Abstract
The Fas receptor and its ligand have been implicated in mediating the bone marrow (BM) suppression observed in graft-versus-host disease and a number of other BM-failure syndromes. However, previous studies have suggested that Fas is probably not expressed on human hematopoietic stem cells (HSCs), but up-regulated as a consequence of their commitment and differentiation, suggesting that progenitors or differentiated blood cells, rather than HSCs, are the targets of Fas-mediated suppression. The present studies confirm that candidate HSCs in human cord blood and BM lack constitutive expression of Fas, but demonstrate that Fas expression on CD34+ progenitor and stem cells is correlated to their cell cycle and activation status. With the use of recently developed in vitro conditions promoting HSC self-renewing divisions, Fas was up-regulated on virtually all HSCs capable of multilineage reconstituting nonobese diabetic/severe combined immunodeficiency (NOD-SCID) mice in vivo, as well as on long-term culture-initiating cells (LTC-ICs). Similarly, in vivo cycling of NOD-SCID repopulating cells upon transplantation, resulted in up-regulation of Fas expression. However, repopulating HSCs expressing high levels of Fas remained highly resistant to Fas-mediated suppression, and HSC function was compromised only upon coactivation with tumor necrosis factor. Thus, reconstituting human HSCs up-regulate Fas expression upon active cycling, demonstrating that HSCs could be targets for Fas-mediated BM suppression. (Blood. 2003;102: 118-126)
Introduction
The continuous and high turnover of cells in the blood system is strictly dependent on the existence of multipotent hematopoietic stem cells (HSCs) with a high self-renewal and regenerative capacity.1 Thus, HSCs are both required and sufficient for reconstitution of the entire hematopoietic system.1 During the last decade, sophisticated methods have been developed allowing phenotypic characterization and prospective isolation of HSCs.2-6 However, HSCs cannot be purified to homogeneity and must therefore ultimately be identified through their unique ability to multilineagereconstitute conditioned recipients.7,8 More recently, in vivo xenograft reconstitution assays have also been developed, facilitating similar studies of candidate human HSCs.5,9-11 Although their functional properties are shared, the cell surface phenotypes of mouse and human HSCs seem to show distinct and important differences. For instance, whereas HSCs in adult mouse bone marrow (BM) are almost exclusively CD34-CD38+,6,12 the majority of reconstituting HSCs in human BM as well as in cord blood (CB) appear to be CD34+CD38-.5,13,14 Furthermore, the expression of important cytokine receptors, such as c-kit and flt3, also in part show a different pattern on mouse and human HSCs.15,16
The self-renewal, commitment, and differentiation of HSCs are tightly regulated processes, controlled in part by interaction between cytokines and their receptors.17-19 A number of growth-promoting cytokines regulating hematopoietic development have been identified.17-19 Of these, the ligands for c-kit, flt3, and c-mpl have all been implicated as nonredundant regulators of the HSC pool and early stages of HSC commitment.15,20-22 Cytokines with inhibitory effects on hematopoiesis have been identified as well, but the effects and roles of these in HSC regulation remain largely unexplored.23-27
Fas, a member of the tumor necrosis factor (TNF) receptor family, has been implicated as playing a role in regulating lymphoid as well as myeloid development.26,28-32 Fas and its ligand have also been documented as suppressing hematopoiesis in acute graft-versus-host disease (GVHD) as well as in a number of chronic BM-failure syndromes.33-35 Whether the inhibitory effect of Fas on normal human hematopoiesis and in BM suppression might involve targeting of the HSC pool is unclear. In adult mice, short and long-term reconstituting HSCs lack constitutive cell surface Fas expression.36,37 Whereas Fas expression is up-regulated on cycling murine hematopoietic progenitors, HSCs remain Fas- upon active cycling, and exposure to high levels of TNF appears required for induction of Fas expression on mouse HSCs.37 Freshly isolated human CD34+ BM progenitor cells have been demonstrated as expressing little or no Fas, but Fas expression is up-regulated on progenitors upon cytokine-induced growth and differentiation.38-40 TNF, a potent inducer of Fas expression, was recently demonstrated as suppressing human reconstituting HSCs.41 However, the status of Fas expression and function on human reconstituting HSCs has not been investigated, neither in steady state nor during HSC cycling. In the present studies, we used advanced fluorescence-activated cell sorting (FACS) and the nonobese diabetic/severe combined immunodeficiency (NODSCID) reconstituting assay, to directly evaluate the expression of Fas on in vitro and in vivo reconstituting multipotent human HSCs, isolated from steady state BM and CB, as well as following in vitro and in vivo conditions efficiently promoting cycling of HSCs with sustained reconstituting ability.
Materials and methods
Isolation of CD34+ and CD34+ CD38- CB and BM cells
CB cells were collected from placentas following normal full-term deliveries, and BM cells were obtained by iliac crest aspirations from healthy adult volunteers, following informed consent and with approval of the ethics committee of the University Hospital of Lund. BM and CB mononuclear cells (MNCs) were isolated by Ficoll-Hypaque gradient centrifugation (Lymphoprep; Nycomed Pharma, Oslo, Norway). Positive selection of CD34+ cells was performed by means of magnetically activated cell sorting (MACS) CD34 isolation kit (Miltenyi Biotec, Bergish Gladbach, Germany), as described before.42 In most experiments, CB CD34+ cells were run through a second column to obtain higher purity of CD34+ cells. The purities of CB and BM CD34+ cells were 53% to 99% (average, 88%), and 63% to 87% (average, 80%), respectively.
CD34+CD38-/low cells were obtained by incubating enriched CD34+ cells with anti–CD34–fluorescein isothiocyanate (FITC) and anti–CD38-phycoerythrin (PE) monoclonal antibodies (mAbs; both from Becton Dickinson [BD], San Jose, CA) and subsequent sorting on a FACSVantage cell sorter (BD). As previously described,42 a conservative approach was taken so that only the 3% to 5% lowest CD38 expressing cells in the CD34+ population were sorted.
Hematopoietic growth factors and Abs
Recombinant human stem cell factor (rhSCF), rh interleukin-3 (rhIL-3), rh granulocyte colony-stimulating factor (rhG-CSF), and rh granulocyte-macrophage CSF (rhGM-CSF) were generously provided by Amgen (Thousand Oaks, CA). Rh thrombopoietin (rhTpo) was kindly provided by Genentech (San Francisco, CA.), rh erythropoietin (rhEpo) by Boehringer Mannheim (Germany), rh flt3 ligand (rhFL) by Immunex (Seattle, WA.), and rh tumor necrosis factor–α (rhTNF-α) from Hoffmann-LaRoche (Basel, Switzerland). A polyclonal neutralizing Ab against human TNF was purchased from R&D Systems (Minneapolis, MN) and used at 2 μg/mL, a concentration that completely blocks the effect of 20 ng/mL rhTNF-α (I.D. and S.E.W.J., unpublished observations, January 2000). An agonistic Ab against human Fas, clone CH11 (monoclonal mouse immunoglobulin M [IgM])43,44 was purchased from Upstate Biotechnology (Lake Placid, NY) and used at 1 μg/mL, on the basis of titration experiments demonstrating similar growth-inhibitory effects of CH11 at 0.5 and 1 μg/mL on Jurkat cells41 as well as on human CD34+ progenitor cells in the presence of TNF (I.D. and S.E.W.J., unpublished observations, December 1999).
Ex vivo expansion cultures
CD34+ CB cells were cultured for 5 to 7 days in serum-free (SF) medium, either x-vivo 15 (BioWhittaker, Walkersville, MD) supplemented with 1% detoxified bovine serum albumin (BSA) (StemCell Technologies, Vancouver, Canada) or Iscove modified Dulbecco medium (IMDM) (BioWhittaker) supplemented with 1% (wt/vol) BSA, 10 μg/mL rh insulin, and 200 μg/mL human transferrin (BIT; StemCell Technologies) as well as 40 μg/mL low-density lipoprotein (LDL) (Sigma, St Louis, MO). CD34+ CB and BM cells were seeded at 10 000 to 40 000 cells per milliliter and cultured in a cocktail of cytokines (100 ng/mL rhSCF, 100 ng/mL rhFL, 100 ng/mL rhTpo, and 20 ng/mL rhIL-3; SFT3) on the basis of previous studies demonstrating that these cytokines efficiently induce proliferation of candidate HSCs with sustained stem cell potential.42,45 TNF and CH11 were added to the expansion cultures as indicated. Following culture, cells were enumerated, and Fas+ and Fas- populations sorted on a FACSVantage.
Flow cytometric analysis and cell sorting
Cell surface expression of Fas was examined on freshly isolated CB and BM cells or following 5 days of culture in SFT3 in SF medium in the absence or presence of 20 ng/mL TNF. Cells were stained with PE-, FITC- or allophycocyanin (APC)–conjugated Abs (all from BD/PharMingen, San Diego, CA) against Fas (CD95), CD34 and lineage antigens (CD3, CD14, CD15, CD19, CD20, CD56, CD66b, and glycophorin A [GpA]). Following blocking of unspecific binding with mouse IgG (Jackson ImmunoResearch Laboratories, West Grove, PA), cells were incubated with Abs for 15 to 20 minutes on ice and analyzed on a FACSCalibur (BD) or sorted on a FACSVantage. Gates were set so that irrelevant isotype-matched control Abs contained fewer than 0.5% positive events. When indicated, 7-amino actinomycin D (7-AAD) was added to gate out dead cells. Data were obtained by means of CellQuest (BD) or FlowJo (Tree Star, San Carlos, CA) software.
Flow cytometric analysis of cell cycle status of freshly isolated and cultured CB progenitors
Freshly isolated and cultured (5 days) CD34+ CB cells stained with anti-CD34 and anti-CD95 were sorted into CD34+Fas- and CD34+Fas+ subpopulations on a FACSVantage as described. Sorted cells were then subjected to high-resolution cell cycle analysis.46,47 Fixation and permeabilization of cells were performed with a Cytfix/Cytoperm Kit (PharMingen). Cells were washed, stained with FITC-conjugated anti–Ki-67 (Immunotech, Westbrook, ME), or an isotype-matched irrelevant control Ab. After 3 hours' incubation on ice in phosphate-buffered saline (PBS) containing 5% fetal calf serum (FCS) (Gibco, Paisley, United Kingdom) and 5 μg/mL 7-AAD, samples were analyzed on a FACSCalibur.
Limiting dilution assay
FACS-purified lineage-negative Fas+ (Lin-Fas+) and Lin-Fas- cells were seeded in Terasaki plates (Nunc, Kamstrup, Denmark) at a density of 1 cell per well in SF medium (x-vivo 15 with 1% BSA), supplemented with a cocktail of cytokines (50 ng/mL rhSCF, 50 ng/mL rhFL, 50 ng/mL rhTpo, and 20 ng/mL rhIL-3; 120 wells per group). Wells were scored for cell growth following 10 to 12 days of incubation at 37°C in a humidified atmosphere with 5% CO2 in air. Since the statistical chance (based on Poisson probability distribution) of a well not receiving any cell is 37% by this method, the maximum expected number of clones was 76.42
Semisolid clonogenic assay
After expansion of CD34+ cells for 6 days in SF medium and SFT3 (described in “Ex vivo expansion cultures”), cells were counted and seeded at a concentration of 500 cells per 1 mL IMDM, 20% FCS, and MethoCult H4100 (StemCell Technologies) supplemented with a cocktail of cytokines at predetermined optimal concentrations (rhSCF, rhGM-CSF, rhG-CSF, rhFL, rhIL-3, all at 10 ng/mL; and 5 U/mL rhEpo) in 35-mm Petri dishes. Cultures were incubated at 37°C and 5% CO2 in air for 10 to 12 days, at which time colonies (larger than 50 cells) were visualized and scored with an inverted microscope. Colonies were classified as granulocyte/macrophage colony-forming units) (CFU-GMs) and erythroid burst-forming units (BFU-Es).
NOD-SCID repopulating assay
NOD/LtSz-SCID mice (originally from The Jackson Laboratory, Bar Harbour, ME) were bred and housed under sterile conditions in microisolator cages and given irradiated food and acidified, autoclaved water. Mice were irradiated with 350 cGy from a 137Cs source at 8 to 12 weeks of age and given 100 mg/L prophylactic ciprofloxacin in the drinking water until analysis. Tail vein transplantations of hematopoietic cells suspended in 0.5 to 1 mL medium were performed within 12 hours of irradiation. In some experiments, 1 × 106 irradiated (1500 cGy) accessory cells (MNCs or CD34-depleted BM or CB cells) were coinjected. At 6 weeks following transplantation, mice were killed by asphyxiation with CO2; femora and tibiae were collected; and engraftment was investigated by flow cytometric analysis (FACSCalibur) as described previously.5,48,49 Briefly, BM cells were blocked with antimouse Fc-block (PharMingen) and whole mouse IgG, and subsequently stained with FITC-conjugated antihuman CD45 and CD71 Abs (BD) as well as antimouse CD45.1–PE Ab (PharMingen). BM cells from mice that did not receive transplants (negative controls) and mixtures of 0.1% to 0.5% human cells in murine BM (positive controls) were always included. If engraftment was detected by CD45/CD71 analysis (detection level, 0.05%), lineage analysis with antihuman CD19-PE (anti-HuCD19–PE) (B cells; BD), anti-HuCD15–PE/CD66b-FITC (myeloid; both PharMingen), and anti-HuCD34–FITC (progenitor cells) combined with anti-HuCD45–APC (BD) were performed. For all samples 7-AAD was included to gate out dead cells. A minimum of 50 000 BM cells were examined for each sample. Only mice with both positive myeloid and lymphoid engraftment (each defined as more than 10 positive events per 50 000 viable BM cells) were evaluated as positive. Gates were set so that samples incubated with irrelevant isotype-matched control Abs had, at maximum, 1 positive event per 50 000 BM cells analyzed. To further confirm myeloid engraftment, 1 × 105 BM cells (4 replicates) were also plated in methylcellulose (described for semisolid clonogenic assay) supplemented with human-specific cytokines (50 ng/mL rhGM-CSF, 25 ng/mL rhIL-3, 25 ng/mL rhSCF) and 5 U/mL rhEpo. CFU-GM and BFU-E colonies were scored after 10 to 12 days. No colonies were observed in the absence of cytokines or from BM of mice that did not receive transplants and that were cultured with the same cytokines (I.D. and S.E.W.J., unpublished observations, December 1999).
Long-term culture–initiating cell (LTC-IC) assay
Long-term-cultures were established and maintained according to previously described procedures.41,50 Briefly, stroma cell feeders were established by seeding a mixture (1:1) of 2 irradiated (8000 cGy) murine fibroblast cell lines (M2-10B4 and sl/sl, kindly provided by D. E. Hogge, Vancouver, BC, Canada), engineered to produce high levels of human G-CSF, IL-3, and SCF,50 into 96-well collagen-coated microtiter plates (Nunc) containing LTC medium (Myelocult; StemCell Technologies) supplemented with freshly dissolved 10-6 M hydrocortisone 21–hemisuccinate (Sigma). The cytokine production from M2-10B4 and sl/sl fibroblasts was routinely tested (by commercially available enzyme-linked immunosorbent assay [ELISA] kits; R&D Systems) and found to be at referenced levels50 (I.D. and S.E.W.J., unpublished observation, September 2000).
Then, 150 to 250 freshly isolated CD34+ or 25 to 75 CD34+CD38- CB or BM cells or their expansion equivalents (EEs) were plated per well (2 to 6 replicates) and incubated at 37°C in a humidified atmosphere with 5% CO2 in air. Cocultures were maintained by weekly 50% medium changes. Following 6 weeks, adherent and nonadherent cells were transferred to methylcellulose cultures containing rhSCF, rhGM-CSF, rhG-CSF, rhFL, rhIL-3 (all at 10 ng/mL), and rhEpo (5 U/mL). To ensure formation of a reliable number of colonies from the LTC, the content of each stroma coculture well was transferred to methylcellulose cultures at both a low (10% to 20% of cells) and a high concentration (80% to 90% of cells). LTC–colony-forming cells (LTC-CFCs) were scored after an additional 10 to 12 days of culture.
Results
Candidate HSCs in CB and BM lack constitutive expression of Fas
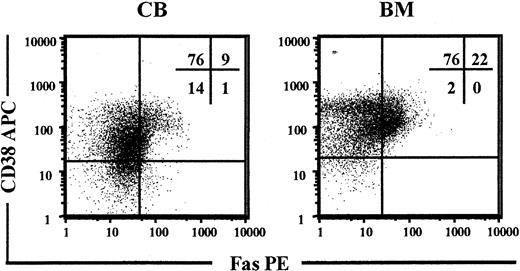
Previous studies have demonstrated low levels of Fas expression on freshly isolated CD34+ BM cells.38-40 CD34+CD38- cells represent only 0.05% to 0.1% of human BM and CB MNCs, but contain most if not all HSC activity.5,13,14 Thus, CB and BM MNCs were investigated for coexpression of CD34, CD38, and Fas with the use of a PE-conjugated anti-Fas Ab. In CB, more than 10% of CD34+CD38+ progenitors had detectable Fas expression, whereas little or no expression was observed on CD34+CD38- candidate HSCs (Figure 1). Similarly, in BM, more than 20% of CD34+CD38+ cells expressed Fas, whereas no expression was observed on CD34+CD38- cells (Figure 1).
Constitutive expression of Fas on candidate human HSCs. Enriched CD34+ CB and BM cells were stained with Abs against CD34, CD38, and Fas and analyzed by flow cytometry. Viable cells expressing CD34 were gated and investigated for coexpression of CD38 and Fas. Panels show one representative experiment for CB and and one for BM cells. Percentages shown in quadrants represent mean values of at least 3 independent experiments.
Constitutive expression of Fas on candidate human HSCs. Enriched CD34+ CB and BM cells were stained with Abs against CD34, CD38, and Fas and analyzed by flow cytometry. Viable cells expressing CD34 were gated and investigated for coexpression of CD38 and Fas. Panels show one representative experiment for CB and and one for BM cells. Percentages shown in quadrants represent mean values of at least 3 independent experiments.
Fas is up-regulated on candidate HSCs in response to cytokine-induced ex vivo cycling
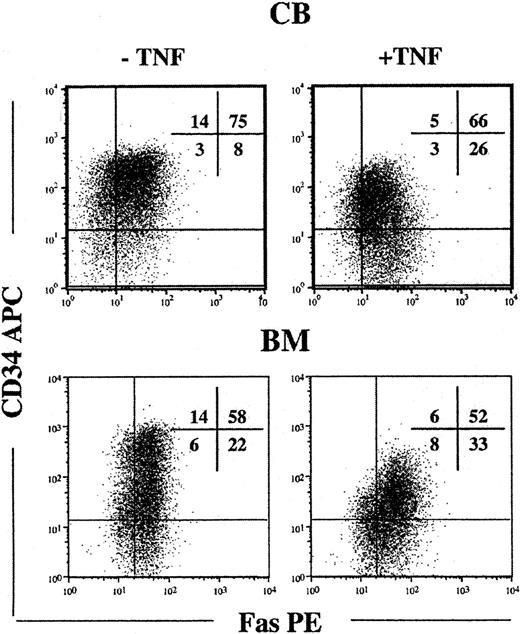
Since candidate HSCs in steady state BM and CB lacked detectable cell surface Fas expression and are predominantly in G0/G1 of the cell cycle,51-54 we next investigated whether cytokine-induced cycling up-regulated Fas expression on candidate HSCs. Toward this aim, CD34+ cells were cultured in SFT3, conditions recently demonstrated to efficiently promote cycling and maintenance of HSCs.42 Following 5 days of culture, at which time virtually all HSCs have divided,42 cells were investigated for cell surface Fas expression. It is noteworthy that the majority of cells that remained CD34+ and Lin- coexpressed high levels of Fas (Figure 2). TNF, a potent inducer of Fas expression38,39 further up-regulated Fas expression on Lin-CD34+ CB and BM cells (Table 1).
Up-regulation of Fas expression on candidate human HSCs in response to in vitro cycling. CD34+ CB and BM cells were cultured in SF medium supplemented with SFT3 for 5 days in the absence or presence of TNF. Lin- cells were gated and investigated for coexpression of CD34 and Fas. Flow profiles are from one representative experiment of CB and BM, respectively, whereas percentages in quadrants represent mean values from 3 independent experiments of CB and BM, respectively.
Up-regulation of Fas expression on candidate human HSCs in response to in vitro cycling. CD34+ CB and BM cells were cultured in SF medium supplemented with SFT3 for 5 days in the absence or presence of TNF. Lin- cells were gated and investigated for coexpression of CD34 and Fas. Flow profiles are from one representative experiment of CB and BM, respectively, whereas percentages in quadrants represent mean values from 3 independent experiments of CB and BM, respectively.
Relative distribution of Fas− and Fas+ cells in Lin−CD34− and Lin−CD34+ CB and BM cell populations, respectively
. | Lin−CD34− . | . | . | . | Lin−CD34+ . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | −TNF . | . | +TNF . | . | −TNF . | . | +TNF . | . | ||||||
| Cell type . | Fas− . | Fas+ . | Fas− . | Fas+ . | Fas− . | Fas+ . | Fas− . | Fas+ . | ||||||
| CB | 27% | 73% | 10% | 90% | 16% | 84% | 7% | 93% | ||||||
| BM | 21% | 79% | 21% | 79% | 19% | 81% | 10% | 90% | ||||||
. | Lin−CD34− . | . | . | . | Lin−CD34+ . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | −TNF . | . | +TNF . | . | −TNF . | . | +TNF . | . | ||||||
| Cell type . | Fas− . | Fas+ . | Fas− . | Fas+ . | Fas− . | Fas+ . | Fas− . | Fas+ . | ||||||
| CB | 27% | 73% | 10% | 90% | 16% | 84% | 7% | 93% | ||||||
| BM | 21% | 79% | 21% | 79% | 19% | 81% | 10% | 90% | ||||||
Data are averages of 3 separate experiments.
Since TNF can up-regulate Fas expression, the high levels of Fas observed on CD34+ cells expanded for 5 days in the absence of TNF might be a result of endogenous TNF production in the cultures (autocrine/paracrine production). However, no difference in Fas expression was seen on Lin-CD34+ CB cells expanded for 5 days in the presence of a neutralizing anti-TNF Ab (Figure 3).
Up-regulation of Fas expression on ex vivo cycling CD34+ CB cells is not mediated through endogenous TNF production. CD34+ CB cells were cultured for 5 days in SF medium supplemented with SFT3 in the absence or presence of a neutralizing anti-TNF Ab as indicated (“Materials and methods”). Lin-CD34+ cells were gated and investigated for coexpression of Fas. Data are presented as the percentages of Lin-CD34+ cells coexpressing Fas in the absence (□) or presence (▪) of anti-TNF (mean values of 3 independent experiments; error bars, standard errors of the mean [SEMs] are shown).
Up-regulation of Fas expression on ex vivo cycling CD34+ CB cells is not mediated through endogenous TNF production. CD34+ CB cells were cultured for 5 days in SF medium supplemented with SFT3 in the absence or presence of a neutralizing anti-TNF Ab as indicated (“Materials and methods”). Lin-CD34+ cells were gated and investigated for coexpression of Fas. Data are presented as the percentages of Lin-CD34+ cells coexpressing Fas in the absence (□) or presence (▪) of anti-TNF (mean values of 3 independent experiments; error bars, standard errors of the mean [SEMs] are shown).
Up-regulation of Fas expression on NOD-SCID repopulating cells following in vivo cycling
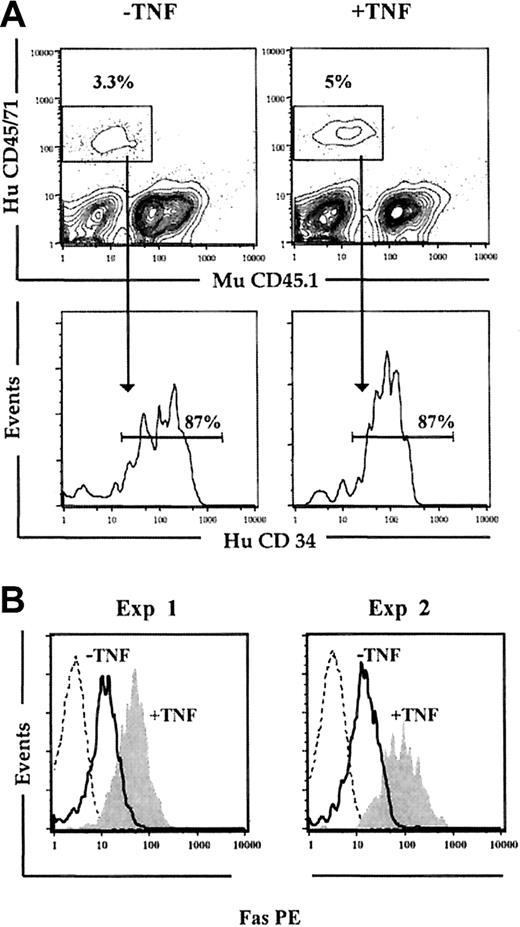
We next wanted to establish that the observed up-regulation of Fas expression on human HSCs was not only an in vitro phenomenon. Previous studies have demonstrated that cycling of HSCs is rapidly induced following in vivo transplantation, and parallels the cycling observed following optimal in vitro stimulation with cytokines.55,56 Thus, NOD-SCID mice received transplants of freshly isolated CD34+ CB cells, and 5 days later the BM was investigated for Fas expression on human CD34+ cells (Figure 4). At this time, 87% of the human engrafting cells remained CD34+ (mean of 2 experiments; Figure 4A), and strikingly, virtually all Lin-CD34+ cells expressed Fas (Figure 4B). In a second group of NOD-SCID mice also given transplants of freshly isolated CD34+ CB cells and receiving 3 injections with 5 μg human TNF (at 24, 64, and 96 hours after transplantation), Fas expression was further up-regulated on Lin-CD34+ cells (Figure 4B). Thus, Fas expression is induced upon in vitro as well as in vivo cycling of candidate human HSCs.
In vivo cycling up-regulates Fas expression on Lin-CD34+ CB cells. Freshly isolated CD34+ CB cells were transplanted into irradiated NOD-SCID mice that subsequently received 3 injections of PBS (-TNF) or 5 μg TNF (+TNF) 24, 64, and 96 hours after transplantation. (A) At 24 hours after the last injection (day 5), mice were killed, and the BM was analyzed for human (HuCD45/71) engraftment. Samples were also stained with murine CD45.1 (MuCD45.1) to distinguish murine and human cells. Cells positive for HuCD45/71 were analyzed for coexpression of HuCD34. Results shown are from 1 of 2 experiments. (B) Engrafted human cells coexpressing CD34 and lacking expression of lineage antigens (Lin-CD34+) were gated and investigated for Fas expression. Broken lines represent engrafted Lin-CD34+ cells stained with isotype control Ab; solid lines, Fas expression on Lin-CD34+ cells from mice injected with PBS; and filled histograms, Fas expression on Lin-CD34+ cells from mice treated with TNF. Results from 2 independent experiments are shown.
In vivo cycling up-regulates Fas expression on Lin-CD34+ CB cells. Freshly isolated CD34+ CB cells were transplanted into irradiated NOD-SCID mice that subsequently received 3 injections of PBS (-TNF) or 5 μg TNF (+TNF) 24, 64, and 96 hours after transplantation. (A) At 24 hours after the last injection (day 5), mice were killed, and the BM was analyzed for human (HuCD45/71) engraftment. Samples were also stained with murine CD45.1 (MuCD45.1) to distinguish murine and human cells. Cells positive for HuCD45/71 were analyzed for coexpression of HuCD34. Results shown are from 1 of 2 experiments. (B) Engrafted human cells coexpressing CD34 and lacking expression of lineage antigens (Lin-CD34+) were gated and investigated for Fas expression. Broken lines represent engrafted Lin-CD34+ cells stained with isotype control Ab; solid lines, Fas expression on Lin-CD34+ cells from mice injected with PBS; and filled histograms, Fas expression on Lin-CD34+ cells from mice treated with TNF. Results from 2 independent experiments are shown.
Fas expression on candidate human HSCs is regulated in a cell cycle–dependent manner
The in vitro and in vivo up-regulation of Fas expression on CD34+ candidate HSCs could be a consequence of cytokine-induced differentiation or could reflect that Fas expression is regulated in an activation or cell cycle–dependent manner. In steady state CB, the majority of CD34+ cells, as expected, were in G0 (53% of Fas+ and 69% of Fas- cells) or G1 (43% of Fas+ and 30% of Fas- cells) of the cell cycle (Table 2).47 Of the very few actively cycling cells in S/G2/M, 3% were Fas+ compared with 1% Fas- cells (Table 2). As anticipated, following ex vivo culture, very few (5% for both Fas+ and Fas-) cells remained in G0, and 49% of CD34+Fas+ cells were in S/G2/M, in contrast to only 23% of CD34+Fas- cells (Table 2). Thus, although Fas is expressed in all phases of the cell cycle, Fas is preferentially up-regulated upon active cycling of CD34+ progenitor/stem cells.
Cell cycle-dependent up-regulation of Fas expression on hematopoletic progenitor/stem cells
. | . | Cell cycle distribution, % (SEM) . | . | . | ||
|---|---|---|---|---|---|---|
| Cell treatment and Fas expression . | % of total CD34+ cells . | G0 . | G1 . | S/G2/M . | ||
| Freshly isolated | ||||||
| Fas+ | 14 | 53 (5) | 43 (6) | 3 (1) | ||
| Fas− | 86 | 69 (5) | 30 (5) | 1 (1) | ||
| SFT3, 5 days | ||||||
| Fas+ | 45 | 5 (1) | 44 (4) | 49 (3) | ||
| Fas− | 55 | 5 (2) | 71 (3) | 23 (2) | ||
. | . | Cell cycle distribution, % (SEM) . | . | . | ||
|---|---|---|---|---|---|---|
| Cell treatment and Fas expression . | % of total CD34+ cells . | G0 . | G1 . | S/G2/M . | ||
| Freshly isolated | ||||||
| Fas+ | 14 | 53 (5) | 43 (6) | 3 (1) | ||
| Fas− | 86 | 69 (5) | 30 (5) | 1 (1) | ||
| SFT3, 5 days | ||||||
| Fas+ | 45 | 5 (1) | 44 (4) | 49 (3) | ||
| Fas− | 55 | 5 (2) | 71 (3) | 23 (2) | ||
Freshly isolated or cultured (SFT3 for 5 days) CD34+ CB cells were sorted into CD34+ Fas+ and CD34+ Fas− subpopulations and investigated for cell cycle distribution following staining with 7-AAD and anti-Ki-67 (“Materials and methods”). Results are from 3 separate experiments for freshly isolated and cultured cells; mean values (SEM).
Cycling LTC-ICs and SCID repopulating cells express Fas
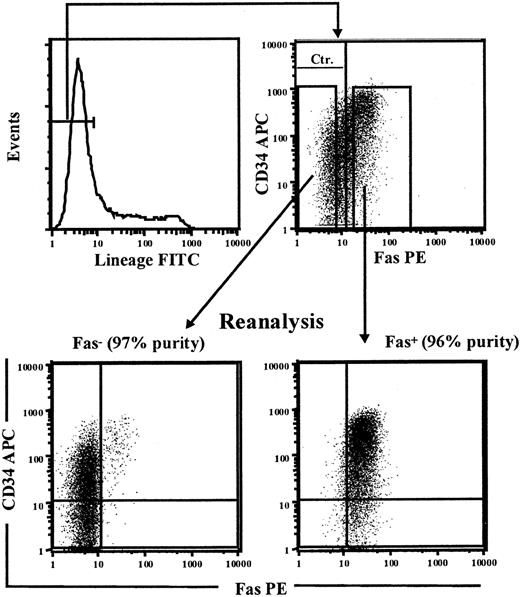
To investigate to what degree CD34+ cells up-regulating Fas expression upon in vitro cycling include reconstituting HSCs, we next performed a series of experiments in which the Fas+ and Fas- fractions of cultured CD34+ cells were sorted and functionally investigated for stem cell activity. Since human Lin-CD34+ as well as Lin-CD34- cells have been demonstrated to possess HSC reconstituting activity,57-61 the total Lin-Fas+ and Lin-Fas- cell populations were purified following 5 days of culture in SFT3 (Figure 5), at which time most Lin- cells coexpressed Fas (52% to 66% for BM and 58% and 86% for CB; Tables 3, 4). Lin-Fas+ CB as well as BM cells were highly enriched for LTC-CFC activity when compared with Lin-Fas- cells (Table 3), and when we corrected for the relative frequencies of Lin-Fas+ and Lin-Fas- cells, more than 90% of LTC-CFC activity proved to be derived from Fas+ cells.
Sorting of Fas+ and Fas- candidate HSCs. CD34+ CB cells were cultured in SF medium supplemented with SFT3 for 5 days before being stained with FITC-conjungated antilineage Abs (CD3, CD14, CD15, CD19, CD20, CD56, CD66b, and glycophorin A), anti-Fas–PE, and anti-CD34–APC. Lin-Fas- and Lin-Fas+ cells were sorted on a FACSVantage and analyzed for purity as indicated (numbers in parenthesis indicate purity of sorted populations with regard to Fas expression). One experiment representative of 3 is shown.
Sorting of Fas+ and Fas- candidate HSCs. CD34+ CB cells were cultured in SF medium supplemented with SFT3 for 5 days before being stained with FITC-conjungated antilineage Abs (CD3, CD14, CD15, CD19, CD20, CD56, CD66b, and glycophorin A), anti-Fas–PE, and anti-CD34–APC. Lin-Fas- and Lin-Fas+ cells were sorted on a FACSVantage and analyzed for purity as indicated (numbers in parenthesis indicate purity of sorted populations with regard to Fas expression). One experiment representative of 3 is shown.
In vitro cycling LTC-ICs up-regulate Fas expression
Experiment, expansion, and sorted cells . | % of total Lin− cells . | LTC-CFCs (replicate values)/seeded cells . |
|---|---|---|
| Exp 1, 28-fold expansion | ||
| BM Lin−Fas− | 34 | 13 (3-23)/7000 |
| BM Lin−Fas+ | 66 | 134 (110-158)/7000 |
| Exp 2, 20-fold expansion | ||
| BM Lin−Fas− | 48 | 5 (4-7)/5000 |
| BM Lin−Fas+ | 52 | 56 (37-67)/5000 |
| Exp 3, 14-fold expansion | ||
| BM Lin−Fas− | 35 | 6 (4-9)/3500 |
| BM Lin−Fas+ | 65 | 52 (37-52)/3500 |
| Exp 4, 16-fold expansion | ||
| CB Lin−Fas− | 14 | 7 (0-5-17)/2400 |
| CB Lin−Fas+ | 86 | 27 (13-15-25)/2400 |
| Exp 5, 19-fold expansion | ||
| CB Lin− | 100 | 40 (23-23-75)/2850 |
| CB Lin−Fas+ | 70 | 140 (45-143-233)/2850 |
Experiment, expansion, and sorted cells . | % of total Lin− cells . | LTC-CFCs (replicate values)/seeded cells . |
|---|---|---|
| Exp 1, 28-fold expansion | ||
| BM Lin−Fas− | 34 | 13 (3-23)/7000 |
| BM Lin−Fas+ | 66 | 134 (110-158)/7000 |
| Exp 2, 20-fold expansion | ||
| BM Lin−Fas− | 48 | 5 (4-7)/5000 |
| BM Lin−Fas+ | 52 | 56 (37-67)/5000 |
| Exp 3, 14-fold expansion | ||
| BM Lin−Fas− | 35 | 6 (4-9)/3500 |
| BM Lin−Fas+ | 65 | 52 (37-52)/3500 |
| Exp 4, 16-fold expansion | ||
| CB Lin−Fas− | 14 | 7 (0-5-17)/2400 |
| CB Lin−Fas+ | 86 | 27 (13-15-25)/2400 |
| Exp 5, 19-fold expansion | ||
| CB Lin− | 100 | 40 (23-23-75)/2850 |
| CB Lin−Fas+ | 70 | 140 (45-143-233)/2850 |
CD34+ CB or BM cells were cultured in SF medium supplemented with SFT3 for 5 days, at which time Lin−Fas− (or total Lin− cells; experiment 5) and Lin−Fas− cells were sorted on a FACSVantage and evaluated for LTC-CFC activity. The expansion equivalents (EEs) of 150 CB or 250 BM CD34+ cells were always investigated. Data shown are mean values, with replicate values in parentheses.
Exp indicates experiment.
In vitro cycling SRCs up-regulate Fas expression
Experiment, expansion, and sorted cells . | % of total Lin− cells . | Cells transplanted per mouse, no. . | Positive mice/total mice, no. . | Human engraftment, % (replicate %) . |
|---|---|---|---|---|
| Exp 1, 15-fold expansion | ||||
| CB Lin−Fas− | 42 | 450 000 | 0/2 | 0 (0-0) |
| CB Lin−Fas+ | 58 | 450 000 | 4/4 | 3 (0.3-0.3-0.4-10) |
| Exp 2, 35-fold expansion | ||||
| CB Lin−Fas− | 38 | 1.8 × 106 | 1/6 | 0.3 (0-0-0-0-0-0-2) |
| CB Lin−Fas+ | 62 | 1.8 × 106 | 7/7 | 7 (0.3-5-5-6-7-10-15) |
| Exp 3, 30-fold expansion | ||||
| CB Lin−Fas− | 40 | 1.5 × 106 | 1/6 | 0.05 (0-0-0-0-0-0.3) |
| CB Lin−Fas+ | 60 | 1.5 × 106 | 5/5 | 2.4 (0.4-0.7-0.7-3-7) |
| Exp 4, 19-fold expansion | ||||
| CB Lin− | 100 | 950 000 | 3/3 | 13 (10-11-18) |
| CB Lin−Fas+ | 70 | 950 000 | 4/4 | 28 (22-23-26-40) |
Experiment, expansion, and sorted cells . | % of total Lin− cells . | Cells transplanted per mouse, no. . | Positive mice/total mice, no. . | Human engraftment, % (replicate %) . |
|---|---|---|---|---|
| Exp 1, 15-fold expansion | ||||
| CB Lin−Fas− | 42 | 450 000 | 0/2 | 0 (0-0) |
| CB Lin−Fas+ | 58 | 450 000 | 4/4 | 3 (0.3-0.3-0.4-10) |
| Exp 2, 35-fold expansion | ||||
| CB Lin−Fas− | 38 | 1.8 × 106 | 1/6 | 0.3 (0-0-0-0-0-0-2) |
| CB Lin−Fas+ | 62 | 1.8 × 106 | 7/7 | 7 (0.3-5-5-6-7-10-15) |
| Exp 3, 30-fold expansion | ||||
| CB Lin−Fas− | 40 | 1.5 × 106 | 1/6 | 0.05 (0-0-0-0-0-0.3) |
| CB Lin−Fas+ | 60 | 1.5 × 106 | 5/5 | 2.4 (0.4-0.7-0.7-3-7) |
| Exp 4, 19-fold expansion | ||||
| CB Lin− | 100 | 950 000 | 3/3 | 13 (10-11-18) |
| CB Lin−Fas+ | 70 | 950 000 | 4/4 | 28 (22-23-26-40) |
CD34+ CB cells were cultured in SF medium supplemented with SFT3 for 5 days, at which time Lin−Fas− (or total Lin− cells; experiment 4) and Lin−Fas+ cells were sorted on a FACSVantage. The expansion equivalents (EEs) of 30 000 to 50 000 CD34+ CB cells were transplanted per mouse. Data shown for human engraftment in NOD-SCID mice are mean values of individual mice per group, with replicate percentages shown in parentheses. Positive mice were defined as mice with human reconstitution of both myeloid and B-cell lineages (“Materials and methods”).
SRC indicates SCID-repopulating cell; exp, experiment.
Similarly, virtually all SCID repopulating cell (SRC) activity was derived from Fas+ cells following culture of CD34+ CB cells (Table 4; Figure 6).
Cytokine-induced cell cycling up-regulates cell surface Fas expression on NOD-SCID repopulating cells. CD34+CB cells were cultured in SF medium supplemented with SFT3 for 5 days and sorted into Lin-Fas- and Lin-Fas+ cells as shown in Figure 5. Irradiated NOD-SCID mice received transplants of 450 000 Lin-Fas- (A) or 450 000 Lin-Fas+ cells (B), representing the EE of 30 000 freshly isolated CD34+ cells. At 6 weeks following transplantation, NOD-SCID BM was analyzed for human multilineage reconstitution (“Materials and methods”). Results are from representative mice that received transplants of Lin-Fas- and Lin-Fas+ cells.
Cytokine-induced cell cycling up-regulates cell surface Fas expression on NOD-SCID repopulating cells. CD34+CB cells were cultured in SF medium supplemented with SFT3 for 5 days and sorted into Lin-Fas- and Lin-Fas+ cells as shown in Figure 5. Irradiated NOD-SCID mice received transplants of 450 000 Lin-Fas- (A) or 450 000 Lin-Fas+ cells (B), representing the EE of 30 000 freshly isolated CD34+ cells. At 6 weeks following transplantation, NOD-SCID BM was analyzed for human multilineage reconstitution (“Materials and methods”). Results are from representative mice that received transplants of Lin-Fas- and Lin-Fas+ cells.
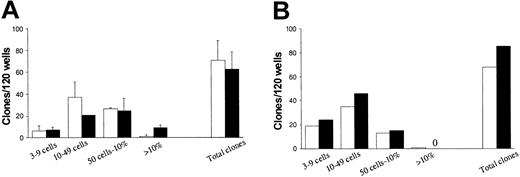
In a last experiment (experiment 5, Table 3; experiment 4, Table 4), in which it was not possible to obtain sufficient Fas- cells, Lin-Fas+ cells were compared with the total Lin- population; this experiment also supported that most LTC-CFCs and SRCs are derived from Fas+ cells. It is noteworthy that although Lin-Fas- cells contained many fewer candidate HSCs (LTC-ICs and SRCs), the frequency of progenitor cells was high and comparable to that of Lin-Fas+ cells (Figure 7). Thus, functionally defined HSCs up-regulate Fas expression upon in vitro cycling.
High and comparable levels of hematopoietic progenitors in Lin-Fas- and Lin-Fas+ cells. CD34+ CB (A) or BM (B) cells were cultured in SF medium supplemented with SFT3 for 5 days and subsequently sorted into Lin-Fas- (□) and Lin-Fas+ (▪) cells (as shown in Figure 5). Both populations were plated at one cell per well in SF medium supplemented with SFT3 (“Materials and methods”). Plates were scored for total clonal growth and clonal size (from 3 cells to 100% of well covered by cells) as indicated. Each group consisted of 120 wells, with a maximum of 76 clones expected per group (“Materials and methods”). Results represent a total of 3 independent experiments (2 CB and 1 BM). A zero indicates no clones observed.
High and comparable levels of hematopoietic progenitors in Lin-Fas- and Lin-Fas+ cells. CD34+ CB (A) or BM (B) cells were cultured in SF medium supplemented with SFT3 for 5 days and subsequently sorted into Lin-Fas- (□) and Lin-Fas+ (▪) cells (as shown in Figure 5). Both populations were plated at one cell per well in SF medium supplemented with SFT3 (“Materials and methods”). Plates were scored for total clonal growth and clonal size (from 3 cells to 100% of well covered by cells) as indicated. Each group consisted of 120 wells, with a maximum of 76 clones expected per group (“Materials and methods”). Results represent a total of 3 independent experiments (2 CB and 1 BM). A zero indicates no clones observed.
Cycling human reconstituting HSCs express Fas, but are resistant to Fas activation
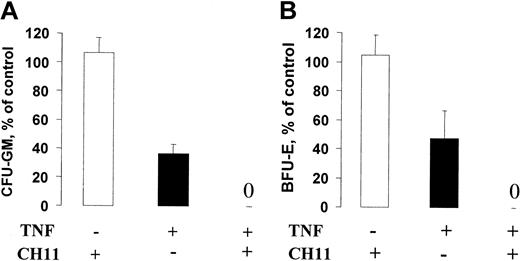
Since SRCs and LTC-ICs up-regulated high levels of Fas expression upon in vitro cycling, we next addressed whether activation of the Fas receptor with an agonistic antibody (CH11) would compromise HSC function. As previously shown, the CH11 agonist efficiently induced apoptosis of the hematopoietic cell line Jurkat.41 In contrast, even when cultured for 5 to 7 days in SFT3 in the presence of CH11, neither SRCs (Table 5) or LTC-ICs (Table 6) were negatively affected by the Fas agonist. However, CD34+ cells exposed to 1 μg/mL CH11 in combination with 2 and 20 ng/mL TNF sustained no LTC-CFC activity, an effect also seen with TNF alone (Table 6). Similarly, in the presence of TNF, CH11 also suppressed CFU-GM and BFU-E colony formation by more than 50% (Figure 8).
Fas-expressing SRCs are resistant to Fas activation
. | Without CH11 . | . | . | With CH11 . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Experiment . | Fold expansion . | Human engraftment, % (replicate %) . | Positive mice . | Fold expansion . | Human engraftment, % (replicate %) . | Positive mice . | ||||
| Exp 1 | 33 | 23 (5-12-18-20-58) | 5/5 | 32 | 22 (0-13-17-38-44) | 4/5 | ||||
| Exp 2 | 21 | 1 (0.4-1-1-3) | 4/4 | 18 | 5 (0-1-3-5-16) | 4/5 | ||||
| Exp 3 | 158 | 11 (3-4-6-10-31) | 5/5 | 160 | 30 (14-25-30-41-64) | 5/5 | ||||
. | Without CH11 . | . | . | With CH11 . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Experiment . | Fold expansion . | Human engraftment, % (replicate %) . | Positive mice . | Fold expansion . | Human engraftment, % (replicate %) . | Positive mice . | ||||
| Exp 1 | 33 | 23 (5-12-18-20-58) | 5/5 | 32 | 22 (0-13-17-38-44) | 4/5 | ||||
| Exp 2 | 21 | 1 (0.4-1-1-3) | 4/4 | 18 | 5 (0-1-3-5-16) | 4/5 | ||||
| Exp 3 | 158 | 11 (3-4-6-10-31) | 5/5 | 160 | 30 (14-25-30-41-64) | 5/5 | ||||
Results are presented as percentage of human engraftment of mice receiving transplants with the expansion equivalent (EE) of 100 000 (exp 1) or 50 000 (exp 2 and exp 3) CD34+ CB cells cultured for 5 (exp 1 and exp 2) or 7 (exp 3) days in SF medium supplemented with SFT3 in the absence or presence of CH11 (1 μg/mL; mean percentages are presented with replicate percentages in parentheses). Also shown are fractions of mice positive for human reconstitution with both B and myeloid cells (“Materials and methods”).
Abbreviations are explained in Table 3.
Fas-expressing LTC-ICs are not compromised by Fas activation
. | Fold expansion . | . | . | . | LTC-CFCs per 50 or 75 CD34+ CD38− cells, (replicate values) . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | Without CH11 . | . | With CH11 . | . | Without CH11 . | With CH11 . | ||||
| Experiment . | Without TNF . | With TNF . | Without TNF . | With TNF . | Without TNF* . | Without TNF† . | ||||
| Exp 1, CB | 23 | 21 | 18 | 7 | 205(120-195-300) | 287(195-215-450) | ||||
| Exp 2, CB | 160 | 100 | 180 | 90 | 50(37-41-52-69) | 40(35-37-39-48) | ||||
| Exp 3, CB | 331 | 109 | 309 | 100 | 290 (75-285-510) | 350 (270-360-420) | ||||
| Exp 4, BM | 52 | 56 | 58 | 60 | 42 (35-37-48-48) | 43 (35-37-47-52) | ||||
| Exp 5, BM | 68 | 42 | 52 | 40 | 43 (22-37-37-41-52-71) | 31 (19-29-34-37-37) | ||||
| Exp 6, BM | 70 | 50 | 46 | 48 | 42 (34-37-45-48-48) | 40 (32-35-37-48-50) | ||||
. | Fold expansion . | . | . | . | LTC-CFCs per 50 or 75 CD34+ CD38− cells, (replicate values) . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | Without CH11 . | . | With CH11 . | . | Without CH11 . | With CH11 . | ||||
| Experiment . | Without TNF . | With TNF . | Without TNF . | With TNF . | Without TNF* . | Without TNF† . | ||||
| Exp 1, CB | 23 | 21 | 18 | 7 | 205(120-195-300) | 287(195-215-450) | ||||
| Exp 2, CB | 160 | 100 | 180 | 90 | 50(37-41-52-69) | 40(35-37-39-48) | ||||
| Exp 3, CB | 331 | 109 | 309 | 100 | 290 (75-285-510) | 350 (270-360-420) | ||||
| Exp 4, BM | 52 | 56 | 58 | 60 | 42 (35-37-48-48) | 43 (35-37-47-52) | ||||
| Exp 5, BM | 68 | 42 | 52 | 40 | 43 (22-37-37-41-52-71) | 31 (19-29-34-37-37) | ||||
| Exp 6, BM | 70 | 50 | 46 | 48 | 42 (34-37-45-48-48) | 40 (32-35-37-48-50) | ||||
The expansion equivalent (EE) of 50 CB or 75 BM CD34+CD38− cells were cultured for 5 (experiments 1,2, 4-6) or 7 days (experiment 3) in SF medium supplemented with SFT3 in the absence or presence of TNF (2 to 20 ng/mL) and/or CH11 (1 μg/mL) as indicated. Cultures were subsequently evaluated for 6-week LTC-CFC activity (“Materials and methods”). Mean values are presented with replicate values in parentheses.
Abbreviations are explained in Table 3.
Data for all cultures without CH11 but with TNF were 0 (0-0-0).
For cultures with both CH11 and TNF, data were 0 (0-0-0) for experiments 1 and 3, and 0 (0-0-0-0) for the other experiments.
Fas activation potently abrogates the growth of CD34+ progenitor cells in the presence of TNF. CD34+ cells were expanded for 6 days in SF medium supplemented with SFT3 (“Materials and methods”) in the absence or presence of CH11 (1 μg/mL) and TNF (20 ng/mL) as indicated. After 6 days, cells were counted and plated at a concentration of 500 cells per milliliter in cytokine-supplemented methylcellulose (“Materials and methods”). After an additional 10 to 12 days of incubation, CFU-GM (A) and BFU-E (B) colonies were scored as described in “Materials and methods.” Results (averages of 3 experiments; 2 with CB and 1 with BM) are presented as percentages of controls (cells expanded in the presence of SFT3; error bars represent SEMs).
Fas activation potently abrogates the growth of CD34+ progenitor cells in the presence of TNF. CD34+ cells were expanded for 6 days in SF medium supplemented with SFT3 (“Materials and methods”) in the absence or presence of CH11 (1 μg/mL) and TNF (20 ng/mL) as indicated. After 6 days, cells were counted and plated at a concentration of 500 cells per milliliter in cytokine-supplemented methylcellulose (“Materials and methods”). After an additional 10 to 12 days of incubation, CFU-GM (A) and BFU-E (B) colonies were scored as described in “Materials and methods.” Results (averages of 3 experiments; 2 with CB and 1 with BM) are presented as percentages of controls (cells expanded in the presence of SFT3; error bars represent SEMs).
Discussion
Previous studies have suggested that most CD34+ progenitor cells in steady state human BM lack expression of Fas.38-40 Using a highly sensitive PE-conjugated anti-Fas Ab, we here demonstrate that Fas is constitutively expressed on a minor fraction of CD34+ cells in human BM and CB, but that Fas+ cells almost exclusively have a CD34+CD38+ progenitor phenotype, whereas the CD34+CD38- candidate HSC pool expresses little or no Fas during steady state hematopoiesis. In agreement with this, Bárcena et al62 demonstrated that although some human fetal liver CD34+CD38- cells express Fas, they are probably preceded by a more primitive CD34+CD38- progenitor lacking Fas expression. Thus, the current and previous studies suggest that the earliest human HSCs express little or no Fas during steady-state hematopoiesis. It is therefore unlikely that HSCs would be targeted by the Fas pathway during steady state hematopoiesis, at which time the majority of HSCs are in G0 or G1 of the cell cycle.51-54
In vitro cytokine stimulation has been demonstrated to up-regulate Fas expression on CD34+ progenitor cells, but it has been hypothesized that this up-regulation most likely reflects cytokine-induced differentiation of CD34+ progenitors,40 a finding supported by the up-regulation of Fas during maturation of different blood cell lineages.63 If so, up-regulation of Fas expression in response to cytokine stimulation would occur entirely outside the HSC pool. However, an alternative hypothesis explored in the present studies would be that HSCs when activated and induced to enter active cell cycling would up-regulate Fas expression. With the use of recently established conditions efficiently promoting in vitro cycling of CD34+CD38- HSCs without loss of HSC function,42,45 Fas expression was found to be up-regulated on candidate BM and CB HSCs. However, since only a minor fraction of CD34+CD38- cells possess HSC multilineage reconstituting ability,14 cultured cells were sorted into Fas+ and Fas- populations to directly investigate whether Fas was up-regulated on SRCs and LTC-ICs. Strikingly, although both Lin-Fas- and Lin-Fas+ cells revealed similar and high progenitor cell activities, the ability to multilineage-reconstitute NOD-SCID mice as well to sustain long-term cultures was almost exclusively a property of Fas+ cells. Furthermore, Fas expression was up-regulated in a similar manner on candidate human HSCs when these were induced to cycle in vivo following transplantation. Thus, these experiments, and the accompanying analysis of Fas expression in relation to different phases of the cell cycle, clearly demonstrated that Fas expression can be up-regulated on HSCs in an activation and/or cell cycle–dependent manner. Consequently, whereas the HSC pool in human BM and CB lacks constitutive Fas expression, most HSCs that are induced to cycle start to express high levels of Fas and therefore become potential targets for Fas suppression.
While the physiologic significance of the up-regulation of Fas on human HSCs remains to be explored in more detail, different studies have implicated involvement of Fas in several BM-suppression syndromes, including aplastic anemia, myelodysplastic syndromes, and acute GVHD,33-35,64,65 although definitive evidence for this is lacking. The present studies would suggest that Fas-mediated suppression under such conditions might in part be mediated through targeting of the HSC pool. It is noteworthy that these BM-failure conditions are also associated with enhanced activity of other proinflammatory mediators such as TNF,35,66 which are documented to be potent activators of Fas responsiveness in hematopoietic progenitors.38,39 Such coactivation might be a prerequisite to efficiently inducing Fas responsiveness of HSCs, since they otherwise appear highly resistant to Fas-induced suppression. The Fas resistance of HSCs, when compared with committed myeloid progenitors,38,39 could be explained by higher levels of expression of members of the B-cell lymphoma 2 (Bcl-2) family or other antiapoptotic pathways in HSCs,62,67 a hypothesis supported by forced overexpression of Bcl-2 in myeloid progenitors resulting in enhanced Fas resistance.26
Whereas in vitro and in vivo activation and cycling were both required and sufficient to up-regulate Fas expression on the vast majority of human SRCs and LTC-ICs in the present studies, we recently demonstrated that efficient cycling of highly purified mouse HSCs under identical conditions was required, but not sufficient, to up-regulate Fas expression on long-term multilineage repopulating HSCs.37 In fact, coactivation with TNF was required to induce Fas expression as well as Fas responsiveness of mouse HSCs.37 Since TNF can be produced by hematopoietic cells, we investigated whether autocrine or paracrine TNF production in the expansion cultures might mediate up-regulation of Fas on candidate HSCs. However, using a neutralizing anti-TNF Ab, we found no evidence for this. Thus, whereas active cycling human HSCs (SRCs and LTC-ICs) become Fas+, up-regulation on murine HSCs requires coactivation by TNF, suggesting a potential distinction between murine and human HSCs as potential targets for Fas activation.
Prepublished online as Blood First Edition Paper, March 13, 2003; DOI 10.1182/blood-2002-07-2286.
Supported by grants from the John and Augusta Persson Foundation; the O and E and Edla Johansson Foundation; the Thelma Zoega's Foundation; the Tobias Foundation; the Greta and Johan Kock's Foundations; Government Public Health Grant; Skånes Landsting; the Swedish Foundation for Strategic Research; the Swedish Cancer Society; Swedish Society of Pediatric Cancer; and the Medical Faculty, University of Lund. I.D. is supported through a fellowship from the Norwegian Cancer Society and the Faculty of Medicine, Norwegian University of Science and Technology.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Donna E. Hogge for kindly providing the murine fibroblast stroma cell lines and helpful advice regarding the use of these. The expert assistance of Dr Lars Nilsson in the NOD-SCID assay; cell sorting assistance by Anna Fossum, Carl-Magnus Högerkorp, Sverker Segrén, and Zhi Ma; as well as the technical assistance and animal care of Gunilla Gärdebring and Lilian Wittman are highly appreciated. We also thank the staff and donors at the Department of Gynecology, Lund University Hospital, and Helsingborg Hospital for help with providing CB; all volunteers for their BM contributions; and the physicians and other staff at the Department of Hematology, Lund University Hospital, for performing the BM aspirations.

![Figure 3. Up-regulation of Fas expression on ex vivo cycling CD34+ CB cells is not mediated through endogenous TNF production. CD34+ CB cells were cultured for 5 days in SF medium supplemented with SFT3 in the absence or presence of a neutralizing anti-TNF Ab as indicated (“Materials and methods”). Lin-CD34+ cells were gated and investigated for coexpression of Fas. Data are presented as the percentages of Lin-CD34+ cells coexpressing Fas in the absence (□) or presence (▪) of anti-TNF (mean values of 3 independent experiments; error bars, standard errors of the mean [SEMs] are shown).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/1/10.1182_blood-2002-07-2286/5/m_h81334535003.jpeg?Expires=1765998438&Signature=clsBpVDmM09zvuhfdXdmGGWRih8sCpqJ-GdSTIDjAJGDxng7gP76SQEUBaAHKLfCwVh0CFDgq6iEevDTsKGKybenTNWWEx-rThBtlKV7NvSuJGHezXrxbi4unqWlsNMnGujEKXIIN1Zg5I684LCPLoDEk8Aas06Ivh4DEio6AoSaV1YZPoT2YtdtQeU7c1vHEZzL6wYm-N39XAZAUS12we6E8zmIq8W54QTftV1l-XiNcQRHFgcO~jwjWVG4C96zUSrvi-jmbi0ceYpsZ9DvJXbzcQkXpL17vKAW4ZXeYk9Gv5KTgirsHvU7yIWGeFenLFAvLnF00yetc6-bF78BgQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal