Abstract
In HIV infection, CD8+ cells show cytotoxic and noncytotoxic anti-HIV activity. The latter function is mediated, at least in part, by a secreted antiviral protein, the CD8+ cell antiviral factor (CAF). Because antiviral effector molecules, such as perforin and granzymes, reside in the exocytic granules of CD8+ T cells, we examined the possibility that granules contain CAF-like activity. CD8+ cells from HIV-infected individuals showing strong CAF-mediated antiviral activity were induced to release their granule constituents into culture media. Within 1 hour of stimulation, high levels of granzyme B (a primary granule constituent) were found in the culture fluids of previously activated CD8+ cells. The same culture fluids contained no or very low amounts of CAF activity, as measured with HIV-infected CD4+ cells. Maximal levels of CAF activity were not observed until 5 or 7 days after stimulation, consistent with typical CAF production kinetics. In addition, extracts of granules purified from antiviral CD8+ cells did not show any CAF activity, whereas the cytoplasmic fraction of these cells showed substantial levels of antiviral activity. These findings suggest that CAF does not reside at appreciable levels in the exocytic granules of antiviral CD8+ T cells. (Blood. 2003;102: 180-183)
Introduction
In HIV infection, 2 types of cellular antiviral responses associated with CD8+ T cells have been described. One response, mediated by cytotoxic T lymphocytes (CTLs), involves the cytolysis of infected cells in a human leukocyte antigen (HLA)—restricted manner.1 Cytolysis results from the targeted release of the contents of exocytic granules from the CD8+ cell. These granules contain perforin and granzymes that act together to induce apoptosis in the targeted cell.2,3 In addition to these effector molecules, other immune factors reside in the exocytic granules of CD8+ T cells, such as granulysin and β-chemokines.4,5 Naive T cells do not have detectable granules, but, once activated, the granule content of the CD8+ T cell increases, reaching a peak level 5 to 10 days later.6
The second type of CD8+ cell—mediated anti-HIV response involves the suppression of HIV replication in cultured CD4+ cells in the absence of cell killing.7 This CD8+ cell noncytotoxic antiviral response (CNAR) is not HLA-restricted, blocks HIV transcription, and is associated with the production of a novel CD8+ cell antiviral factor (CAF).7-10 CAF lacks identity with other known cytokines, including granzymes and chemokines.7,11,12 Its peak production occurs 5 to 9 days after the activation of the CD8+ cells.7,11 Because of the antimicrobial content of CD8+ T cell granules and the similarity in kinetics in the synthesis of granules6 and CAF production, we sought to determine whether CAF resides in the exocytic granules of CD8+ T cells of HIV-infected persons.
Materials and methods
Subjects
Heparinized peripheral blood samples were obtained by venipuncture from HIV-1—seropositive donors previously characterized for CD8+ cell anti-HIV activity. HIV-infected subjects were clinically healthy males with CD4+ T-cell counts of more than 500 cells/μL and viral loads less than 6000 copies/mL. Two subjects were taking antiretroviral therapy. Blood samples from HIV-seronegative donors were provided by Blood Centers of the Pacific (San Francisco, CA). This study received the approval of the Committee on Human Research, University of California, San Francisco.
Induction of granule release from antiviral CD8+ cells
CD8+ T cells from HIV-infected subjects, previously shown to be good producers of CAF, were isolated from heparinized blood by immunomagnetic bead (IM; Dynal, Lake Success, NY) separation as described.13 Purity of the isolated CD8+ cells was always greater than 95% as determined by flow cytometry. Cells were washed 3 times and were cultured in complete RPMI 1640 medium (Mediatech, Herndon, VA) containing 10% heat-inactivated (56°C, 30 minutes) fetal calf serum (FCS), 2 mM glutamine, 1% antibiotics (100 μg/mL penicillin; 100 μg/mL streptomycin) (Tissue Culture Facility, University of California, San Francisco), and 100 U/mL recombinant interleukin-2 (rIL-2; Glaxo Wellcome, Research Triangle Park, NC). Cells were then plated in 24-well plates at a density of 4 × 106 cells/mL in the presence of 25 ng/mL phorbol ester (PMA) plus 100 ng/mL ionomycin (Sigma Chemical, St Louis, MO) or anti-CD3 IM beads (at a 4:1 bead-cell ratio),11 or they were left untreated. After 1 and 3 hours of culture, all culture fluid was collected and stored at -80°C, and the cultures were replenished with fresh medium. Untreated and anti-CD3 IM bead-treated CD8+ cell cultures were maintained with passing and collecting fluids on day 3 and every 2 days thereafter.11 In all instances, the anti-CD3 IM beads were removed from the cultures after the initial 3-day activation period.
Previously activated CD8+ cells were obtained from the cultures stimulated with anti-CD3 IM beads, as described in the previous paragraph, 9 to 11 days after the initial activation. They were washed 3 times and then given a second round of stimulation analogous to the first round. Viability (measured by trypan blue dye exclusion) of these previously stimulated CD8+ cells was greater than 85%.
Subcellular fractionation and isolation of CD8+ cell granules
Antiviral CD8+ cells were stimulated for 3 days with anti-CD3 IM beads, then separated from the IM beads and passaged at a density of 2 × 106 cells/mL into serum-free AIM-V medium (Gibco-BRL, Gaithersburg, MD) supplemented with 200 U/mL rIL-2. Cultures were passed every 2 days thereafter, and samples were collected at each passage for monitoring of CAF activity. On day 7 or 9, the CD8+ cells (80%-93% viable) were washed twice with phosphate-buffered saline (PBS) and then disrupted in a cavitation bomb as described.14 In brief, the cells were adjusted to approximately 5 × 108 cells/mL in cold relaxation buffer before placement in a chilled cavitation bomb (Parr Instrument, Moline, IL) on ice. Cavitated material was then processed through several centrifugation steps to remove cell fragments, debris, and nuclei. The resultant supernatant was centrifuged to pellet the granules, leaving the cytoplasmic supernatant. Pelleted granules were resuspended in 1 to 1.5 mL PBS, then put through 4 freeze/thaw cycles to burst the granules. Ultracentrifugation of this material resulted in a fraction containing soluble granule contents and a pellet of granule membranes. Because various proteins, including granzymes, have been associated with the granule membrane, we treated this fraction with 1 M NaCl to dissociate bound proteins. These solubilized proteins were recovered after ultracentrifugation to remove the granule membranes. The 3 fractions of interest—cytoplasm, soluble granule contents, and granule membrane—associated proteins—were all immediately frozen at -80°C before analysis.
Quantification of granzyme B
Assay for CAF activity
The extent of anti-HIV activity in CD8+ cell culture fluids was measured using a standardized acute virus infection assay.11 In brief, phytohemagglutinin-P (3 μg/mL; Sigma Chemical)—stimulated CD4+ cells cultured from HIV-seronegative subjects were acutely infected for 1 hour with 4000 tissue-culture infectious dose-50 (TCID50) HIV-1SF2, a β-chemokine—insensitive isolate.16 Infected CD4+ cells (more than 90% pure by flow cytometry) were washed to remove free virus, and 105 cells were plated per well in a 96-well culture plate. Unless noted otherwise, target cells were cultured in triplicate in a 50% dilution of the test fluid (ie, CD8+ cell culture fluid, subcellular CD8+ cell fraction, or control medium or buffer). Cultures were passed every 2 days and monitored for reverse transcriptase (RT) activity.17 Fresh CD8+ cell supernatant, test material, or control medium was added at each passage. Using this assay for CAF activity, the maximum amount of reduction typically observed in RT activity was between 50% and 80%.7,11
Results
Induction of exocytic granule release from CD8+ cells
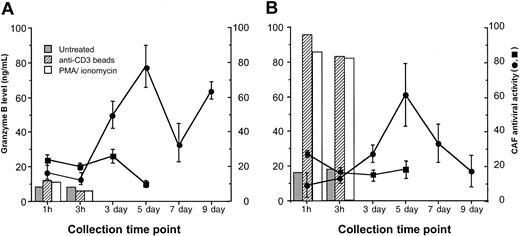
Freshly isolated CD8+ T cells from HIV-infected subjects were induced to release exocytic granules by short-term stimulation with PMA plus ionomycin, or anti-CD3 IM beads. Both stimuli have been shown to induce high levels of granule release from murine and human CTLs within 1 hour of treatment.6 After 1 and 3 hours of exposure to either of these activating stimuli, the levels of granzyme B (a major constituent of T-cell exocytic granules) in the culture fluids were relatively low (less than 15 ng/mL) and not substantially different from the levels found in untreated CD8+ cells (Figure 1A). In the same 1-hour and 3-hour fluids, little anti-HIV activity was detected (25% or less reduction in HIV replication). However, maximal levels of CAF production were observed at days 5 and 9 in the anti-CD3 IM-bead—treated cultures, as expected for the typically cyclical kinetics of CAF production.7,11 Previous studies have shown that granzyme B levels in CD8+ cell culture supernatants from days 5 to 13 after stimulation with α-CD3 IM beads are relatively low or absent.12 Stimulation with PMA plus ionomycin led to massive death in the CD8+ cells after 1 to 2 days of culture (as expected), so CAF production could not be observed similarly in these cultures.
Granule release (granzyme B levels) and CAF production from antiviral CD8+ cells. Freshly isolated CD8+ cells (A) from an HIV-infected patient showing strong CAF activity were left untreated (▪) or were activated with anti-CD3 IM beads (•) and were cultured as described in “Materials and methods.” (B) On day 9 or 11, CD8+ cells from anti-CD3 IM bead-activated cultures were washed and then treated as in panel A. At the indicated time points, samples of culture fluid were taken and frozen at -80°C. Granzyme B levels in the culture fluids were measured by ELISA (detection limit, 50 pg/mL). CAF activity in the culture fluids, indicated as the percentage suppression of HIV replication ± SD, was measured in a standardized β-chemokine—insensitive virus acute infection assay (see “Materials and methods”). Data are representative of 2 experiments, each with a different subject.
Granule release (granzyme B levels) and CAF production from antiviral CD8+ cells. Freshly isolated CD8+ cells (A) from an HIV-infected patient showing strong CAF activity were left untreated (▪) or were activated with anti-CD3 IM beads (•) and were cultured as described in “Materials and methods.” (B) On day 9 or 11, CD8+ cells from anti-CD3 IM bead-activated cultures were washed and then treated as in panel A. At the indicated time points, samples of culture fluid were taken and frozen at -80°C. Granzyme B levels in the culture fluids were measured by ELISA (detection limit, 50 pg/mL). CAF activity in the culture fluids, indicated as the percentage suppression of HIV replication ± SD, was measured in a standardized β-chemokine—insensitive virus acute infection assay (see “Materials and methods”). Data are representative of 2 experiments, each with a different subject.
Because of the apparent lack of granule release from the freshly isolated CD8+ T cells from HIV-infected subjects, we evaluated granule release from the CD8+ cells previously activated with α-CD3 IM bead treatment. CD8+ cells actively producing CAF, taken 9 to 11 days after initial activation, were restimulated to induce granule release in a fashion analogous to the treatment of the freshly isolated cells (Figure 1B). PMA plus ionomycin and α-CD3 IM beads induced the release of high levels of granzyme from the CD8+ cells within 1 hour of treatment (generally from 85 to > 200 ng/mL), suggesting effective granule exocytosis. CD8+ cells in the untreated cultures, however, produced low levels (17-25 ng/mL) of granzyme B (Figure 1B). This likely resulted from residual synthesis after the initial stimulation, which was between 20 and 31 ng/mL at days 9 and 11 (data not shown). CAF levels in the fluids from untreated and restimulated cultures were minimal after 1 hour and 3 hours, never reaching more than 25% reduction of HIV replication (Figure 1B). In contrast, strong CAF production was seen in the cultures 5 days after CD8+ cell stimulation (Figure 1B). This finding is again consistent with the kinetics of CAF production from freshly isolated, stimulated CD8+ cells (Figure 1A).7,11 These results suggest that CAF is likely not present at appreciable levels in exocytic granules but instead is secreted late after activation.
CAF activity in subcellular fractions of CD8+ cells
To further evaluate the possibility that CD8+ cell granules contain CAF antiviral activity, we analyzed extracts of granules purified from antiviral CD8+ cells. Purified CD8+ cells from HIV-infected subjects were ruptured by cavitation, and the constituents were processed to yield subcellular fractions representing the cytoplasm, the soluble granule contents, and the granule membrane-associated components (see “Patients, materials, and methods”). The latter fraction was prepared (using high salt extraction) because some of the proteins residing in granules associate with the granule membrane. Granzyme B levels were used as indicators of granule purification efficiency and were measured by an assay dependent on the enzymatic activity of this protein. Results indicate that the fractionation procedure was mild enough to leave proteins (eg, granzyme B) functionally intact. Representative results from 3 of 6 subjects studied are presented in Table 1. Two showed typical CAF production at 5 to 7 days (subjects A and B), and one lacked production of CAF (subject C). In the CD8+ cell extracts, low or no granzyme B was detected in any of the cytoplasmic fractions, whereas the highest levels were found in the soluble granule fractions, indicating ideal granule isolation (Table 1). The amount of granzyme B in the soluble granule fractions ranged from approximately 650 to 5000 ng. This amount is approximately 10% to 60% of that released by a CD8+ CTL cell line (on a per-cell basis) after the induction of granule exocytosis15 (Figure 1; Table 1).
Results of CAF activity in subcellular fractions of CD8+ cells
Subject . | CAF activity, % suppression* . | Fraction . | Granzyme B concentration, nM . | Total volume, μL . | Total granzyme B yield, ng . | CAF activity† . |
|---|---|---|---|---|---|---|
| A | 46 | Cytoplasm, organelle free | 0 | 2000 | 0 | + |
| Soluble granule contents | 158 | 1500 | 7580 | − | ||
| Granule membrane-associated | 448 | 200 | 2870 | − | ||
| B | 61 | Cytoplasm, organelle free | 1 | 1000 | 30 | + |
| Soluble granule contents | 62 | 1000 | 1973 | − | ||
| Granule membrane-associated | 193 | 100 | 617 | − | ||
| C | 7 | Cytoplasm, organelle free | 25 | 1000 | 792 | − |
| Soluble granule contents | 154 | 1000 | 4937 | − | ||
| Granule membrane-associated | 1514 | 100 | 4845 | − |
Subject . | CAF activity, % suppression* . | Fraction . | Granzyme B concentration, nM . | Total volume, μL . | Total granzyme B yield, ng . | CAF activity† . |
|---|---|---|---|---|---|---|
| A | 46 | Cytoplasm, organelle free | 0 | 2000 | 0 | + |
| Soluble granule contents | 158 | 1500 | 7580 | − | ||
| Granule membrane-associated | 448 | 200 | 2870 | − | ||
| B | 61 | Cytoplasm, organelle free | 1 | 1000 | 30 | + |
| Soluble granule contents | 62 | 1000 | 1973 | − | ||
| Granule membrane-associated | 193 | 100 | 617 | − | ||
| C | 7 | Cytoplasm, organelle free | 25 | 1000 | 792 | − |
| Soluble granule contents | 154 | 1000 | 4937 | − | ||
| Granule membrane-associated | 1514 | 100 | 4845 | − |
Results are representative of 6 subjects studied.
Granzyme B concentration was measured using an enzymatic assay (“Materials and methods”).
Total granzyme B yield was calculated from the respective concentration and total volume.
Total volume of each fraction was approximately 170- to 300-fold (1700- to 3000-fold in membrane-associated fraction) less per number of cells than that used to obtain the CAF-active culture supernatants.
See also Figure 2 regarding CAF activity in the subcellular fractions.
Activity of CAF was measured as described in “Materials and methods.” Fluids were collected at 9 days (subject A) and 7 days (subjects B and C) after CD8+ cell stimulation. 11 Total number of CD8+ cells processed was 13.7 × 108 (subject A) and 5.0 × 108 (subjects B and C).
Activity measured in subcellular fractions.
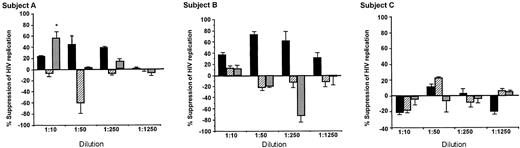
Appreciable CAF activity was only found in dilutions of cytoplasmic fractions, and then only in those fractions from CD8+ cells that produced CAF in culture (Figure 2; Table 1, subjects A and B). Sometimes the granule fractions showed enhancement of HIV replication, but these findings were not dose dependent, nor were they found consistently in the subjects. The antiviral activity in one granule membrane—associated fraction was associated with toxicity of the CD4+ cells and thus does not reflect CAF activity (Figure 2A). Except for this one instance, dilutions of the various subcellular fractions did not affect target CD4+ cell viability or growth (data not shown).
Antiviral activity of subcellular fractions isolated from CD8+ cells of HIV-infected subjects. Subcellular fractions, obtained during the purification of intracellular granules, were diluted in their respective fractionation buffer and added to acutely infected CD4+ cells in a standardized β-chemokine—insensitive virus assay for CAF activity. ▪ indicates cytoplasmic fraction; ▨, soluble components of granules fraction; and ▦, granule membrane. Percentage suppression of HIV replication ± SD was determined by dividing the amount of peak RT activity (day 7) in the culture fluids treated with the test sample by that treated with the respective buffer control, × 100. The amount of peak RT activity in the control infected CD4+ cell cultures was always greater than 200 000 cpm/0.1 mL. Some toxicity was noted in the least diluted granule membrane extract of subject A (*), which explains the reduction in RT activity observed. CD8+ cells from subjects A and B showed production of CAF in cell culture; those from subject C did not (Table 1). Negative values indicate enhancement of HIV replication.
Antiviral activity of subcellular fractions isolated from CD8+ cells of HIV-infected subjects. Subcellular fractions, obtained during the purification of intracellular granules, were diluted in their respective fractionation buffer and added to acutely infected CD4+ cells in a standardized β-chemokine—insensitive virus assay for CAF activity. ▪ indicates cytoplasmic fraction; ▨, soluble components of granules fraction; and ▦, granule membrane. Percentage suppression of HIV replication ± SD was determined by dividing the amount of peak RT activity (day 7) in the culture fluids treated with the test sample by that treated with the respective buffer control, × 100. The amount of peak RT activity in the control infected CD4+ cell cultures was always greater than 200 000 cpm/0.1 mL. Some toxicity was noted in the least diluted granule membrane extract of subject A (*), which explains the reduction in RT activity observed. CD8+ cells from subjects A and B showed production of CAF in cell culture; those from subject C did not (Table 1). Negative values indicate enhancement of HIV replication.
Discussion
CD8+ T lymphocytes can suppress HIV replication in a noncytotoxic manner. At least part of this activity is mediated by the production of a novel CD8+ cell antiviral factor, CAF.7 The present studies were conducted to determine whether CAF resides in granules in which other effector components, such as perforin, granzymes, and granulysin, can be found. The results indicate that granules of CD8+ T cells from HIV-infected subjects do not contain the antiviral activity associated with CAF (Figures 1, 2). Instead, anti-HIV activity was found in the cytoplasmic fractions of these antiviral CD8+ cells (Figure 2; Table 1). This antiviral activity observed in the cytoplasmic fraction was only associated with CD8+ cells that produced CAF, suggesting that the anti-HIV activity observed in these fractions resulted from CAF. This conclusion is supported by the lack of demonstrable CAF-like activity associated with granule exocytosis immediately after the mitogenic stimulation of CD8+ cells (Figure 1). CAF production, however, did become detectable 3 to 5 days later, as has been commonly demonstrated with activated CD8+ cells from healthy HIV-infected persons (Figure 1).7,11 These findings suggest CAF is not stored at substantial levels in the cell but requires new synthesis for its production.
The present results are consistent with previous work in our laboratory showing that the granule constituents, granzymes A and B and granulysin, do not show anti-HIV activity and that CAF activity in CD8+ cell culture fluids does not correlate with the granyzme concentrations in these fluids.12,18 Moreover, these studies also indicate that CAF does not appear to be produced with typical kinetics of cytokine production by CD8+ T cells, which is highest early after activation and then decreases after 1 to 3 days.
The absence of CAF activity in the extracts of exocytic granules likely does not result from inactivation of the antiviral factor during protein purification. The recovered granyzme B was still enzymatically active, and we have observed stability of CAF activity after several freeze-thaw procedures with CD8+ cell supernatants (C.E.M., unpublished observations, 1999). The results of this study, therefore, support the conclusion that CAF does not reside in appreciable amounts in exocytic granules but is directly secreted by CD8+ cells.
Prepublished online as Blood First Edition Paper, March 20, 2003; DOI 10.1182/blood-2002-10-3034.
Supported by National Institutes of Health grants RO1AI30350 and RO1AI42519 (J.A.L.) and grant RO1AI44941 (C.J.F.) and by National and Chicago Area Chapter Arthritis grants (C.J.F.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Michael Luther of Glaxo-Wellcome Corporation for providing the recombinant IL-2 used in these studies. We also thank Ann Murai and Kaylynn Peter for their help in the preparation of the manuscript.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal