown previously, also synthetic aminobisphosphonates such as pamidronate are potent γδ T-cell–stimulatory compounds.18 In addition, we could demonstrate that pamidronate-activated γδ T cells produce cytokines (ie, interferon γ [IFN-γ]), exhibit specific cytotoxicity against lymphoma or myeloma cell lines, and lead to reduced survival of autologous myeloma cells.8 It was confirmed that Vγ9Vδ2 T cells recognize and kill a broad spectrum of B-cell lymphomas in vitro.9 Furthermore, pamidronate enhances recognition of many other tumor cell lines by γδ T lymphocytes.10 For an immunotherapeutic application it is important that γδ T cells have the potential for polyclonal expansion without prior priming.19 An immunotherapeutic approach of inducing antitumor response via stimulation of γδ T cells in vivo has not been performed in humans so far.
The aim of this pilot study is to evaluate the feasibility of activation and/or expansion of γδ T cells in vivo using the combination of pamidronate and interleukin 2 (IL-2) in patients with refractory/relapsed lymphoma or myeloma, to determine the most effective IL-2 dose, to assess the toxicity of this regimen, and to evaluate its ability to exert antitumor effects.
Patients, materials, and methods
Patients
Adults suffering from low-grade B-cell lymphoma (NHL) or MM, refractory or relapsing after salvage therapy, were entered on the protocol. All patients signed an informed consent according to guidelines of the local ethics committee. Eligibility criteria required (1) an Eastern Cooperative Oncology Group (ECOG) score of less than 3 and (2) no severe impairment of cardiac, renal, or hepatic function. Pretreatment evaluation and follow-up studies included a history and physical examination, complete blood counts, extensive immunologic monitoring by fluorescence-activated cell sorter (FACS) analysis of peripheral blood lymphocytes, computed tomography (CT) scans, ultrasound, positron emission tomography (PET) scans, or bone marrow biopsy whichever was appropriate.
Response criteria
Complete remission required disappearance of all lymphoma manifestations for at least 4 weeks. Partial remission was defined as 50% reduction or more of all measurable lymphoma manifestations for at least 4 weeks. In addition, no single manifestation should have shown enlargement of 25% or more in size, and no new lesions should have appeared during that period. Stable disease meant less than 50% reduction or no measurable change in lymphoma manifestations was present. Progressive disease (PD) was defined as increase of frequency and severity of disease-associated symptoms, occurrence of new nodal or extranodal lesions, increase of preexisting lymphoma manifestations by more than 25%, or any combination. Adverse events were assessed according to World Health Organization (WHO) criteria.
γδ T-cell proliferation assay
γδ T-cell proliferation assay was described previously.8 Briefly, 5 × 104 peripheral blood mononuclear cells (PBMCs) were cultivated in triplicate in 100 μL RPMI 1640 medium per well (Invitrogen, Karlsruhe, Germany), 10% pooled human AB serum, and 100 IU/mL IL-2 in round-bottom microtiter plates (Nunc, Wiesbaden, Germany). For determination of γδ T-cell activity, pamidronate (Novartis, Nuernberg, Germany) was added in concentrations between 1 μM and 100 μM on day 0. Cells were harvested on day 7 and were double or triple stained with fluorescein isothiocyanate (FITC)–or phycoerythrin (PE)–conjugated monoclonal CD69, HLA-DR, CD3, CD56, TCR pan αβ, TCR pan γδ, TCR Vγ9, TCR Vδ2 (Coulter-Immunotech, Krefeld, Germany), or TCR Vδ1 (T Cell Diagnostics, Woburn, MA) antibodies. For NK cells (CD3-CD56+) triple staining was performed using allophycocyanin (APC)–labeled CD3 antibodies. Cells (5 × 103) from each sample were analyzed using a FACScan supported with Cellquest as acquisition and data analysis software (Becton Dickinson, Heidelberg, Germany). The lymphocytes were gated using forward/sideward scatter analysis.
Increase of γδ T cells was calculated by counting the number of viable cells per well and by cytofluorimetric identification of γδ T cells using FACS analysis on day 7 of culture. The stimulation index was determined according to the following calculation: (γδ T-cell number in pamidronate/IL-2 culture) - (γδ T-cell number in medium/IL-2)/(γδ T-cell number in medium/IL-2) × 100. A stimulation index greater than 2 was considered a significant increase of γδ T cells. More than 5% of γδ T cells in PBMC culture on day 7 were required to avoid nonproportional increases at lower numbers.
FACS analysis of peripheral blood
Blood samples of patients were collected before each treatment course and on days 2 or 3, and 7 after start of treatment. PBMCs were stained with FITC-, PE-, or APC-conjugated antibodies and analyzed by flow cytometry as described earlier. For analysis of activation markers, CD69 was determined on day 2 or 3, HLA-DR on day 7 after infusion. Results represent percentage increase of antigen-expressing cells analyzed by double or triple staining (NK cells) according to the following calculation: (antigen expressing cells after pamidronate/IL-2 infusion) - (antigen-expressing cells before treatment)/(antigen-expressing cells before treatment) × 100. For a significant increase, more than 5% antigen-expressing cells were required to avoid unspecific staining and nonproportional increases at lower numbers.
For analysis of lymphocyte subset (αβ T cell, NK cell, γδ T cell) proliferation, absolute numbers of each lymphocyte subset before and on day 7 after treatment with pamidronate/IL-2 were counted. Results were shown as increase in percentage (%) according to the following calculation: (lymphocyte subset number on day 7 after pamidronate/IL-2 infusion) - (lymphocyte subset number before treatment)/(lymphocyte subset number before treatment) × 100. For significant increase, more than 1% (and > 10/μL) of cells of a lymphocyte subset on day 7 were required to avoid unspecific staining and nonproportional increases at lower numbers.
Cytokine assays
IFN-γ, tumor necrosis factor α (TNF-α), and IL-4 concentrations in serum were determined, using commercial enzyme-linked immunosorbent assay (ELISA) systems from Pharmingen (San Diego, CA). Sera were collected before and on day 1 or 2 after therapy and stored at -80°C until analysis.
Statistical analysis
For statistical comparison Fisher exact test was performed. P < .05 was considered statistically significant.
Results
Nineteen patients, with a median age of 61 years (range, 36-83 years), were enrolled in this pilot study (Table 1). Eleven patients (58%) had a diagnosis of low-grade B-NHL (4 follicle center lymphoma [FCL], 4 chronic lymphocytic leukemia [CLL], 2 mantle zone lymphoma [MZL], 1 immunocytoma [IC]), and 8 patients had a diagnosis of MM (42%). The majority of patients had advanced disease and received pamidronate/IL-2 as their second or subsequent salvage therapy (74%). Only patients with progressive disease were eligible for inclusion in the study.
Patient characteristics and response to therapy
. | . | . | . | . | . | . | γδ T-cell Proliferation∥ . | . | . | |
|---|---|---|---|---|---|---|---|---|---|---|
| Patient . | Age, y/sex . | Diagnosis/stage* . | Prior therapy (no. of cycles)† . | Off therapy, mo‡ . | IL-2 dose level (no. of cycles) . | Side effects§ . | In vitro . | In vivo . | Response¶ . | |
| Cohort A | ||||||||||
| 1A | 83/M | MM/III | MP (15) | 2 | 0.25-0.5 × 106 IU/m2 (2) | − | − | − | PD | |
| 2A | 79/M | CLL/IV | CLB (10) | 11 | 0.25-3 × 106 IU/m2 (6) | F(1), T(3) | +♯ | − | SD | |
| 3A | 67/F | CLL/IV | PM (10), CLB (5) | 2 | 0.25-0.5 × 106 IU/m2 (2) | I(1), F(1), T(2) | − | − | PD | |
| 4A | 57/M | IC/IV | CLB/P (24), LR (30 Gy) | 14 | 0.5 × 106 IU/m2 (1) | T(2) | − | − | PD | |
| 5A | 76/F | MM/III | MP (9) | 10 | 0.5 × 106 IU/m2 (1) | I(2), F(2), T(2) | − | ND | NE | |
| 6A | 63/F | CLL/IV | CLB/P (25), MCP (6), F (12), R (4) | 5 | 0.5 × 106 IU/m2 (1) | F(2), I(2) | ND | − | PD | |
| 7A | 68/M | CLL/IV | CLB/P (4), COP (6), CLB (12) | 2 | 1-2 × 106 IU/m2 (2) | I(2), F(2), T(3) | ND | − | PD | |
| 8A | 66/M | MM/III | MP (15), VID (6) | 2 | 1 × 106 IU/m2 (1) | − | ND | − | PD | |
| 9A | 58/M | MZL/III | MCP (6), TBI/CY + PBSCT (1) | 29 | 1 × 106 IU/m2 (1) | F(2), T(2) | ND | − | PD | |
| 10A | 79/F | MM/III | CHOP (2), LR (50 Gy) | 10 | 2-3 × 106 IU/m2 (2) | F(1), T(2) | ND | − | PD | |
| Cohort B | ||||||||||
| 1B | 73/M | MM/III | MP (2), VID (6) | 3 | 0.25 × 106 IU/m2 (1) | − | + | − | PD | |
| 2B | 59/M | MM/III | MP (16), VID (6), HD-M + PBSCT (1), IFN | 2 | 0.25 × 106 IU/m2 (2) | T(2) | + | − | PD | |
| 3B | 52/M | FCL/IV | MCP (4), CHOP (2), Dexa-BEAM (2), IFN, LR (44 Gy), I131-R | 4 | 0.25 × 106 IU/m2 (1) | F(1), T(2) | + + | − | PD | |
| 4B | 43/F | FCL/III | TNI (49 Gy) | 24 | 0.5-1 × 106 IU/m2 (4) | F(2), T(2) | + + + | + + + | SD | |
| 5B | 57/M | MM/II | VID (4) | 2 | 0.5-2 × 106 IU/m2 (9) | F(1), T(2) | + + + | + | PR | |
| 6B | 36/F | MM/II | LR (30 Gy) | 3 | 0.5 × 106 IU/m2 (1) | F(1), S(1) | + | + + | PD | |
| 7B | 46/M | MZL/IV | TNI (44 Gy) | 29 | 1 × 106 IU/m2 (4) | F(2), T(2) | + + + | − | SD | |
| 8B | 51/F | FCL/IV | COP (10), LR (30 Gy), MCP (4) | 48 | 2 × 106 IU/m2 (4) | F(1), T(2), S(2) | + + + | + | PR | |
| 9B | 55/M | FCL/III | CHOP (4), Dexa-BEAM (2), TBI/CY + PBSCT (1), LR (30 Gy) | 6 | 1-2 × 106 IU/m2 (8) | F(1) | + | + | PR | |
. | . | . | . | . | . | . | γδ T-cell Proliferation∥ . | . | . | |
|---|---|---|---|---|---|---|---|---|---|---|
| Patient . | Age, y/sex . | Diagnosis/stage* . | Prior therapy (no. of cycles)† . | Off therapy, mo‡ . | IL-2 dose level (no. of cycles) . | Side effects§ . | In vitro . | In vivo . | Response¶ . | |
| Cohort A | ||||||||||
| 1A | 83/M | MM/III | MP (15) | 2 | 0.25-0.5 × 106 IU/m2 (2) | − | − | − | PD | |
| 2A | 79/M | CLL/IV | CLB (10) | 11 | 0.25-3 × 106 IU/m2 (6) | F(1), T(3) | +♯ | − | SD | |
| 3A | 67/F | CLL/IV | PM (10), CLB (5) | 2 | 0.25-0.5 × 106 IU/m2 (2) | I(1), F(1), T(2) | − | − | PD | |
| 4A | 57/M | IC/IV | CLB/P (24), LR (30 Gy) | 14 | 0.5 × 106 IU/m2 (1) | T(2) | − | − | PD | |
| 5A | 76/F | MM/III | MP (9) | 10 | 0.5 × 106 IU/m2 (1) | I(2), F(2), T(2) | − | ND | NE | |
| 6A | 63/F | CLL/IV | CLB/P (25), MCP (6), F (12), R (4) | 5 | 0.5 × 106 IU/m2 (1) | F(2), I(2) | ND | − | PD | |
| 7A | 68/M | CLL/IV | CLB/P (4), COP (6), CLB (12) | 2 | 1-2 × 106 IU/m2 (2) | I(2), F(2), T(3) | ND | − | PD | |
| 8A | 66/M | MM/III | MP (15), VID (6) | 2 | 1 × 106 IU/m2 (1) | − | ND | − | PD | |
| 9A | 58/M | MZL/III | MCP (6), TBI/CY + PBSCT (1) | 29 | 1 × 106 IU/m2 (1) | F(2), T(2) | ND | − | PD | |
| 10A | 79/F | MM/III | CHOP (2), LR (50 Gy) | 10 | 2-3 × 106 IU/m2 (2) | F(1), T(2) | ND | − | PD | |
| Cohort B | ||||||||||
| 1B | 73/M | MM/III | MP (2), VID (6) | 3 | 0.25 × 106 IU/m2 (1) | − | + | − | PD | |
| 2B | 59/M | MM/III | MP (16), VID (6), HD-M + PBSCT (1), IFN | 2 | 0.25 × 106 IU/m2 (2) | T(2) | + | − | PD | |
| 3B | 52/M | FCL/IV | MCP (4), CHOP (2), Dexa-BEAM (2), IFN, LR (44 Gy), I131-R | 4 | 0.25 × 106 IU/m2 (1) | F(1), T(2) | + + | − | PD | |
| 4B | 43/F | FCL/III | TNI (49 Gy) | 24 | 0.5-1 × 106 IU/m2 (4) | F(2), T(2) | + + + | + + + | SD | |
| 5B | 57/M | MM/II | VID (4) | 2 | 0.5-2 × 106 IU/m2 (9) | F(1), T(2) | + + + | + | PR | |
| 6B | 36/F | MM/II | LR (30 Gy) | 3 | 0.5 × 106 IU/m2 (1) | F(1), S(1) | + | + + | PD | |
| 7B | 46/M | MZL/IV | TNI (44 Gy) | 29 | 1 × 106 IU/m2 (4) | F(2), T(2) | + + + | − | SD | |
| 8B | 51/F | FCL/IV | COP (10), LR (30 Gy), MCP (4) | 48 | 2 × 106 IU/m2 (4) | F(1), T(2), S(2) | + + + | + | PR | |
| 9B | 55/M | FCL/III | CHOP (4), Dexa-BEAM (2), TBI/CY + PBSCT (1), LR (30 Gy) | 6 | 1-2 × 106 IU/m2 (8) | F(1) | + | + | PR | |
MM indicates multiple myeloma; CLL, chronic lymphocytic leukemia; IC, immunocytoma; MZL, mantle zone lymphoma. Staging according to Durie and Salmon (MM), Rai (CLL), Ann-Arbor (IC; MZL).
MP indicates melphalan/prednisone; CLB, chlorambucil; PM, prednimustine; COP, cyclophosphamide/vincristine/prednisone; VID, vincristine/idarubicin/dexamethasone; MCP, mitoxantrone/chlorambucil/prednisone; CHOP, cyclophosphamide/vincristine/prednisone; F, fludarabine; R, rituximab; TBI/CY, total body irradiation/high-dose cyclophosphamide followed by peripheral blood stem cell transplantation (PBSCT); LR, local radiotherapy (dose); I131-R; radioimmunotherapy with iodine131-rituximab; Dexa-BEAM, dexamethasone/carmustine/etoposide/cytarabine/melphalan; HD-M, high-dose melphalan followed by PBSCT; TNI, total nodal irradiation (dose); and IFN, INF-α (maintenance therapy).
Months between last chemotherapy/radiotherapy and first pamidronate/IL-2 treatment.
T indicates thrombophlebitis; F, fever; S, skin-erythema; I, infection; (2) WHO grade 2.
In vitro results represent percentages of control culture according to the following calculation: (γδ T-cell number in pamidronate/IL-2 culture)−(γδ T-cell number in medium/IL-2)/(γδ T-cell number in medium/IL-2) × 100. In vivo results represent percentage of increase according to the following calculation: (Vy9δ2 T-cell number on day 8 after pamidronate/IL-2 infusion)−(Vγ9δ2 T-cell number before treatment)/(Vγ9δ2 T-cell number before treatment) × 100. − Indicates <20%; +, 20% to 100%; + +, >100% to 200%; + + +, >200% increase of γδ T-cell number; ND, not done.
SD indicates stable disease; PR, partial remission; PD, progressive disease; NE, not evaluable.
No absolute counts are available; however, percentage of γδ T cells increased from 7% to 19.5%.
Treatment schedule
The treatment schedule was adopted from our in vitro experience, where up to 50-fold expansion of γδ T cells was achieved in the presence of pamidronate and IL-2. The consequences of a selective activation of γδ T cells in vivo were not known at the beginning of the study, and pamidronate alone could have induced a cytokine-mediated acute phase reaction. Therefore, the first 10 patients (cohort A, Table 1) received increasing dose levels of continuous 24-hour IV infusions of low-dose IL-2 (0.25-3 × 106 IU/m2) from day 3 to day 8 after an initial pamidronate infusion on day 1 (90 mg/3 h). The subsequent 9 patients (cohort B, Table 1) received IL-2 from day 1 to day 6 directly after the pamidronate infusion (90 mg/3 h) in the form of increasing dose levels (0.25-2 × 106 IU/m2) of a 6-hour IV bolus infusion.
A minimum of 3 patients were included at each dose level and observed for at least 3 weeks prior to starting additional patients at an increased dose. Dose escalations in subsequent patients were in 100% increments until toxicity greater than grade 2 based on the National Cancer Institute (NCI) criteria was reached. Patients who showed no toxicity greater than grade 2 were allowed to continue receiving the next dose level. Maximal tolerated dose (MTD) was defined as the dose that caused grade 3 toxicity in 2 of the first 3 to 6 patients at a particular dose level. Maximal effective dose was defined as the dose that was able to induce significant γδ T-cell proliferation and/or activation without significant concomitant αβ T-cell or NK-cell stimulation.
Toxicity
Both treatment schedules of pamidronate/IL-2 were generally well tolerated. Fourteen patients (74%) developed low-grade fever and/or chills (grade 1-2) during IL-2 therapy, which peaked on day 2 and 3 in cohort A and on day 3 and 4 in cohort B, respectively. These side effects were transient and easily controlled by oral paracetamol. Thirteen patients (68%) developed postinfusional thrombophlebitis (grade 2). Two patients (10%) developed a local erythema (grade 1-2) at the infusion site. Three patients (16%) experienced mild infections (grade 1-2) that were not considered to be related to the study medication. Only 2 patients (10%) experienced grade 3 toxicity (10%): a catheter-associated jugular vein thrombosis and a recurrent femoral vein thrombosis that occurred at an IL-2 dose level of 2 × 106 IU/m2 and 3 × 106 IU/m2, respectively. Because only one toxicity greater than grade 2 was observed on each particular IL-2 dose level, IL-2 was escalated up to 3 × 106 IU/m2 per day. No dose-limiting toxicity for the combination of pamidronate and IL-2 could be defined in this study.
Activation and proliferation of γδ T cells
None of the first 10 patients (cohort A, patients 1A-10A) showed a measurable γδ T-cell response during pamidronate/IL-2 treatment in vivo, even at the highest IL-2 dose level (Table 1). Although in vitro proliferation of γδ T cells in response to pamidronate/IL-2 was not regularly examined in this patient group, the majority of tested patients (4 of 5) had negative in vitro proliferation assays (Table 1). Therefore, in vitro testing of γδ T cells in response to pamidronate/IL-2 was performed for all further eligible patients, and only patients with significant in vitro proliferation qualified for study entry. Compared with an age-matched group of healthy donors in which 88% exhibited an in vitro response to pamidronate/IL-2, only 49% of patients with lymphoid malignancies (n = 41) showed significant in vitro γδ T-cell proliferation (Table 2). In accordance with the data observed in vivo, the proportion of patients with B-CLL showing γδ T-cell proliferation to pamidronate/IL-2 in vitro was quite low. Thus, the underlying disease (eg, B-CLL) seems to have an effect on γδ T-cell reactivity. Furthermore, in vitro testing revealed that addition of IL-2 on day 1 instead of day 3 significantly increased proliferation of γδ T cells in response to pamidronate (data not shown).
In vitro proliferation of γδ T cells in response to pamidronate/IL-2
. | Healthy donors (%) . | All patients (%) . | MM (%) . | NHL (%) . | B-CLL (%) . |
|---|---|---|---|---|---|
| Positive | 14/16 (88) | 20/41 (49) | 12/26 (46) | 8/10 (80) | 1/5 (20) |
| Negative | 2/16 (12) | 21/41 (51) | 14/26 (54) | 2/10 (20) | 4/5 (80) |
. | Healthy donors (%) . | All patients (%) . | MM (%) . | NHL (%) . | B-CLL (%) . |
|---|---|---|---|---|---|
| Positive | 14/16 (88) | 20/41 (49) | 12/26 (46) | 8/10 (80) | 1/5 (20) |
| Negative | 2/16 (12) | 21/41 (51) | 14/26 (54) | 2/10 (20) | 4/5 (80) |
On the basis of these results, we changed the treatment schedule (IL-2 start on day 1), and only patients with significant in vitro proliferation of γδ T cells to pamidronate/IL-2 were defined to be eligible. In this patient group (cohort B, patients 1B-9B) significant in vivo proliferation of γδ T cells could be achieved in 5 of 9 patients (55%) (Table 1). The pronounced effect of the pamidronate/IL-2 combination on γδ T lymphocytes in vivo becomes even more evident when expression of activation markers was analyzed (Table 3). In contrast to αβ T cells and NK cells, we found a significant increase of CD69 and/or HLA-DR activation antigens on γδ T cells. Concomitant expression of early (CD69) and late (HLA-DR) activation markers demonstrates a more specific stimulation. Both antigens were up-regulated on γδ T cells in a dose-dependent manner, whereas on αβ T cells and NK cells this effect was achieved only at the highest dose level of IL-2 in a significant proportion of patients (Table 3).
Expression of activation antigens after pamidronate/IL-2 infusion of patients from cohort B
. | . | αβ T cells . | . | . | NK cells . | . | . | γδ T cells . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dose level, IL-2 IU/m2 . | Patient, n . | CD69, % mean (range) . | HLA-DR, % mean (range) . | CD69+ HLA-DR, n/patients (%) . | CD69, % mean (range) . | HLA-DR, % mean (range) . | CD69+ HLA-DR, n/patients (%) . | CD69, % mean (range) . | HLA-DR, % mean (range) . | CD69+ HLA-DR, n/patients (%) . | ||||||
| 0.25 × 106 | 3 | 3 (−56-71) | 10 (0-15) | 0/3 (0) | 24 (−17-43) | 0 (0-123) | 0/3 (0) | 0 (−23-101) | 163 (−7-375) | 1/3 (33) | ||||||
| 0.5 × 106 | 3 | 33 (−23-95) | 37 (27-86) | 1/3 (33) | 0 (−26-81) | 0 (0-67) | 0/3 (0) | 53 (38-167) | 400 (0-831) | 2/3 (66) | ||||||
| 1 × 106 | 4 | 62 (3-153) | 45 (0-122) | 0/4 (0) | 23 (18-27) | 8 (−38-54) | 0/2 (0) | 183 (82-426) | 322 (0-1253) | 3/4 (75) | ||||||
| 2 × 106 | 3 | 65 (52-98) | 60 (19-121) | 2/3 (66) | 81 (−37-103) | 93 (79-291) | 2/3 (66) | 186 (62-230) | 124 (122-571) | 3/3 (100) | ||||||
. | . | αβ T cells . | . | . | NK cells . | . | . | γδ T cells . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dose level, IL-2 IU/m2 . | Patient, n . | CD69, % mean (range) . | HLA-DR, % mean (range) . | CD69+ HLA-DR, n/patients (%) . | CD69, % mean (range) . | HLA-DR, % mean (range) . | CD69+ HLA-DR, n/patients (%) . | CD69, % mean (range) . | HLA-DR, % mean (range) . | CD69+ HLA-DR, n/patients (%) . | ||||||
| 0.25 × 106 | 3 | 3 (−56-71) | 10 (0-15) | 0/3 (0) | 24 (−17-43) | 0 (0-123) | 0/3 (0) | 0 (−23-101) | 163 (−7-375) | 1/3 (33) | ||||||
| 0.5 × 106 | 3 | 33 (−23-95) | 37 (27-86) | 1/3 (33) | 0 (−26-81) | 0 (0-67) | 0/3 (0) | 53 (38-167) | 400 (0-831) | 2/3 (66) | ||||||
| 1 × 106 | 4 | 62 (3-153) | 45 (0-122) | 0/4 (0) | 23 (18-27) | 8 (−38-54) | 0/2 (0) | 183 (82-426) | 322 (0-1253) | 3/4 (75) | ||||||
| 2 × 106 | 3 | 65 (52-98) | 60 (19-121) | 2/3 (66) | 81 (−37-103) | 93 (79-291) | 2/3 (66) | 186 (62-230) | 124 (122-571) | 3/3 (100) | ||||||
Results represent means of increase of the percentages of positive cells analyzed by double or triple staining (NK cells). CD69 was determined on day 2 or 3 and HLA-DR on day 7 after infusion. For analysis of patients expressing CD69 and HLA-DR, a threshold of 50% positive cells on day 8 was defined.
In addition, significant expansion of Vγ9δ2 T cells, which are the target population of pamidronate stimulation, was observed in vivo (Table 4). Although at the first IL-2 dose level there was no significant proliferation of Vγ9δ2 T cells, absolute numbers of this γδ T-cell subset increased at a dose level of 0.5 × 106 IU/m2 IL-2 and reached a maximum increase of 128% compared with before treatment. However, at the highest IL-2 dose level, absolute increase of Vγ9δ2 T cells was less pronounced, which might be a secondary effect because of activation of bystander cells. We also found a similar dose-response curve in vitro with an inferior capacity of γδ T cells to proliferate at higher IL-2 concentrations. The absolute numbers of Vγ9Vδ2 T cells in patients who showed positive in vivo proliferation after the first cycle of pamidronate/IL-2 continued to increase after subsequent cycles (data not shown).
Change of cell number of lymphocyte subpopulations after pamidronate/IL-2 infusion of patients from cohort B
. | . | . | . | Increase γδ T cells, % mean (range) . | . | |
|---|---|---|---|---|---|---|
| Dose level, IL-2 IU/m2 . | Patients, n . | Increase αβ T cells, % mean (range) . | Increase NK cells, % mean (range) . | Vδ1 (range) . | Vγ9δ2 (range) . | |
| 0.25 × 106 | 3 | 47 (−3-82) | 44 (42-152) | 64 (0-114) | 0 (0) | |
| 0.5 × 106 | 3 | 19 (11-23) | 122 (19-136) | 0 (−3-0) | 128 (24-257) | |
| 1 × 106 | 4 | 35 (7-70) | 30 (0-98) | 0 (0-36) | 89 (−15-210) | |
| 2 × 106 | 3 | 12 (−5-14) | 37 (26-71) | 0 (0-8) | 57 (21-95) | |
. | . | . | . | Increase γδ T cells, % mean (range) . | . | |
|---|---|---|---|---|---|---|
| Dose level, IL-2 IU/m2 . | Patients, n . | Increase αβ T cells, % mean (range) . | Increase NK cells, % mean (range) . | Vδ1 (range) . | Vγ9δ2 (range) . | |
| 0.25 × 106 | 3 | 47 (−3-82) | 44 (42-152) | 64 (0-114) | 0 (0) | |
| 0.5 × 106 | 3 | 19 (11-23) | 122 (19-136) | 0 (−3-0) | 128 (24-257) | |
| 1 × 106 | 4 | 35 (7-70) | 30 (0-98) | 0 (0-36) | 89 (−15-210) | |
| 2 × 106 | 3 | 12 (−5-14) | 37 (26-71) | 0 (0-8) | 57 (21-95) | |
Results represent means of increase in absolute cell numbers on day 7.
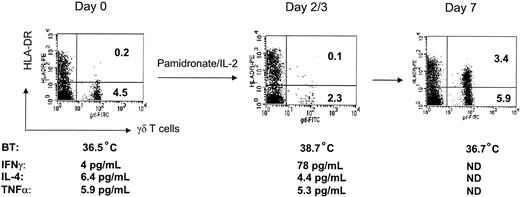
A remarkable in vivo response from patient 4B is depicted in Figure 1: after infusion of pamidronate/IL-2, γδ T cells disappeared from peripheral blood presumably because of activation-induced transmigration through the endothelial layer and reappeared several days later in a highly activated status and increased in number. Simultaneously, the patient developed fever and showed significant increase of serum IFN-γ. Similar results were observed in other patients from cohort B who showed significant activation and proliferation of γδ T cells in vivo. Measurement of Th1 and Th2 cytokines revealed that IFN-γ concentrations (but not IL-4 or TNF-α levels) were significantly increased in the serum of 7 of 9 patients of cohort B (data not shown).
Representative example demonstrating activation and expansion of γδ T cells during pamidronate/IL-2 treatment. Peripheral blood lymphocytes (PBLs) of patient 4B were stained with FITC- and PE-labeled antibodies and analyzed by flow cytometry before and after infusion of pamidronate and IL-2. Percentages of cells are given per quadrant. Body temperature (BT) was measured 2 times daily. Cytokine concentrations were determined in serum using ELISA systems. ND indicates not done.
Representative example demonstrating activation and expansion of γδ T cells during pamidronate/IL-2 treatment. Peripheral blood lymphocytes (PBLs) of patient 4B were stained with FITC- and PE-labeled antibodies and analyzed by flow cytometry before and after infusion of pamidronate and IL-2. Percentages of cells are given per quadrant. Body temperature (BT) was measured 2 times daily. Cytokine concentrations were determined in serum using ELISA systems. ND indicates not done.
Clinical response to therapy
None of the 9 analyzable patients of cohort A (patients 1A-10A) achieved an objective tumor response (Table 1). In one patient (patient 2A) stable disease was observed which lasted 6 months.
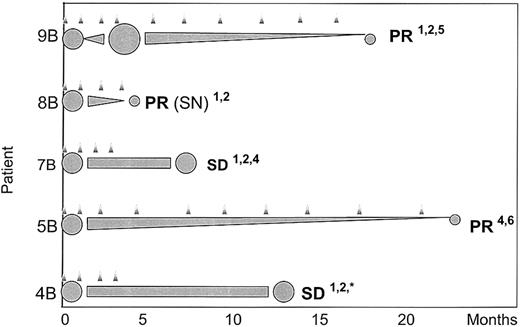
In contrast, 3 of 9 patients in cohort B (patients 5B, 8B, 9B) achieved a partial remission (PR), giving an objective response rate of 33% (Table 1). Two additional patients achieved stable disease, still ongoing after 7 months (patient 7B) and lasting 13 months in another patient (patient 4B). Responding patients received IL-2 at a dose level of 1 × 106 IU/m2 to 2 × 106 IU/m2. The response profile of these 5 patients revealed that the time interval from start of therapy until maximum response was quite long, ranging from 4 months to 23 months (Figure 2).
Time course of responding patients. Response confirmed by (1) CT scan, (2) ultrasound, (3) PET scan, (4) blood tests, (5) lymph node, or (6) bone marrow biopsy.  indicates treatment cycle; ⬢, relative tumor mass; SN, subcutaneous nodules; * lost to follow up.
indicates treatment cycle; ⬢, relative tumor mass; SN, subcutaneous nodules; * lost to follow up.
Time course of responding patients. Response confirmed by (1) CT scan, (2) ultrasound, (3) PET scan, (4) blood tests, (5) lymph node, or (6) bone marrow biopsy.  indicates treatment cycle; ⬢, relative tumor mass; SN, subcutaneous nodules; * lost to follow up.
indicates treatment cycle; ⬢, relative tumor mass; SN, subcutaneous nodules; * lost to follow up.
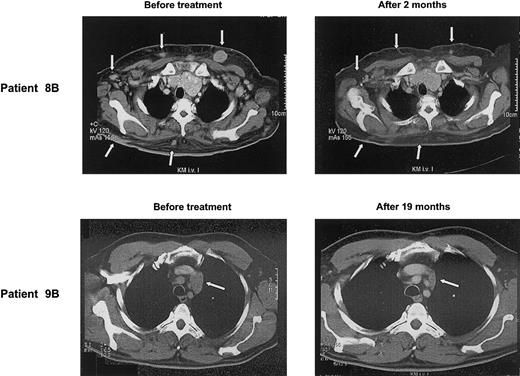
A more detailed description of the patients achieving an objective tumor response gives an impression of how γδ T-cell–mediated immunotherapy might operate: patient 9B with a follicle center lymphoma had relapsed despite high-dose chemotherapy. After 3 cycles of pamidronate/IL-2, CT scan revealed insignificant changes of most lymph nodes, except one that had increased in size. Biopsy of this lymph node revealed a predominant fibrosis with only small numbers of lymphoma cells left. Thereafter, tumor mass steadily declined and, after 19 months of treatment, a PR has been reached (Figure 3).
Clinical response to treatment. CT scans of patients 8B and 9B before and after several cycles of pamidronate/IL-2 therapy show regression of skin metas tases and mediastinal lymph node (arrows), respectively.
Clinical response to treatment. CT scans of patients 8B and 9B before and after several cycles of pamidronate/IL-2 therapy show regression of skin metas tases and mediastinal lymph node (arrows), respectively.
Patient 8B had relapsed with a follicle center lymphoma and developed multiple subcutaneous manifestations in addition to multiple lymph node involvement. After the start of pamidronate/IL-2 treatment, the subcutaneous nodules rapidly disappeared (Figure 3), whereas nodal manifestations regressed slowly. Two months later, treatment was stopped because of noncompliance of the patient.
Patient 5B suffered from an IgAκ (immunoglobulin Aκ) multiple myeloma (stage IIA) refractory to conventional chemotherapy. After 9 cycles of immunotherapy the patient's bone marrow was cleared from tumor cells and IgA level decreased from 3400 mg/dL to 1740 mg/dL.
The in vivo expansion of γδ T cells of all analyzable patients from cohorts A and B correlated with the response to therapy, confirming a γδ T-cell–mediated effect. It seems that expansion of γδ T cells in vivo is a necessary prerequisite for tumor regression (3 of 5 patients responded; P = .015; Table 5). Because none of the patients without γδ T-cell stimulation achieved an objective tumor response, the negative predictive value was 100%.
In vivo proliferation of γδ T cells and response to treatment with pamidronate/IL-2*
. | In vivo γδ T-cell proliferation . | . | . | |
|---|---|---|---|---|
| Objective response . | + . | − . | Σ . | |
| + | 3 | 0 | 3 | |
| − | 2 | 13 | 15 | |
| Σ | 5 | 13 | 18 | |
. | In vivo γδ T-cell proliferation . | . | . | |
|---|---|---|---|---|
| Objective response . | + . | − . | Σ . | |
| + | 3 | 0 | 3 | |
| − | 2 | 13 | 15 | |
| Σ | 5 | 13 | 18 | |
All analyzable patients (n = 18) according to Table 1 were included.
P = .015, Fisher exact test.
Discussion
There has been no study published so far on in vivo stimulation of γδ T cells in humans, and the consequences of a selective activation of γδ T cells in vivo were not known. Therefore, evaluation of toxicity was one major end point of this study. We started with a low IL-2 dose of 0.25 × 106 IU IL-2/m2 and subsequently increased the IL-2 dose to 3 × 106 IU IL-2/m2 in cohort A and to 2 × 106 IU IL-2/m2 in cohort B. Overall, the combination of pamidronate and IL-2 was well tolerated, and no dose-limiting toxicity was observed. Most of the patients developed self-limiting fever and thrombophlebitis at the infusion site. Local thrombophlebitis has been described as a rare side effect in patients receiving pamidronate alone.20,21 The high frequency of local thrombophlebitis in patients receiving pamidronate in combination with IL-2 might reflect immune-mediated effects on endothelial cells. It has also been recently shown that aminobisphosphonates have dose-dependent effects on proliferation-inhibition and apoptosis-induction of human endothelial cells in vitro.22
Next we asked whether the combination of pamidronate and IL-2 induces activation and proliferation of γδ T cells in vivo. None of the first 10 patients included in this pilot study (cohort A, Table 1) developed a measurable γδ T-cell response in vivo. The inability to induce γδ T-cell proliferative response in vivo correlated with the negative in vitro proliferation of γδ T cells in response to pamidronate/IL-2 in 4 of 5 analyzable patients. Therefore, extensive prior in vitro testing was initiated for all further eligible patients. Using this strategy, we found that a much lower proportion of patients with hematologic malignancies showed positive in vitro proliferation of γδ T cells in response to pamidronate/IL-2 compared with a control group of healthy donors (49% versus 88%). Although the exact mechanisms of this defect are currently under investigation, a severe immunodeficiency caused by extensive prior chemotherapy in these relapsed/refractory patients and/or the underlying disease itself may account for this observation. Indeed, the type of underlying disease seems to influence the in vitro proliferative response to pamidronate/IL-2 (Table 2). The failure of patients with B-CLL to develop a measurable γδ T-cell proliferative response may be a result of the very small number of γδ T cells in peripheral blood, which were often below the detection limit in our series. However, a larger number of patients with distinct disease entities and at different disease stages (eg, untreated versus treated) need to be evaluated to support this observation and to identify additional clinical parameters influencing γδ T-cell reactivity. Furthermore, extensive prior in vitro testing in eligible patients revealed that γδ T-cell proliferation in response to pamidronate can be significantly enhanced by concomitant addition of IL-2 to PBMC cultures on day 1 instead of day 3 (as previously done).
Thus, for all further patients the treatment schedule was changed (concomitant administration of IL-2 on day 1), and only patients with significant in vitro proliferation of γδ T cells in the presence of pamidronate and IL-2 were included (cohort B, Table 1). After these modifications, significant in vivo expansion of γδ T cells could be observed in 5 of 9 patients (55%) (Table 1). In vivo proliferation of γδ T cells was associated with a robust up-regulation of early (CD69) and late (HLA-DR) activation markers, whereas pamidronate and IL-2 failed to induce comparable effects on αβ T cells and NK cells (Table 3). These data support in vitro findings that the action of pamidronate is highly specific and, except for Vγ9Vδ2 T cells, it does not activate other immune effector cells.8,23,24 However, at higher IL-2 doses unspecific stimulation effects of IL-2 became more evident because a proportion of patients showed a moderate up-regulation of activation markers on αβ T cells and NK cells at the highest dose level of IL-2 tested in this study. On the basis of the analysis of activation marker expression and proliferation we conclude that 1 × 106 IU IL-2/m2 IL-2 per day seems to be the most effective dose with respect to specific and effective γδ T-cell stimulation in vivo.
Another aim of our study was to assess the clinical response. None of the 9 analyzable patients of cohort A (Table 1) achieved an objective tumor response. After change of protocol and inclusion criteria (cohort B, Table 1) 3 of 9 patients (33%) achieved an objective tumor response (3 PR). Clinical response could be associated with γδ T-cell proliferation in vivo, because all 4 patients from cohort B without γδ T-cell proliferation in vivo did not experience an objective tumor response, and 4 of 5 patients with γδ T-cell proliferation in vivo responded (3 PR, 1 stable disease [SD]). These results suggest that the observed tumor regression in our patients is dependent on γδ T-cell activation and proliferation. The relevance of this correlation is underlined by the fact that pamidronate-stimulated γδ T cells possess an increased capacity for killing tumor cells in vitro.8,10 It is still open which mechanisms may have been responsible for the clinical responses. Several other antitumor effects have been attributed to aminobisphosphonates. However, at pharmacologically achievable concentrations in vivo, only the specific stimulation of Vγ9Vδ2 T cells can be observed.8 Alternatively, the occurrence of clinical remissions may be attributed to an IL-2–mediated effect on other immune effector cells. However, our immunologic monitoring indicates that the combination of pamidronate and low-dose IL-2 does not induce specific activation and expansion of αβ T cells or NK cells compared with the effect on γδ T cells. In addition, the concentrations of IL-2 used here are much lower than the doses required in other immunotherapeutic approaches for these malignancies.25-27
The important question of what precise mechanisms are involved in tumor recognition and eradication by γδ T cells is out of the scope of this study and will require further in vitro and in vivo studies. However, tumor cell recognition by γδ T cells seems to be modulated by a balance of positive and negative signals.28 Although killer inhibitory receptors (KIRs) are obviously involved in the mediation of negative signals, the positive signals are only incompletely understood. One example of such a positive signal is the NKG2D-DAP10 receptor complex, which is known to interact with stress-induced ligands on tumor cells such as MICA and Rae-1.29 The very slow response profiles of most of the patients in our series strongly argue for an indirect influence on lymphoma cells rather than a sole cytotoxic effect. One possible mechanism may be secretion of cytokines, which influence tumor cells or their microenvironment.30 We have already shown that IFN-γ is the major cytokine secreted by pamidronate-activated γδ T cells.8,31 IFN-γ has multiple antitumor effects such as direct inhibition of tumor growth, blocking angiogenesis, or stimulation of macrophages.32 Recently, a significant negative correlation between angiogenetic factors (ie, VEGF) and IFN-γ serum levels was described in patients treated with pamidronate.33 Therefore, IFN-γ might be one of the key cytokines involved in the γδ T-cell–mediated antitumor response.
In conclusion, this study indicates for the first time that in vivo γδ T-cell stimulation by pamidronate and low-dose IL-2 is a safe and promising immunotherapy approach in the treatment of patients with low-grade B-NHL and MM. Further studies are necessary to confirm the clinical efficacy of this novel strategy. Our immunologic and clinical monitoring data provide further insight into the capacity of γδ T cells to induce an antitumor immune response. However, this study also reveals that the function of γδ T cells can be impaired in some patients with lymphoid malignancies. Therefore, the results of this study provide principles relevant to the design of future trials, including appropriate prior in vitro testing.
Prepublished online as Blood First Edition Paper, March 6, 2003; DOI 10.1182/blood-2002-12-3665.
Supported by Interdisziplinaeres Zentrum für Klinische Forschung Wuerzburg (Grant no. 01KS9603) and Dr Mildred Scheel Stiftung fuer Krebsforschung (Grant no. 10-1897-Ku 2).
M.W. and V.K. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Karin Kelm for her expert technical assistance, J. Sandstede, MD, and D. Hahn, MD, for providing CT scans, D. Kraemer, MD, for assistance with the production of figures, and S. Guelgoenen for reading the manuscript.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal