Abstract
The goal of our study was to raise monoclonal antibodies (mAbs) against endothelial cell—surface proteins specific for tumor vasculature. Here, we describe the generation and intensive characterization of mAb AA98, including its functional properties and its antigen identification. In our study, an enhanced mAb AA98 immunoreactivity was observed on stimulated human umbilical vein endothelial cells (HUVECs). In addition, mAb AA98 showed remarkably restricted immunoreactivity against intratumoral neovasculature compared with blood vessels of normal tissues. We identified the AA98 antigen as human CD146, an adhesion molecule belonging to the immunoglobulin superfamily. Data from in vitro experiments imply structural and signaling functions for endothelial CD146; however, the role of CD146 in vivo is largely unknown. Here, we show that mAb AA98 displays antiangiogenic properties in vitro and in vivo. Proliferation and migration of HUVECs were inhibited by mAb AA98 as was angiogenesis in chicken chorioallantoic membrane (CAM) assays and tumor growth in 3 xenografted human tumor models in mice. Our data provide new insights into the function of CD146 on endothelial cells, validate CD146 as a novel target for antiangiogenic agents, and demonstrate that mAb AA98 has potential as a diagnostic and therapeutic agent in vascular and cancer biology. (Blood. 2003;102:184-191)
Introduction
The formation of blood vessels is mediated by vasculogenesis and angiogenesis. Vasculogenesis is responsible for the development of the vascular system during embryogenesis and is defined by the in situ differentiation of angioblasts into endothelial cells that aggregate into a primary capillary plexus. The formation of new capillaries from pre-existing microvasculature, angiogenesis, occurs both during development and in postnatal life during physiologic, reparative, and pathologic processes such as cancer and chronic inflammatory disorders.1-3 Furthermore, recent studies have suggested that circulating endothelial cells derived from endothelial progenitor cells of bone marrow and blood vessel endothelium of adults can participate in new blood vessel formation in normal and pathologic states, including tumor “neoangiogenesis.”4-6 The ultimate biomedical aim in vascular biology is to suppress blood vessel formation during pathologic processes, including tumorigenesis, and to promote new blood vessel formation when needed, for example, to prevent and treat ischemic events. To achieve this goal, it is important to identify and elucidate the molecular mechanisms that are crucial to both normal and disease-linked angiogenesis and vasculogenesis. Numerous mechanical, hormonal, humoral, and growth factors are implicated in these processes, which, by stimulation and inhibition, keep tight control of the growth of vasculature.1,2,7 Angiogenesis and vascular homeostasis are linked to the functional state of interendothelial junctions that are modulated by the state of growth and activation of the endothelial cells.8,9 It is conceivable that diseases linked to altered endothelial permeability or vascular morphogenesis are associated with alterations in the composition of intercellular junctions of endothelial cells. The typical transmembrane protein in adherens junctions is vascular endothelial (VE)—cadherin, while occludin is present in tight junctions. In addition, other adhesive molecules not directly located in these types of intercellular junctions have been found to be concentrated at endothelial cell-cell contacts. These include platelet endothelial cell adhesion molecule (PECAM), CD34, endoglin, and S-Endo-1/MUC18/CD146.8 CD146 was initially identified as a progression marker of melanoma (MUC18), melanoma-associated antigen (A32), melanoma cell adhesion molecule (Mel-CAM, MCAM) and endothelia-associated antigen (S-Endo-1).10-14 Like a number of other adhesion molecules, CD146 belongs to the immunoglobulin superfamily and contains 5 extracellular immunoglobulin (Ig)—like domains (V-V-C2-C2-C2), 1 transmembrane region, and a short cytoplasmic tail.15 CD146 is expressed predominantly in malignant melanocytes, but is also detectable in intermediate trophoblasts and blood vessels, in the thymic microenvironment, and as an activation marker on activated peripheral T lymphocytes and T leukemia cells.16-20 Enforced expression of CD146 on melanoma cells increases tumor growth and metastasis,21 and decreasing CD146 expression results in reduced tumorigenicity,22 indicating that CD146 may play an important role in promoting melanoma progression. However, the same molecule may serve as a tumor suppressor in breast cancer.23
Another important set of data has identified CD146 on circulating endothelial cells as a marker of endothelium and implicated CD146 as a structural component of interendothelial junctions.12,24-31 Although the ligand for CD146 has not been identified, engagement of CD146 with anti-CD146 antibodies has profound effects on cellular functions. It has been shown that CD146 can mediate Ca2+-independent homotypic and heterotypic cell-cell interactions on cultured endothelial cells and is involved in the control of intercellular permeability.28 Additional studies indicated that CD146 can induce the association of tyrosine kinase p59fyn with the cytoplasmic tail of CD146 and the phosphorylation of p125FAK and paxillin in human umbilical vein endothelial cells (HUVECs). This indicates that CD146 is not merely a “molecular glue,” but is actively involved in outside-in signaling and may be involved in the control of cell-cell contact.32,33 However, little is known about the in vivo function of CD146 in vascular biology and tumor growth.34 One way to investigate the expression and biologic roles of cell-surface proteins is to develop antibodies that specifically recognize and/or interfere with the proteins' functions.35
In the present study, we used an antibody-based approach and identified mAb AA98 as a novel anti-CD146 antibody. mAb AA98 showed restricted binding to CD146 expressed in blood vessels of tumors. This antibody efficiently inhibited the proliferation and migration of HUVECs and angiogenesis in chicken chorioallantoic membrane (CAM) assays and human tumor growth in 3 xenografted tumor models in mice.
Materials and methods
Primary antibodies
The primary antibodies used were mouse antihuman CD31 (PECAM-1), rat antimouse CD31 mAb MEC 13.3 (Pharmingen, Heidelberg, Germany), and isotype-matched IgG (Sigma, Beijing, China). Mouse mAb AA98 (this study) was used either as hybridoma culture supernatant for biochemical analysis or protein A—Sepharose purified from ascites for functional assays. Corresponding species-specific horseradish-peroxidase (HRP)—biotinylated, HRP-conjugated (Pierce, Beijing, China) or Cy2- and/or Cy3-conjugated secondary antibodies (Dianova, Hamburg, Germany) were used.
Cells, tissues, and animals
MCAM/CD146-transfected SBcl-2 cells and parental CD146- SBcl-2 cells were kindly supplied by Dr Judith P. Johnson (Institute of Immunology, University of Munich, Germany). HUVECs, human microvascular endothelial cells (HMVECs), and endothelial cell growth medium (EGM) were purchased from CellSystems (Biotechnologie Vertrieb GmbH, Katharinen, Germany). Cell lines A375, A431, HT1080, Pc-1, HBL100, SK-LMS-1, SW1990, hepatocarcinoma SSMC 7721, SKOV3, and Hela, and other cell lines (Table 1), were obtained from American Type Culture Collection (Rockville, MD). Human tumor tissues were obtained from the tissue bank of the 301 Hospital in Beijing. Human normal tissues were obtained from the Beijing Legal Medical Institute. BALB/c normal mice and nude mice were obtained from the Animal Center of the Chinese Academy of Medical Science, Beijing. Fertilized eggs of white Leghorn chicken were supplied by the Chicken Center of the Chinese Agriculture University, Beijing.
FACS analysis of mAb AA98 immunoreactivity to human cell lines
Cell line . | mAb AA98 . |
|---|---|
| HUVEC | + |
| HMVEC | + |
| Hepatocarcinoma ALEX | − |
| Osteosarcoma Saos-2 | − |
| Epidermoid cells A431 | − |
| Melanoma A375 | + |
| Melanoma SBcl | − |
| Lung cancer cell A549 | − |
| Colon cancer LS-174 | − |
| Colon cancer SW1116 | − |
| Embryonal kidney cell 293 | − |
| Leiomyosarcoma SK-LMS-1 | − |
| Hepatocarcinoma 7721 | − |
| Bladder cancer cell T24 | − |
| Leukemia T-cell Jurkat | − |
| Myeloblastic leukemia KG-1 | − |
| Pancreatic cancer SW1990 | − |
| Fibrosarcoma HT1080 | − |
| Mammary epithelial HBL 100 | − |
| Ovary carcinoma SKOV3 | − |
| Blood lymphocytes | − |
| Fibroblast | − |
| Prostastic cell Pc-1 | − |
| Choriocarcinoma JAR | − |
| Pancreatic cancer SW1990 | − |
| Cervix cancer HeLa | − |
Cell line . | mAb AA98 . |
|---|---|
| HUVEC | + |
| HMVEC | + |
| Hepatocarcinoma ALEX | − |
| Osteosarcoma Saos-2 | − |
| Epidermoid cells A431 | − |
| Melanoma A375 | + |
| Melanoma SBcl | − |
| Lung cancer cell A549 | − |
| Colon cancer LS-174 | − |
| Colon cancer SW1116 | − |
| Embryonal kidney cell 293 | − |
| Leiomyosarcoma SK-LMS-1 | − |
| Hepatocarcinoma 7721 | − |
| Bladder cancer cell T24 | − |
| Leukemia T-cell Jurkat | − |
| Myeloblastic leukemia KG-1 | − |
| Pancreatic cancer SW1990 | − |
| Fibrosarcoma HT1080 | − |
| Mammary epithelial HBL 100 | − |
| Ovary carcinoma SKOV3 | − |
| Blood lymphocytes | − |
| Fibroblast | − |
| Prostastic cell Pc-1 | − |
| Choriocarcinoma JAR | − |
| Pancreatic cancer SW1990 | − |
| Cervix cancer HeLa | − |
+ indicates positive for mAb AA98; −, negative.
Generation of monoclonal antibody
Primary HUVECs were obtained from human umbilical veins as described.36 For stimulation, HUVECs were grown in conditioned Dulbecco modified Eagle medium (DMEM) from hepatoma cell line SMMC 7721 for 3 weeks. Stimulated HUVECs (1 × 107) were injected intraperitoneally with Freund complete adjuvant into 6-week-old BALB/c mice and boosted 4 times weekly, and then their spleens were taken for hybridoma preparation as described.37
Screening of antibodies, ELISA, and immunohistochemistry
The enzyme-linked immunosorbent assay (ELISA) was used to select antibodies binding to stimulated HUVECs. Immunohistochemistry was then applied to assess the specificity of antibodies for neovasculature on hepatoma tissue. In ELISA, hybridomas were screened with freshly prepared primary HUVECs and stimulated HUVECs (previous paragraph). Cells were grown in 96-well plates and incubated with hybridoma culture supernatants at 25°C for one hour. Plates were washed 3 times in washing buffer (phosphate-buffered saline [PBS] with 0.05% Tween 20), and the bound antibodies were detected by incubation with HRP-conjugated anti—mouse IgG at 25°C for another hour. After careful washing, 3,3′,5,5′-tetramethylbenzydine (Sigma, Deisenhofen, Germany) was added as substrate. The color reaction was measured at 450 nm with a BioRad ELISA reader (Richmond, CA). Standard techniques were applied to examine tissues and tumors.
For immunohistochemistry, specimens from normal and pathologic tissues were cryosectioned, fixed in acetone at -20°C for 10 minutes, and then preincubated with 0.3% H2O2 in methanol for 30 minutes to quench endogeneous peroxidases. After every incubation step the sections were washed with PBS. Next the sections were blocked with 5% goat serum in PBS at room temperature for 2 hours. Then they were incubated with primary antibodies in PBS for another hour. mAb AA98 hybridoma culture supernatant was used at a 1:5 dilution. Appropriate biotin-conjugated secondary antibodies were then applied followed by HRP-conjugated streptavidin (Dianova). For negative controls, the primary antibodies were omitted or isotype-match control IgGs were used. The sections were finally counterstained with hematoxylin or hematoxylin and eosin.
Flow cytometric analysis
Detached cells were processed to obtain single-cell suspensions followed by staining with primary antibodies (mAb AA98, antihuman CD31 mAb, or isotype-matched control IgG as negative control at saturating concentrations) on ice for 40 minutes. After 3 washes in PBS containing 0.1% bovine serum albumin (BSA), cells were incubated with corresponding fluorescein isothiocyanate or phycoerythrin-conjugated secondary antibodies (Dianova) on ice for 40 minutes, then washed and analyzed using a FACSCalibur flow cytometry system (Becton Dickinson, San Jose, CA).
Immunoprecipitation and immunoblotting
HUVEC monolayers were cell-surface biotinylated with sulfo—N-hydroxysulfosuccinimide (NHS)—biotin according to the manufacturer's protocol (Pierce, Rockford, IL). Cells were scraped with a rubber policeman in 0.5 mL ice-cold lysis buffer (150 mM NaCl, 1 mM EDTA (ethylenediaminetetraacetic acid), 50 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), 10 mM sodium phosphate, pH 7.6, 10% glycerol, 1% Triton X-100, 1 mM phenylmethylsulfonyl fluoride, 25 μg/mL aprotinin, 2 μg/mL leupeptin, and 2 μg/mL pepstatin). Supernatants of cell lysates were precleared by incubation with protein A—Sepharose (Sigma, Deisenhofen, Germany) and incubated with mAb AA98 at 4°C for 2 hours, followed by an incubation with protein A—Sepharose for 1 hour. Immunoprecipitates were boiled for 5 minutes and analyzed by 10% sodium dodecyl sulfate—polyacrylamide gel electrophoresis (SDS-PAGE) under reducing or nonreducing conditions and immunoblotting. The membranes were probed either directly with HRP-conjugated streptavidin or with mAb AA98 followed by HRP-conjugated anti—mouse IgG. Protein bands were visualized with enhanced chemiluminescence reagent (Amersham Pharmacia Biotech, Freiburg, Germany).
Antigen purification and amino acid sequence
The cellular lining of human umbilical veins was collected by scraping and homogenized in lysis buffer (previous paragraph). The supernatant of the prepared cell extract was applied to a mAb AA98—protein A—Sepharose column. The bound proteins were eluted with 100 mM glycine, pH 3.0, followed by neutralization with 1 M Tris (tris(hydroxymethyl)aminomethane), pH 8.0. The eluates were separated by SDS-PAGE under nonreducing conditions and then electrotransferred onto polyvinylidene difluoride (PVDF) membranes (Millipore, Beijing, China). After staining with Coomassie blue, distinct bands of proteins were excised and their NH2-terminal sequence was determined by automated Edman degradation on an ABI Model 492 protein sequencer (Applied BioSystems, Weiterstadt, Germany). The FASTA program (William R. Pearson, University of Virginia, Charlottesville) was used for database searches.
Cell proliferation assay and cell death detection by ELISA
Proliferation of cells was measured using a [3H]-thymidine incorporation assay.38 Cells (2 × 103 cells/well) were seeded on 96-well culture plates, cultured until the cells reached 70% to 80% confluency, and then serum starved in DMEM for 12 to 20 hours. Endothelial cells were stimulated with 10 ng/mL vascular endothelial growth factor (VEGF) and 10 ng/mL basic fibroblast growth factor (bFGF) (Life Technologies, Gaithersburg, MD) in serum-free medium with mAb AA98 or control mIgG. For stimulation of nonendothelial cells, DMEM containing 10% fetal bovine serum (FBS) (Life Technologies) was used. After 48 hours, cells were pulsed with [3H]-thymidine (Amersham, Buckinghamshire, United Kingdom) for 4 hours. Cells were harvested and their [3H]-thymidine incorporation was measured in the liquid scintillation counter LKB1219.
To quantify apoptosis, cells were cultured as described in the presence or absence of mAb AA98 or control mIgG at various concentrations. The extent of histone-associated DNA fragments in the cytoplasmic fraction of cell lysates was measured by Cell Death Detection ELISAPLUS kit (Roche Diagnostics GmbH, Mannheim, Germany). This assay is based on the quantitative ELISA principle using mouse mAbs directed against DNA and histones. The presence of mono- and oligonucleosomes is a feature of cells undergoing apoptosis.
Cell migration assay
Cell migration was assayed using a modified Boyden chamber assay as described by Kuzuya and Kinsella using transwells (8-μm pore size; Corning Costar, NY).39 The transwells were coated with type I collagen solution at 100 μg/mL and then blocked with 1% BSA in PBS. Cells were trypsinized, washed, and resuspended in serum-free medium containing 1% BSA. Cells were then added to the upper chamber (5000 cells per well) in the presence of either mAb AA98 or control mIgG at various concentrations (1-25 μg/mL). Lower chambers contained serum-free medium containing 10 ng/mL human VEGF and bFGF or 1% BSA (negative control). After incubation for 4 hours at 37°C, cells remaining at the upper surface of the membrane were removed using a swab, while the cells that migrated to the lower membrane surface were fixed with ethanol and stained with Giemsa solution. The number of cells migrating through the filter was counted and plotted as the number of migrating cells per optic field (× 20).
CAM angiogenesis assay40
Fertilized eggs of white Leghorn chicken were incubated at 37°C at 65% to 70% humidity. The 6-day-old embryos with intact yolks were placed in a bowl and incubated at 37°C with 3% CO2. Small filter disks carrying either 1, 5, 10, or 20 μg purified mAb AA98 or control mIgG were separately applied to the CAM of individual embryos. After 24 hours of incubation, neovascularization in chicken CAMs was observed with a stereomicroscope. Vessels were counted in the vicinity of the implanted disks under a magnifier.
Animal experiments
Female 6-week-old BALB/c nude mice with a body weight of approximately 20 g were used and kept under specific pathogen-free conditions. Xenografts of human tumor cell lines were produced by injecting tumor cells (1× 107 resuspended in PBS) subcutaneously into the back of mice. When tumors reached a diameter of 3 to 5 mm, the mice were grouped (10 mice per group) and administered intraperitoneally purified mAb AA98 or mIgG at a dose of 10 mg/kg, or PBS, twice per week for 18 to 28 days. Isotype-matched mIgG or saline served as controls. Tumor size was measured twice per week and tumor volume was determined according to the equation: tumor size = width2 × length × (π/6). mAb AA98 or control mIgG were labeled with [131I] (Amersham) by using the iodogen method.41 [131I]-labeled mAb AA98 or [131I]-labeled mIgG with an activity of 18.5 Mbq [131I]/97 μg was injected intraperitoneally once to treat tumor-bearing mice.
Statistical analysis
The 2-tailed Student t test was used for statistical analysis. A P value less than .05 was considered statistically significant.
Results
Generation and screening of mAb AA98
We prepared primary HUVECs and stimulated them by culturing in conditioned medium from hepatocarcinoma SSMC 7721 cells. This medium was confirmed to contain a number of additional angiogenic factors, including VEGF, bFGF, transforming growth factor β (TGF-β), and platelet-derived growth factor, compared with normal medium containing 10% serum (data not shown). Stimulated HUVECs were used as immunogen to generate mouse monoclonal antibodies. In 2 steps, approximately 300 hybridomas were screened for clones producing antibodies that (1) bound to the cell surfaces of stimulated HUVECs but not primary HUVECs and (2) recognized tumor neovasculature in hepatocarcinoma, but not blood vessels from normal liver tissues. From this screen, 6 clones were selected for further characterization. The hybridoma clone AA98 was found to produce an IgG2a/κ antibody that satisfied best the 2 criteria.
Specificity of mAb AA98
The binding of mAb AA98 to stimulated HUVECs, but not to freshly prepared primary HUVECs, was confirmed by flow cytometric analysis. At the same time, both stimulated and primary HUVECs stained positive for CD31 (Figure 1Ai-ii, and data not shown). mAb AA98 also bound HMVECs in culture, whereas it did not bind to more than 20 different nonendothelial human cell lines with the exception of melanoma A375 (Table 1). In addition, we found that AA98 reactivity to primary HUVECs was increased after culture for several days in DMEM supplemented with 10% fetal calf serum, and it could be reversed by shifting the cell culture conditions to serum-free medium (Figure 1Aiii, and data not shown).
AA98 antigen expression analyses and identification of the AA98 antigen as CD146. (A) Flow cytometry analysis of AA98 antigen expression on HUVECs. (Ai) AA98 antigen is not detectable on primary HUVECs (pHUVEC) immediately after isolation. (Aii) mAb AA98 recognizes its antigen on the stimulated HUVECs (sHUVEC) cultured in conditioned medium from hepatocarcinoma SMMC 7721 cells. (Aiii) An increase in mAb AA98 immunoreactivity is detected on primary HUVECs cultured in the presence of 10% FBS and is decreased after shifting the cells to serum-free medium for at least 12 hours. (B) Immunohistochemical analysis of the AA98 antigen on frozen sections from human tumor and normal tissues. AA98 antigen is readily detectable in tumor neovasculature of hepatocarcinoma (Bii), thyroid tumor (Biv), and brain tumor (Bvi), whereas there was no significant mAb AA98 staining in corresponding normal tissue, such as liver (Bi), thyroid (Biii), and brain (Bv). Original magnification, × 200. (C) Characterization, purification, and identification of the AA98 antigen as CD146. (Ci-ii) Western blot analysis of cell surface—biotinylated proteins of HUVECs immunoprecipitated by mAb AA98, separated under nonreducing or reducing conditions by SDS-PAGE, and detected by either HRP-conjugated streptavidin (Ci) or mAb AA98 followed by HRP-conjugated anti—mouse IgG (Cii). Positions of molecular-weight standard marker proteins are shown at the right margin. (Ciii) Comparison of the first 20 amino acids of AA98 antigen with the amino acid sequence of human CD146. The first 20 amino acid residues of AA98 antigen are identical to the amino acid sequence of positions 24 to 43 of the human CD146 protein (Swissprot accession P43121). (Civ) Flow cytometric analysis shows the binding of mAb AA98 to stably CD146-transfected SBcl-2 cells (CD146-SBcl-2) but not to parental CD146-SBcl-2 cells.
AA98 antigen expression analyses and identification of the AA98 antigen as CD146. (A) Flow cytometry analysis of AA98 antigen expression on HUVECs. (Ai) AA98 antigen is not detectable on primary HUVECs (pHUVEC) immediately after isolation. (Aii) mAb AA98 recognizes its antigen on the stimulated HUVECs (sHUVEC) cultured in conditioned medium from hepatocarcinoma SMMC 7721 cells. (Aiii) An increase in mAb AA98 immunoreactivity is detected on primary HUVECs cultured in the presence of 10% FBS and is decreased after shifting the cells to serum-free medium for at least 12 hours. (B) Immunohistochemical analysis of the AA98 antigen on frozen sections from human tumor and normal tissues. AA98 antigen is readily detectable in tumor neovasculature of hepatocarcinoma (Bii), thyroid tumor (Biv), and brain tumor (Bvi), whereas there was no significant mAb AA98 staining in corresponding normal tissue, such as liver (Bi), thyroid (Biii), and brain (Bv). Original magnification, × 200. (C) Characterization, purification, and identification of the AA98 antigen as CD146. (Ci-ii) Western blot analysis of cell surface—biotinylated proteins of HUVECs immunoprecipitated by mAb AA98, separated under nonreducing or reducing conditions by SDS-PAGE, and detected by either HRP-conjugated streptavidin (Ci) or mAb AA98 followed by HRP-conjugated anti—mouse IgG (Cii). Positions of molecular-weight standard marker proteins are shown at the right margin. (Ciii) Comparison of the first 20 amino acids of AA98 antigen with the amino acid sequence of human CD146. The first 20 amino acid residues of AA98 antigen are identical to the amino acid sequence of positions 24 to 43 of the human CD146 protein (Swissprot accession P43121). (Civ) Flow cytometric analysis shows the binding of mAb AA98 to stably CD146-transfected SBcl-2 cells (CD146-SBcl-2) but not to parental CD146-SBcl-2 cells.
To investigate the specificity of mAb AA98 for tumor blood vessels, we screened 193 tumor tissues and 48 normal tissues by immunohistochemistry. mAb AA98 did not stain, or only slightly stained, blood vessels in normal tissues, including liver, brain, pancreas, and thyroid, with a frequency of staining of only 18.8% (9/48; Table 2). Remarkably, a strong mAb AA98—positive staining pattern was observed in 97.4% (188/193) of blood vessels from various tumor tissues (Table 2; Figure 1B; and data not shown). In tumor tissues, mAb AA98 strongly stained (CD31+) endothelial cells of capillaries, and in the few cases where mAb AA98 recognized normal tissues, the immunoreaction was localized mainly on perivascular cells of large arteries and veins (data not shown).
Immunohistochemical analysis of the specificity of mAb AA98 for blood vessels in normal and tumor human tissues
Tissues . | mAb AA98+/cases . |
|---|---|
| Normal tissues | |
| Liver | 0/14 |
| Brain | 0/6 |
| Adrenal gland | 0/4 |
| Pancreas | 0/5 |
| Stomach | 2/2 |
| Colon | 2/2 |
| Breast | 1/1 |
| Lung | 2/2 |
| Ovary | 2/2 |
| Cardiac muscle | 0/2 |
| Thyroid | 0/2 |
| Lymph node | 0/2 |
| Bone marrow | 0/2 |
| Sinuses | 0/2 |
| Tumors | |
| Hepatocarcinoma | 20/20 |
| Brain tumor | 9/9 |
| Kidney cancer | 2/2 |
| Pancreas cancer | 6/6 |
| Gastric cancer | 10/10 |
| Colon cancer | 15/15 |
| Breast cancer | 20/20 |
| Lung cancer | 18/18 |
| Ovary cancer | 9/9 |
| Leiomyosarcoma | 13/13 |
| Thyroid cancer | 16/20 |
| Throat cancer | 4/4 |
| Squamous cancer | 3/3 |
| Fibroangioma | 4/4 |
| Uterus cancer | 15/15 |
| Others | 24/25 |
Tissues . | mAb AA98+/cases . |
|---|---|
| Normal tissues | |
| Liver | 0/14 |
| Brain | 0/6 |
| Adrenal gland | 0/4 |
| Pancreas | 0/5 |
| Stomach | 2/2 |
| Colon | 2/2 |
| Breast | 1/1 |
| Lung | 2/2 |
| Ovary | 2/2 |
| Cardiac muscle | 0/2 |
| Thyroid | 0/2 |
| Lymph node | 0/2 |
| Bone marrow | 0/2 |
| Sinuses | 0/2 |
| Tumors | |
| Hepatocarcinoma | 20/20 |
| Brain tumor | 9/9 |
| Kidney cancer | 2/2 |
| Pancreas cancer | 6/6 |
| Gastric cancer | 10/10 |
| Colon cancer | 15/15 |
| Breast cancer | 20/20 |
| Lung cancer | 18/18 |
| Ovary cancer | 9/9 |
| Leiomyosarcoma | 13/13 |
| Thyroid cancer | 16/20 |
| Throat cancer | 4/4 |
| Squamous cancer | 3/3 |
| Fibroangioma | 4/4 |
| Uterus cancer | 15/15 |
| Others | 24/25 |
mAb AA98 recognizes human CD146
To identify the AA98 antigen, cell extracts from cell-surface—biotinylated stimulated HUVECs were immunoprecipitated with-mAb AA98 and immunoblotted with HRP-conjugated streptavidin. Figure 1Ci shows that mAb AA98 immunoprecipitated a protein with an apparent molecular mass of 110 kDa analyzed by SDS-PAGE under reducing conditions and of 100 kDa under nonreducing conditions. However, mAb AA98 did not react with the 110-kDa protein under reducing conditions in Western blot experiments (Figure 1Cii), suggesting that mAb AA98 recognized a conformation-dependent epitope of this protein. We next purified the AA98 antigen from cell extracts of human umbilical veins using a mAb AA98—protein A—Sepharose column. Concentrated eluates were analyzed by SDS-PAGE under nonreducing conditions and electroblotting. Coomassie blue staining of the corresponding blots revealed the expected distinct mAb AA98—reactive 100-kDa protein (data not shown). The first 20 amino acid residues of the purified AA98 antigen were identified by NH2-terminal sequencing. Protein database searches showed that they were identical to the amino acid sequence positions 24 to 43 of the human CD146 protein (Figure 1Ciii), indicating the identity of the AA98 antigen with human CD146 and the removal of a 23 residue signal peptide in the AA98 antigen.10 The binding of mAb AA98 to CD146-transfected SBcl-2 melanoma cells, but not to CD146- parental SBcl-2 cells in FACS analysis (Figure 1Civ), confirmed the identity of AA98 antigen to CD146.
mAb AA98 inhibits proliferation and migration of HUVECs but does not induce apoptosis
We examined the functional properties of mAb AA98 in angiogenic processes, particularly in the proliferation and migration of HUVECs. The [3H]-thymidine assay was used to test the effects of mAb AA98 on the proliferation of CD146+ HUVECs and A375 melanoma cells. mAb AA98 inhibited the incorporation of [3H]thymidine into DNA of HUVECs in a dose-dependent manner (28.5% at 1 μg/mL, 43.2% at 10 μg/mL, and 62.4% at 100 μg/mL), whereas the isotype-matched control antibody displayed very low levels of inhibition (5%-7%). The inhibitory effects of mAb AA98 compared with mIgG control on the proliferation of A375 cells did not differ significantly over a broad range of antibody concentrations (Figure 2A). mAb AA98 did not inhibit the proliferation of other tumor cells including hepatocarcinoma SMMC 7721 cells, cervix tumor Hela cells, and ovary tumor SKOV3 cells (data not shown), confirming that the antiproliferative effect of mAb AA98 on CD146+ HUVECs was specific and not due to cytotoxic effects.
mAb AA98 inhibits proliferation and migration of HUVECs and does not promote apoptosis. (A) HUVECs or A375 melanoma cells were incubated with mAb AA98 in culture medium at indicated concentrations for 48 hours followed by incubation with [3H]-thymidine for 4 hours. The inhibition of [3H]-thymidine incorporation by mAb AA98 (100 μg/mL) compared with control mIgG (100 μg/mL) showed that mAb AA98 had a significant inhibitory effect on the proliferation of HUVECs, whereas proliferation of A375 cells was not affected. The assays were performed 2 times in duplicate and the data represent the average of duplicate determinations from a single representative experiment. The range of the values for the duplicate determinations was less than 10%. (B) Migration of HUVECs toward VEGF/bFGF in the presence of 10 μg/mL mAb AA98 or control mIgG. The migration assay was allowed to proceed for 4 hours, and the number of cells reaching the lower chamber—side of the membrane were counted at high-power fields (original magnification, × 200). Graphic representation of migrated cells is shown as the mean ± SD of 10 fields on duplicated filters. (C) mAb AA98 does not promote apoptosis of proliferating HUVECs or A375 cells. Apoptosis assays were performed with cultured HUVECs and A375 cells in the presence or absence (untreated) of antibodies mAb AA98 or mIgG (negative control). Cells cultured in the presence of the apoptosis-promoting agent doxorubicin (DOX) at 1 μM served as positive apoptosis control. Concentrations of antibodies are indicated on the x-axis. Absorbance on the y-axis is directly proportional to the extent of apoptosis. Each point represents the mean of triplicate determinations; error bars, ± SD.
mAb AA98 inhibits proliferation and migration of HUVECs and does not promote apoptosis. (A) HUVECs or A375 melanoma cells were incubated with mAb AA98 in culture medium at indicated concentrations for 48 hours followed by incubation with [3H]-thymidine for 4 hours. The inhibition of [3H]-thymidine incorporation by mAb AA98 (100 μg/mL) compared with control mIgG (100 μg/mL) showed that mAb AA98 had a significant inhibitory effect on the proliferation of HUVECs, whereas proliferation of A375 cells was not affected. The assays were performed 2 times in duplicate and the data represent the average of duplicate determinations from a single representative experiment. The range of the values for the duplicate determinations was less than 10%. (B) Migration of HUVECs toward VEGF/bFGF in the presence of 10 μg/mL mAb AA98 or control mIgG. The migration assay was allowed to proceed for 4 hours, and the number of cells reaching the lower chamber—side of the membrane were counted at high-power fields (original magnification, × 200). Graphic representation of migrated cells is shown as the mean ± SD of 10 fields on duplicated filters. (C) mAb AA98 does not promote apoptosis of proliferating HUVECs or A375 cells. Apoptosis assays were performed with cultured HUVECs and A375 cells in the presence or absence (untreated) of antibodies mAb AA98 or mIgG (negative control). Cells cultured in the presence of the apoptosis-promoting agent doxorubicin (DOX) at 1 μM served as positive apoptosis control. Concentrations of antibodies are indicated on the x-axis. Absorbance on the y-axis is directly proportional to the extent of apoptosis. Each point represents the mean of triplicate determinations; error bars, ± SD.
We next examined the effect of mAb AA98 on the migration of HUVECs in a modified Boyden chamber assay. To that end, the migration of HUVECs toward the chemoattractants VEGF and bFGF in serum-free medium was analyzed in the presence of mAb AA98 or mIgG control antibody. Figure 2B shows that the number of migrating cells in the presence of mAb AA98 resulted in a 75% reduction compared with mIgG-treated cells (Figure 2B).
Because mAb AA98 inhibited cell proliferation and migration of HUVECs, we further studied whether mAb AA98 induced apoptosis of HUVECs, A375, or Hela cells. Cells were incubated in the presence or absence of AA98 or control mIgG. The apoptosis-promoting agent doxorubicin42,43 was added to the cultures used as positive controls. Apoptosis was analyzed using a sensitive ELISA recognizing histone-associated DNA fragments in cytoplasmic cell lysates. We could not detect any significant apoptotic activity of mAb AA98 compared with mIgG in cultures of proliferating HUVECs, A375, or Hela cells using antibody concentrations of 1 to 100 μg/mL (Figure 2C, and data not shown).
Inhibition of angiogenesis in CAM assays by mAb AA98
Activation, migration, and proliferation of endothelial cells play important roles in angiogenesis.1,2 We therefore investigated the effect of mAb AA98 on angiogenesis in the CAM assay. The function of CD146 in such experimental settings has not been addressed in previous studies. Placed on CAMs of day-6 embryos were 1, 5, 10, or 20 μg mAb AA98 or control mIgG absorbed on small filter disks. We used 20 embryos per group for mAb AA98 treatment and 10 embryos per group for mIgG treatment. After 12 hours of incubation, areas surrounding the applied mAb AA98 were nearly avascular. These avascular zones became larger with increasing incubation time. At 24 hours of incubation, the zone size reached about 10 mm2, and no evidence of any inflammatory reaction or hemorrhage was seen in the implant area (Figure 3A). In mAb AA98—treated CAMs, the frequency of avascular zones at the implantation sites was observed to increase with increasing amounts of antibody: 13 of 20 CAMs at 1 μg, 15 of 20 CAMs at 5 μg, 18 of 20 CAMs at 10 μg, and 20 of 20 CAMs at 20 μg. The mAb AA98—treated areas were conspicuous by the scarcity of sprouting or branching capillaries. Their blood vessel density was reduced approximately 7-fold compared with mIgG-treated CAMs (Figure 3C).
mAb AA98 inhibits angiogenesis in CAM assays. (A-B) CAM angiogenesis assays showing an almost avascular area in the CAM where the disk carrying 10 μg mAb AA98 was implanted and incubated for 24 hours (A). Normal vascularized CAMs were observed with 10 μg mIgG for 24 hours (B). White arrowheads point to implanted disk, and black arrowheads indicate the borders of the almost avascular area. (C) The number of small branching capillaries was counted within a defined area of 72 mm2surrounding the implanted disk. In each group, 10 embryos were used and the data represent the mean values (± SEM).
mAb AA98 inhibits angiogenesis in CAM assays. (A-B) CAM angiogenesis assays showing an almost avascular area in the CAM where the disk carrying 10 μg mAb AA98 was implanted and incubated for 24 hours (A). Normal vascularized CAMs were observed with 10 μg mIgG for 24 hours (B). White arrowheads point to implanted disk, and black arrowheads indicate the borders of the almost avascular area. (C) The number of small branching capillaries was counted within a defined area of 72 mm2surrounding the implanted disk. In each group, 10 embryos were used and the data represent the mean values (± SEM).
Inhibition of human tumor growth in xenografted mice by mAb AA98
The possibility that mAb AA98 may retain its antiangiogenic properties in tumor-bearing mice prompted us to study endothelial CD146 as a target for anticancer therapy. We generated 3 human xenografted tumor models by subcutaneously injecting SK-LMS-1 cells (leiomyosarcoma), SW1990 cells (pancreatic cancer), or hepatocellular carcinoma SSMC 7721 cells into the back of BALB/c nude mice. When tumors reached a size of 0.3 to 0.5 cm in diameter, purified mAb AA98, control mIgG, or PBS was injected intraperitoneally into mice, twice per week. Tumor growth was monitored by determination of relative tumor volume. After 18, 24, or 28 days of treatment, the sizes of all 3 types of tumors were significantly reduced in mAb AA98—treated groups compared with control, either mIgG or saline (Figure 4A-C). The inhibition is 72% in hepatocarcinoma, 50% in leiomyosarcoma, and 41% in pancreatic tumors. In the course of our experiments, we did not observe any toxic effects associated with administration of mAb AA98. No metastasis was found in the mAb AA98—treated group by histopathologic analysis, whereas in the control group 60% to 80% of the mice developed metastases in lung and lymph nodes and 20% died at days 14 to 16 after administration (data not shown).
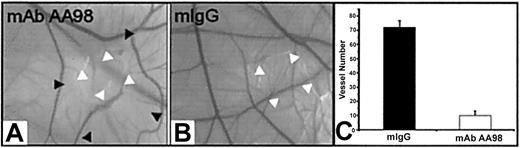
Tumor growth inhibition and reduction of blood vessel density in tumors following the administration of mAb AA98. Mean tumor volumes at specific time points after injection of human hepatocarcinoma SSMC 7721 (A), leiomyosarcoma SK-LMS-1 (B), or pancreatic cancer SW1990 (C-D) cells subcutaneously into the back of BALB/c nude mice are shown. Mean tumor volumes of mice carrying human hepatocarcinoma (n = 10; P < .05), leiomyosarcoma (n = 10; P < .05), or pancreatic cancer (n = 10; P < .05) treated with mAb AA98 (A-C) or with [131I]-labeled mAb AA98 (D) (n = 10; P < .05) were significantly smaller when compared with mean tumor volumes from tumor-bearing mice treated with isotope-matched mIgG (B-C), [131I]-mIgG (D), and/or PBS (A-D) as controls (n = 10; P < .05). (E-F) Sections from a mAb AA98 or control IgG-treated hepatocellular carcinoma stained with anti-CD31 mAbs are shown. (G) The numbers of blood vessels in pancreatic tumors treated with mIgG or mAb AA98 and [131I]-mIgG or [131I]-mAb AA98 were counted from 3 sections within × 200 fields. Data are the average values of 3 high-power fields at × 200 per section. (H) Hematoxylin and eosin—stained section from a mAb AA98—treated leiomyosarcoma. Black arrows indicate blood vessels with narrowed lumen surrounded by an increased eosinophilic layer of extracellular material.
Tumor growth inhibition and reduction of blood vessel density in tumors following the administration of mAb AA98. Mean tumor volumes at specific time points after injection of human hepatocarcinoma SSMC 7721 (A), leiomyosarcoma SK-LMS-1 (B), or pancreatic cancer SW1990 (C-D) cells subcutaneously into the back of BALB/c nude mice are shown. Mean tumor volumes of mice carrying human hepatocarcinoma (n = 10; P < .05), leiomyosarcoma (n = 10; P < .05), or pancreatic cancer (n = 10; P < .05) treated with mAb AA98 (A-C) or with [131I]-labeled mAb AA98 (D) (n = 10; P < .05) were significantly smaller when compared with mean tumor volumes from tumor-bearing mice treated with isotope-matched mIgG (B-C), [131I]-mIgG (D), and/or PBS (A-D) as controls (n = 10; P < .05). (E-F) Sections from a mAb AA98 or control IgG-treated hepatocellular carcinoma stained with anti-CD31 mAbs are shown. (G) The numbers of blood vessels in pancreatic tumors treated with mIgG or mAb AA98 and [131I]-mIgG or [131I]-mAb AA98 were counted from 3 sections within × 200 fields. Data are the average values of 3 high-power fields at × 200 per section. (H) Hematoxylin and eosin—stained section from a mAb AA98—treated leiomyosarcoma. Black arrows indicate blood vessels with narrowed lumen surrounded by an increased eosinophilic layer of extracellular material.
To test if the efficacy of treatment increases when AA98 is combined with another anticancer agent, we prepared [131I]-labeled AA98 and used it as a single administration to treat pancreatic tumors when their sizes were about 0.3 cm in diameter. The tumor volumes were monitored over a period of 18 days. Figure 4D shows that [131I]-labeled mAb AA98 significantly inhibited tumor growth by 75% compared with [131I]-mIgG as a negative control.
We further examined the correlation between vascularity and tumor growth by counting blood vessels on the treated tumor sections using an anti-CD31 antibody as a marker for blood vessels (Figure 4E-F). We observed a reduction of microvessel density in the range of approximately 70% in tumors treated with mAb AA98 compared with tumors treated with mIgG (Figure 4G, and data not shown). This is consistent with the observation that the surfaces of mAb AA98—treated tumors appeared pale in contrast to the bloody surfaces of tumors from control mice (data not shown). Furthermore, histologic analysis of tumors revealed that blood vessels with narrowed lumina surrounded by enhanced eosinophilic material, most likely extracellular matrix proteins, were present in mAb AA98—treated tumors, and some blood vessels appeared to be occluded by a thrombus. Tumors of control animals did not show these features (Figure 4H, and data not shown).
Discussion
In the present study, we used an antibody-based approach to identify endothelial cell—surface proteins specific for tumor vasculature. We successfully selected mAb AA98 that bound to stimulated HUVECs and preferentially recognized tumor blood vessels, and identified AA98 antigen as human endothelial CD146.10 Using this antibody, we detected a dramatic increase of CD146 expression on HUVECs stimulated with either conditioned medium or serum containing medium. In contrast, on shifting the cell culture conditions back to serum-free medium, the endothelial CD146 expression was remarkably decreased. Other groups have reported the similar finding that an increase in CD146 expression was found on human microvascular endothelial cells and confluent HUVECs compared with nonconfluent HUVECs.27,28 Strikingly, our immunohistologic analyses of tissues revealed that mAb AA98 preferentially stained almost all blood vessels of the examined tumor tissues as opposed to only a limited number of blood vessels of normal tissues. In some cases, we also observed a low intensity mAb AA98 immunoreactivity on tumor cells themselves.
This rather restricted AA98/CD146 expression on tumor blood vessels is not inconsistent with earlier studies where immunoreactivity of other anti-CD146 mAbs on blood vessels, endothelium, and endothelia-derived tumors was noted.23,34 However, most previous studies have focused more on the distribution of CD146 in normal tissues and tumor cells, particularly melanomas, rather than on blood vessels of the corresponding tissues.10-12,15,16,34 While CD146 is presently recognized as an endothelial marker on theentire vascular tree and on circulating endothelial cells,12,26-28 the specificity of mAb AA98 for tumor neovasculature may either reflect higher expression levels of AA98/CD146 on the microvascular endothelium of tumor compared with other endothelia, including large vessels of normal tissues, or mAb AA98 may recognize a specific activation epitope of the CD146 molecule. Specific activation epitopes have been described for other cell adhesion molecules, including integrin β1, prostate-specific membrane antigen, and CD44.44-48 The differences in reported reactivity of various anti-CD146 mAbs in immunohistologic studies are likely due to their different epitopes, as discussed earlier by Solovey et al.28 Therefore, mapping the epitopes of anti-CD146 mAbs will provide a better understanding of the observed variation in CD146 expression and the functional properties of these mAbs.
Adhesion molecules such as integrins and cadherins mediate cell morphogenesis, proliferation, and survival via their coodinated interactions with cytoskeletal and signaling molecules.49 Functional studies using various anti-CD146 antibodies have implicated CD146 as playing an important role in many of these processes, including cell-cell interaction, control of intercellular permeability, cell migration, and/or promotion of focal adhesion assembly via phosphorylation of p59fyn, p125FAK, and paxillin.27,28,32,33 In this study, we demonstrate that mAb AA98 has a pronounced antiproliferative effect on HUVECs, but not on CD146+ A375 melanoma cells or various other tumor cells in vitro and show, for the first time, that CD146 is involved in the control of endothelial cell proliferation. However, since we performed our proliferation studies with cultured A375 cells on substrate, we cannot rule out the possibility that mAb AA98 also inhibits the anchorage-independent growth of melanoma in a tissue context as shown by Satyamoorthy et al22 using Mel-CAM/CD146-specific genetic suppressor elements. Importantly, the engagement of mAb AA98 with CD146 on HUVECs or A375 cells does not lead to apoptotic cell death, indicating the inhibitory roles of mAb AA98 are apoptosis independent, although the underlying mechanisms are not yet known. The same applies to our data gained from migration assays. Here, we have demonstrated that soluble mAb AA98 inhibits migration of HUVECs independent of apoptosis in response to chemoattractants VEGF/bFGF. The involvement of CD146 in the control of cell migration has been suggested previously by 2 different approaches. First, Solovey et al have shown that only beads of immobilized anti-CD146 mAb P1H12, but not soluble P1H12 mAb, can inhibit in vitro migration of HUVECs in “wound closure” experiments, proposing a block of movement by contact inhibition.28 Second, it has been shown that overexpression of chicken CD146 (HEMCAM/gicerin) in fibroblasts can inhibit their integrin β1—mediated adhesion and migratory properties.50 Considering the importance of changes in the actin cytoskeleton and integrin-mediated adhesions for cells to control the transition from a migratory to a proliferative state in order to enter the cell cycle,51 and the increase of p125FAK observed in migratory cells52,53 and in cell cultures with increasing cell density,54 it will be important to test whether the engagement of mAb AA98 with CD146 exerts a negative effect on the phosphorylation events of p59fyn, p125FAK, and paxillin.27,28,32,33 Taking these in vitro studies into account, we suggest that CD146 is functionally connected to structural and signaling networks that control migration, proliferation, and endothelial monolayer formation. It will be necessary to determine whether CD146 promotes or represses proliferation and migration for each different cell type, and consequently, whether mAb AA98 acts as an agonist or antagonist of CD146 in each case.
Despite the proposed structural and signaling functions for CD146, the in vivo roles of CD146 on endothelia are still largely unknown.27,28,33 Our data, using mAb AA98 as a function-blocking agent, provide the first in vivo evidence that CD146 is functionally involved in angiogenesis. In CAM-angiogenesis assays, mAb AA98 inhibits blood vessels from forming secondary and tertiary vascular branches, suggesting that mAb AA98 interferes with relatively early angiogenic processes instrumental for sprouting of new blood vessels rather than for vessel maintenance.1,2
In our animal studies, we have shown that the administration of mAb AA98 significantly inhibits tumor growth associated with an efficient decrease in microvessel density. Given the fact that mAb AA98 inhibits endothelial cell migration and proliferation in vitro, it is most likely that these early angiogenic processes required for vascular sprouting were also targeted by mAb AA98 in our in vivo studies, leading to the inhibition or reduction of neovascularization, which is naturally needed to supply the CAM or the rapidly growing tumor mass.1,2 These findings are novel and the functional importance of CD146 on endothelial cells, but not on tumor cells, for tumor angiogenesis, as shown by the antiangiogenic effects of mAb AA98, is supported by the fact that the xenografted tumor cells do not express CD146. However, at present, we cannot exclude functional heterophilic interactions of endothelial CD146 with neighboring tumor cells, as was shown for CD146 by coculture studies of melanoma cells and endothelial cells.21 It will be of crucial importance to identify downstream events induced by the homophilic and/or heterophilic engagement of CD146 in order to understand the exact mechanisms of CD146 participation in angiogenesis in vivo. It is tempting to speculate that one possible mechanism may involve the regulation of matrix metalloproteinase activity, since it was shown that enforced expression of CD146 in melanoma cells resulted in a significant increase in matrix metalloproteinase 2 activity.21 Moreover, the process of extracellular matrix remodelling by capillary endothelial cells has been shown to promote migration, proliferation, and capillary tube formation required for neovascularization.55
In summary, our studies show that mAb AA98 can efficiently inhibit the migration and proliferation of HUVECs, inhibit angiogenesis in CAM assays and tumors, and inhibit the growth of 3 human xenograft tumors in mice. In the future, it will be of interest to investigate whether these effects are limited to tumor angiogenesis or if the same mechanisms apply to other situations including embryonic development,17,18 circulating bone marrow—derived endothelial and hematopoietic precursor cells,5,26,29,30 and tumor progression of human melanoma.11,15,21,56
While this paper was under revision, inhibition of tumor growth and metasasis of human melanoma by antibodies to MCAM/MUC18/CD146 was reported by Mills et al.57 The characterization of the CD146 epitope recognized by mAb AA98 and the identification of the unknown CD146 ligand(s) may lead to the development of other agents with high therapeutic potential for the inhibition of angiogenesis and, therefore, tumor growth and progression.
Prepublished online as Blood First Edition Paper, February 27, 2003; DOI 10.1182/blood-2002-04-1004.
Supported by the National 863 and 973 grant, a Chinese Academy of Sciences grant, the National Natural Sciences Foundation of China (X.Y.), and a scientific exchange program between the Chinese Academy of Sciences and the Max-Planck-Society (X.Y., B.L.B).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Rupert Timpl for his support and suggestions on the manuscript; Dr Markus Keller and Albert Ries for technical advice; Dr J. Johnson for supplying the CD146-transfected SBcl-2 and parental SBcl-2 cells; and Prof Yumei Wen, Dr Andrew Perrett, and Dr Kathryn Rodgers for critical reading of the manuscript.


![Figure 2. mAb AA98 inhibits proliferation and migration of HUVECs and does not promote apoptosis. (A) HUVECs or A375 melanoma cells were incubated with mAb AA98 in culture medium at indicated concentrations for 48 hours followed by incubation with [3H]-thymidine for 4 hours. The inhibition of [3H]-thymidine incorporation by mAb AA98 (100 μg/mL) compared with control mIgG (100 μg/mL) showed that mAb AA98 had a significant inhibitory effect on the proliferation of HUVECs, whereas proliferation of A375 cells was not affected. The assays were performed 2 times in duplicate and the data represent the average of duplicate determinations from a single representative experiment. The range of the values for the duplicate determinations was less than 10%. (B) Migration of HUVECs toward VEGF/bFGF in the presence of 10 μg/mL mAb AA98 or control mIgG. The migration assay was allowed to proceed for 4 hours, and the number of cells reaching the lower chamber—side of the membrane were counted at high-power fields (original magnification, × 200). Graphic representation of migrated cells is shown as the mean ± SD of 10 fields on duplicated filters. (C) mAb AA98 does not promote apoptosis of proliferating HUVECs or A375 cells. Apoptosis assays were performed with cultured HUVECs and A375 cells in the presence or absence (untreated) of antibodies mAb AA98 or mIgG (negative control). Cells cultured in the presence of the apoptosis-promoting agent doxorubicin (DOX) at 1 μM served as positive apoptosis control. Concentrations of antibodies are indicated on the x-axis. Absorbance on the y-axis is directly proportional to the extent of apoptosis. Each point represents the mean of triplicate determinations; error bars, ± SD.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/1/10.1182_blood-2002-04-1004/5/m_h81334571002.jpeg?Expires=1767764962&Signature=QHriqvbMYM-itln8EumV0Yp9u1ltLm0YLWY~bcL2nBmabFVPTiVgx~LAFhn2UuIk3F~4IxHayAf2K4OAC~hKMfs0yx-rMrI22FYiErPKTejFPWXEllFyH5gAgRWsSUVRkYv3~GmrayrAtaSZN7WzhOerAiHTF9pghALUh~8Z2p6cNv7LwEPLkB2hPW-~pgMP~P1YO9d0PB~d4ENnUhci7veUApHZViWCN7HG4zTygiSLK4VTCfuaA1Wo47a6HhWIeNJwj4YgRbo525QVx~X4GkSTaNmmz89gzVq5R5pqOsdB8XyFlbvUpHoeGA1bQUOJ6npWJhlyYlLodleBjRUnjw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. Tumor growth inhibition and reduction of blood vessel density in tumors following the administration of mAb AA98. Mean tumor volumes at specific time points after injection of human hepatocarcinoma SSMC 7721 (A), leiomyosarcoma SK-LMS-1 (B), or pancreatic cancer SW1990 (C-D) cells subcutaneously into the back of BALB/c nude mice are shown. Mean tumor volumes of mice carrying human hepatocarcinoma (n = 10; P < .05), leiomyosarcoma (n = 10; P < .05), or pancreatic cancer (n = 10; P < .05) treated with mAb AA98 (A-C) or with [131I]-labeled mAb AA98 (D) (n = 10; P < .05) were significantly smaller when compared with mean tumor volumes from tumor-bearing mice treated with isotope-matched mIgG (B-C), [131I]-mIgG (D), and/or PBS (A-D) as controls (n = 10; P < .05). (E-F) Sections from a mAb AA98 or control IgG-treated hepatocellular carcinoma stained with anti-CD31 mAbs are shown. (G) The numbers of blood vessels in pancreatic tumors treated with mIgG or mAb AA98 and [131I]-mIgG or [131I]-mAb AA98 were counted from 3 sections within × 200 fields. Data are the average values of 3 high-power fields at × 200 per section. (H) Hematoxylin and eosin—stained section from a mAb AA98—treated leiomyosarcoma. Black arrows indicate blood vessels with narrowed lumen surrounded by an increased eosinophilic layer of extracellular material.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/1/10.1182_blood-2002-04-1004/5/m_h81334571004.jpeg?Expires=1767764962&Signature=g06u6W31X9DwELIDibjyPX5AO97Elw~n5Z6mBCcm34n24SssF8HGCQcQix~-w5VTFZRTP3uy2DK-f7FcRUl-ugB6xitMqrzDx~aG8~X3D3Jt-SQxRqeoluM7AG~VNGjzdvl2MMGg7YRvXYb~NBYgVguLMri~LoUnJDEPvl~FvRC-Pk-zIubvZFLTky01WlTcQ~Ua9jcEg~c7Guk3UvZxYqjfw0sGJFEcC4ampMf68VfdcPrpLLis1Y46~lPM1PU7TXWy-j5FgPMCsioAodJaDKvMhmZvlC6cam9ZhU5q8Bkl9FYpR0MdXlEiIa6hd59DyiZyJOMji3mmeRhLuoz6gA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal