Human T-cell leukemic virus type 1 (HTLV-1)–infected T cells express the fucosyltransferase (Fuc-T) VIIgene involved in the biosynthesis of the leukocyte sialyl Lewis X, which may be related to tissue infiltration in patients with malignant adult T-cell leukemia. HTLV-1 induces Fuc-T VIItranscription through the viral transactivator Tax, although the underlying molecular mechanism remains unknown. In the present study, we analyzed the role of the cis-activating element in Tax activation using reporter constructs bearing the 5′-regulatory region of Fuc-T VII in Jurkat T cells. A sequence (GGCTGTGGGGGCGTCATATTGCCCTGG) covering a half-palindromic cyclic adenosine monophosphate (cAMP)–responsive element (CRE) was found to be required for Tax activation of the Fuc-T VII promoter. We further demonstrated that transcription factors of the CRE-binding protein (CREB)/activating transcription factor (ATF) family bind to this CRE-like sequence and that Tax binds in association with CREB and the coactivator CREB-binding protein (CBP) in Jurkat T cells. This element, containing the G+C–rich flanking sequences, is homologous to the Tax-responsive viral CREs in the HTLV-1 long terminal repeat (LTR)–promoter. Furthermore, CREMα, an isoform of CREB deficient in the glutamine-rich domains, was found to activate the Fuc-T VII promoter in a phosphorylation-independent manner, similar to the viral CRE in HTLV-1 LTR but in contrast to the phosphorylation-dependent activation of the cellular CREs by Tax. These findings indicate that the Fuc-T VII promoter is transactivated by Tax in concert with CBP through a CRE-like sequence in a manner similar to that of viral CRE in HTLV-1 LTR.
Introduction
We have previously shown that leukemia cells in patients with adult T-cell leukemia (ATL), and cell lines derived therefrom, more strongly express sialyl Lewis X, a major ligand for selectins in leukocytes, than other types of human lymphocytic leukemia cells.1,2 ATL is a malignancy of peripheral CD4+ cells initiated by infection with human T-cell leukemic virus type 1 (HTLV-1).3,4 Infiltration by leukemic cells into various tissues is a frequent manifestation of ATL5 and often causes serious clinical problems. The degree of expression of sialyl Lewis X on leukemic cells in ATL significantly correlates with their degree of extravascular infiltration.2 The evidence suggests that deregulated expression of sialyl Lewis X determinant on leukemic cells is involved in the infiltration of ATL cells because the binding of sialyl Lewis X on leukocytes with selectins on endothelial cells is known to trigger extravasation.6-8
Human fucosyltransferase VII (Fuc-T VII) has proved to be crucial for the synthesis of sialyl Lewis X,9,10 the expression of which is regulated during hematopoietic differentiation, inflammation, and malignant transformation.11-14 Recent studies revealed that the synthesis of sialyl Lewis X in leukocytes and leukemic cells is controlled principally at the level of transcription of Fuc-T VII.9,10,12 15-21
Therefore, it is important to determine how the transcription ofFuc-T VII is enhanced in ATL cells. We previously found that the expression of sialyl Lewis X and Fuc-T VII is transactivated by HTLV-1 Tax,22 which is involved in the proliferation and transformation of T cells in ATL through the control of viral gene expression.23-25 Tax also exerts its transactivating effects on promoters of various cellular genes.26,27 In the present study, undertaken to investigate the mechanism underlying transactivational effects of Tax on the Fuc-T VII promoter, we identified a Tax-responsive element in the Fuc-T VII promoter that contains a half-palindromic cAMP-responsive element (CRE) binding to CRE-binding protein/activating transcription factor (CREB/ATF) factors. The family of CREB/ATF transcription factors, a member of the basic leucine zipper (bZIP) transcription factor group, has been shown to bind the Tax-responsive element in the 21–base pair (bp) repeats of the HTLV-1 long-terminal repeat (LTR) promoter and to modulate viral promoter activity.28 Further examination revealed that this CRE-like element highly resembles the Tax-responsive element in the 21-bp repeats, called the viral CRE, similarly flanked by G+C–rich sequences.29
Materials and methods
Cells and plasmids
JPX-9 (kindly provided by Drs K. Ohtani and M. Nakamura, Tokyo Medical and Dental University) is a Jurkat T-cell subline generated by stable introduction of a gene that contains the Tax-coding region preceded by a murine metallothionein promoter.32 JPX-9 cells, a human T cell line (Jurkat), an HTLV-1–infected T-cell line (ED40515-N),22 and a natural killer (NK)–like cell line (YT)15 were maintained in RPMI 1640 medium with 10% fetal calf serum. F9 cells were maintained in Dulbecco modified Eagle medium with the same supplement. Construction of the expression plasmid of Tax, pCGTax, and of Tax mutants (d3 and d7/16) were described previously.33-35 The Tax mutant M2236 was a gift from Dr W. C. Greene. Rous sarcoma virus (RSV) enhancer-driven expression plasmids RSV-CREMα and RSV–protein kinase A (PKA) were kindly provided by Dr Richard Goodman.37 Chloramphenicol acetyltransferase (CAT) reporter constructs pU3R-CAT38 and somatostatin-CAT39 plasmids, which were also gifts of Dr Richard Goodman, were digested with XhoI andHindIII or with SalI and HindIII, respectively, and were ligated to pGL2 (Promega, Madison, WI) to get pU3R-Luc and somatostatin-Luc reporter plasmids.
Construction of 5′-deletion and site-directed mutants
A genomic clone containing the exons of human Fuc-T VII and identifying transcriptional start points was generated (N.H. et al, manuscript submitted). An 895-bp element of theFuc-T VII promoter region (from −791 to +104, just 5′ of the ATG codon; numbers correspond to the principal transcription start point) was polymerase chain reaction (PCR)–amplified from the genomic clone using a sense primer containing 5′ KpnI site (primer −791 to −769) and an antisense primer with 3′ HindIII site (primer +78 to +104) to facilitate subcloning. The PCR products were purified from agarose gels, digested, and subcloned into theKpnI/HindIII site immediately 5′ of the luciferase cDNA segment of the promoterless luciferase reporter gene plasmid, pGL2, to create p(−791). The same approach was used to produce the deletion reporter constructs. To generate the constructs of mutated p(−791), featuring changes in the CRE-like element or the Sp1 consensus site, PCR was used by overlap extension.40 To construct the multisite mutants, the first site mutant cloned in pGL2-Basic was used as a template. The second-step PCR product was cloned, and sequential overlap extensions were performed as described above.40 The absence of secondary mutations introduced by PCR was confirmed for all constructs by DNA sequencing and comparison with the sequence of the original genomic clone.
Promoter activity determinations
Cultures at 50% to 60% confluence grown in 6-well culture plates were rinsed in serum-free medium and exposed to SV40-CAT (0.5 μg) and reporter constructs (4 μg), with or without pCGTax (4 μg) with 0.5% DMRIE-C (Life Technologies, Grand Island, NY) for 5 hours. Cells were refed medium supplemented with 10% fetal calf serum and cultured for 48 to 72 hours to reach confluence, rinsed twice with phosphate-buffered saline, and extracted with 200 μL cell lysis buffer (Promega). Nuclei were cleared by centrifugation at 12 000g for 5 minutes. Luciferase assays were performed according to the manufacturer's protocol for the reporter assay system (Promega) with a single-photon channel of a scintillation counter (Beckman, Somerset, NJ) and CAT activity in supernatants. For F9 embryonic teratocarcinoma, a 1:15 dilution was made at 50% confluence, and after 6 hours the cells were transfected with Lipofectamine (Life Technologies) with a constant amount of DNA. Cultures were grown for 25 to 30 hours before harvesting and were assayed for luciferase activity as described above, normalized to vector-dependent CAT activity.
Purification of histidine fusion proteins
Escherichia coli BL21 (DE3) cells transformed with Tax expression plasmid 6HisT-pET11 derivatives were grown and induced with isopropyl-β-D-thiogalactoside. Histidine fusion proteins were purified from cell lysates on nitrilotriacetic acid–agarose column (Qiagen, Düsseldorf, Germany) by stepwise elution with buffer containing 20 to 100 mM imidazole. Fractions containing histidine fusion proteins were dialyzed against 20 mM HEPES (N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic acid)–KOH buffer, pH 7.9, 80 mM KCl, 0.2 mM EDTA (ethylenediaminetetraacetic acid), 0.5 mM dithiothreitol (DTT), 10% glycerol, and 0.1 mM phenylmethylsulfonyl fluoride (PMSF) and were stored frozen at −80°C.
DNAP assay
DNA affinity precipitation (DNAP) assays were performed as described previously.28,34 Briefly, biotinylated DNA probe carrying a tetramer of the CRE-like element was prepared, and aliquots (1 μg) was mixed with a cell lysate containing poly(dI-dC) (15 μg). Streptavidin–Dynabeads (Dynal, Great Neck, NY) were added, collected with a magnet, and washed twice with buffer. The trapped proteins were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) followed by Western blotting. Bands were enhanced with a chemiluminescence detection system (Amersham, Piscataway, NJ). The monoclonal antibody Lt-4 used against Tax was previously reported.41 Anti–CREB-1 (specific for the bZIP domain), anti–CREB-2, anti-ATF4, and anti-CBP antibody were purchased from Santa Cruz Biotechnology (Santa Cruz, CA), along with a bZIP domain peptide derived from CREB-1.
Electrophoretic mobility shift assays
Nuclear extracts of unstimulated or stimulated Jurkat T cells were prepared by the method described by Dignam et al.42The final extract was dialyzed overnight at 4°C in a mixture of 20 mM HEPES (pH 7.8), 20% glycerol, 0.1 M KCl, 0.2 mM EDTA, 0.5 mM DTT, and PMSF, centrifuged at 10 000g to remove any precipitation, and stored at −80°C in aliquots. The protein concentration ranged between 2 and 4 mg/mL. Electrophoretic mobility shift assays (EMSAs) were carried out as described previously43 with electrophoresis in 5% nondenaturing polyacrylamide gels. The probe used from the Fuc-T VII promoter corresponded to the sequence from −166 bp to −144 bp—GTGGGGGCGTCATATTGCCCTGG. Oligonucleotide competition analysis was conducted with a 20- to 50-fold molar excess of unlabeled wild-type or mutant probe oligonucleotides (mut-probe, GTGGGGGCcaCATATTGCCCTGG) and the 21-bp enhancer sequence in HTLV-1 LTR.28 The CRE-consensus sequence was purchased from Promega. Gels were dried and subjected to autoradiography and analysis with BAS2000 (Fuji Film, Tokyo, Japan). For supershift analysis, 1-μg aliquots of specific antibodies (obtained from Santa Cruz Biotechnology) were used as specified in the figure legends.
Results
Tax transactivation of the Fuc-T VII promoter is dependent on the CRE-like element
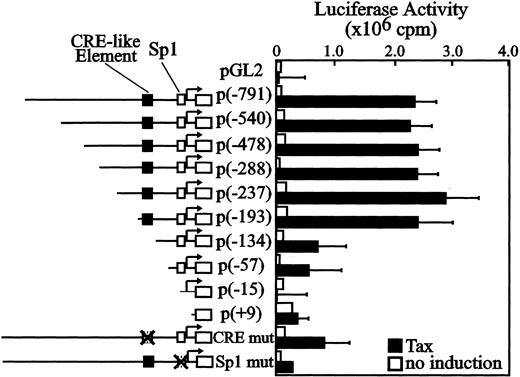
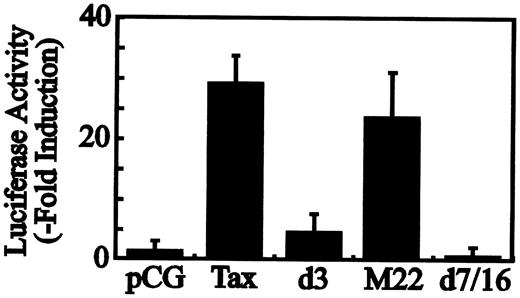
We reported that Tax induces the expression of Fuc-T VII in vitro and in vivo.22 Previously we cloned a 991-bp fragment of the 5′ flanking region of humanFuc-T VII and identified transcriptional start sites (N.H. et al, manuscript submitted). The sequence of the fragment is shown in Figure 1 (the nucleotide sequence reported in this paper has been deposited in the DNA Data Bank of Japan (DDBJ) databases, with accession numberAB012668). As judged by the sequence, there is no conventional TATA box upstream of the principal transcription start site, but an initiator element is juxtaposed with an Sp1 consensus site for the accurate initiation of RNA transcription. We subcloned this fragment into the luciferase reporter plasmid and prepared several deletion and mutated constructs. Transfection of Tax with the promoter construct p(−791) resulted in remarkable transactivation (Figure2). Site-specific Sp1 consensus site mutants exhibited abrogated Fuc-T VII promoter activity, showing that the Sp1 site juxtaposed to an initiator element is critical for the efficiency of initiation and for positioning of transcription start sites in TATA-less promoters.44,45 The reporter constructs p(−791) through p(−193) are activated by Tax, but transactivation was suppressed with the p(−134) reporter construct. Because suppression was seen in the experiments with the smaller reporter constructs, the minimal Tax-responsive element should reside between nucleotides −193 and −134. In this region, we found the sequence GGGGCGTCATATTGCCC, which contains the CRE motif CGTCA (a half-palindromic CRE motif in comparison with the 8-bp full CRE palindrome, TGACGTCA), but did not detect the TRE-2 sequence (a holding protein binding sequence, GGAACCACCCA), which is involved in responding to Tax trans activation in the HTLV-1 LTR promoter.43 However, the TRE-2 sequence contains Sp1 and an Ets-like site, GGAA, side by side. In addition, as shown in Figure2, we found in the Fuc-T VII promoter/enhancer that they play a critical role in induction and may be related to the TRE-2 structure and its binding factors.46 Because CRE elements are known to be important targets for Tax transactivation of the LTR (viral CRE) and for certain cellular genes (cellular CRE), for confirmation a mutated reporter construct was generated with the half-palindromic CRE motif. Tax-induced transactivation in theFuc-T VII promoter was significantly suppressed. To confirm the role of the CREB/ATF transcription pathway in Tax-mediated transactivation of the Fuc-T VII promoter, reporter assays were performed with cotransfection of wild-type or selected Tax mutants. The wild-type and M22, which preferentially activates the CRE-dependent pathway but not the NF-κB/Rel-dependent pathway, fully activated the reporter construct (Figure3). We next tested mutants d3 and d7/16. The d3 mutant is almost completely inactive regarding the LTR in HTLV-1 and ATF/CRE sites but is almost fully active with the NF-κB site.34 The d7/16 mutant has none of abilities to activate all above.34 These mutants failed to transactivate theFuc-T VII promoter. These results imply that the CRE-like element has the ability to enhance the Tax-induced expression of Fuc-T VII in Jurkat T cells.
Genomic sequence of the 5′-untranslated region of the
Fuc-T VII gene. The number in the left column pertains to the nucleotide location of the first letter in each lane with respect to the +1 site, which corresponds to the principal transcription start site, and the initiator sequence regarding this transcription start site is underlined with a wavy line. Uppercase letters indicate exon sequences, and lowercase letters indicate untranscribed sequences. The ATG codon is in boldface. The CRE-like element is also underlined (half-palindromic CRE motif is in boldface). Another putativecis-acting motif, Sp1, just 5′ to Ets-like site (GGAA), is represented by a closed box.
Genomic sequence of the 5′-untranslated region of the
Fuc-T VII gene. The number in the left column pertains to the nucleotide location of the first letter in each lane with respect to the +1 site, which corresponds to the principal transcription start site, and the initiator sequence regarding this transcription start site is underlined with a wavy line. Uppercase letters indicate exon sequences, and lowercase letters indicate untranscribed sequences. The ATG codon is in boldface. The CRE-like element is also underlined (half-palindromic CRE motif is in boldface). Another putativecis-acting motif, Sp1, just 5′ to Ets-like site (GGAA), is represented by a closed box.
Identification of the Tax-responsive region in theFuc-T VII promoter.
Reporter constructs, full-length, and 5′ deletions of the Fuc-T VII sequence cloned upstream of luciferase reporter gene (pGL2) through +104 nucleotide, were transfected into Jurkat T cells and either not cotransfected (■) or cotransfected with pCGTax (▪). The transcription start site and its location are indicated with an arrow in each construct. Reporter constructs shown are described in “Materials and methods.” The CRE-like element is represented by closed boxes, and the Sp1 consensus element is represented by open boxes. Promoter activity was expressed as the fold induction of the observed reporter activity of the construct cotransfected with pCGTax over that of the negative control, the full-length construct without pCGTax. CRE-mut has a mutation of bases −159 to −155 (from CGTCA to TTTAA) in the half-palindromic CRE motif of the CRE-like element, and Sp1-mut has a mutation of bases −39 to −32 (from GGGGCGGG to GTTTCAGG) in the Sp1 consensus element. Crossed-out boxes depict the site of these mutations. Results are expressed as relative light units normalized for CAT activity. Mean values for 3 independent assays are presented, and the corresponding standard deviation is indicated on the top of each bar.
Identification of the Tax-responsive region in theFuc-T VII promoter.
Reporter constructs, full-length, and 5′ deletions of the Fuc-T VII sequence cloned upstream of luciferase reporter gene (pGL2) through +104 nucleotide, were transfected into Jurkat T cells and either not cotransfected (■) or cotransfected with pCGTax (▪). The transcription start site and its location are indicated with an arrow in each construct. Reporter constructs shown are described in “Materials and methods.” The CRE-like element is represented by closed boxes, and the Sp1 consensus element is represented by open boxes. Promoter activity was expressed as the fold induction of the observed reporter activity of the construct cotransfected with pCGTax over that of the negative control, the full-length construct without pCGTax. CRE-mut has a mutation of bases −159 to −155 (from CGTCA to TTTAA) in the half-palindromic CRE motif of the CRE-like element, and Sp1-mut has a mutation of bases −39 to −32 (from GGGGCGGG to GTTTCAGG) in the Sp1 consensus element. Crossed-out boxes depict the site of these mutations. Results are expressed as relative light units normalized for CAT activity. Mean values for 3 independent assays are presented, and the corresponding standard deviation is indicated on the top of each bar.
Differential activation of the Fuc-T VIIreporter with Tax mutants.
Jurkat T cells were cotransfected with 4 μg reporter construct of theFuc-T VII promoter and 4 μg pCGTax/Tax mutants. Resultant activities are plotted as fold induction over that in the absence of Tax. Results are the mean values of fold induction for 3 independent assays normalized for CAT activity, and the corresponding standard deviation is indicated on the top of each bar.
Differential activation of the Fuc-T VIIreporter with Tax mutants.
Jurkat T cells were cotransfected with 4 μg reporter construct of theFuc-T VII promoter and 4 μg pCGTax/Tax mutants. Resultant activities are plotted as fold induction over that in the absence of Tax. Results are the mean values of fold induction for 3 independent assays normalized for CAT activity, and the corresponding standard deviation is indicated on the top of each bar.
Transcription factors of the ATF/CREB family bind to the CRE-like sequence in the Fuc-T VII promoter
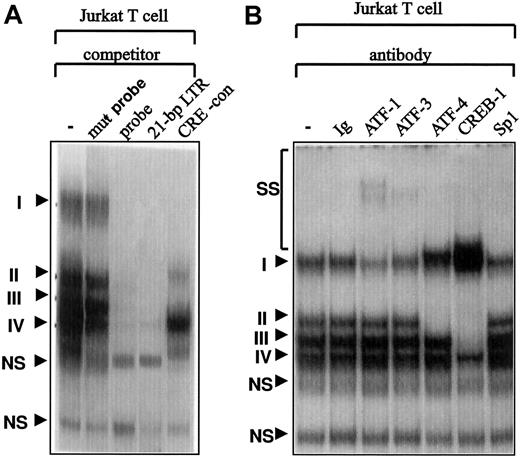
Electrophoretic mobility shift assays showed that transcription factors of the CREB/ATF family interact with the CRE-like sequence (Figure 4), as evidenced by competition analysis and antibody recognition. Incubation of a 27-bp oligonucleotide, including a half-palindromic CRE-like site with Jurkat T-cell nuclear extracts, resulted in the formation of 4 major sequence-specific DNA-binding complexes (Figure 4A). The complex with the slowest mobility was identified as ATF1 and ATF2 complexes based on interaction with specific antibodies but was not totally supershifted, suggesting that it might contain other proteins (Figure 4B). Two of the faster migrating complexes were identified as ATF4 (CREB-2) and CREB-1, respectively (Figure 4B). These 3 major bands competed with the probe with the 21-bp enhancer, and with the CRE consensus sequence. We could not identify components of the fastest sequence-specific DNA-binding complex, which was also competed with the probe and the 21-bp enhancer but not with the CRE-consensus sequence (Figure 4A).
Binding of transcription factors of the CREB/ATF family to the CRE-like sequence in the Fuc-T VII promoter.
Electrophoretic mobility shift assays were conducted with Jurkat T cell nuclear extracts, as described in “Materials and methods.” (A) A 27-bp oligonucleotide containing the CRE-like sequence was used as a probe in the absence and presence of competitor oligonucleotides. Complexes were resolved on a 4% nondenaturing gel. Sequence-specific binding species obtained were competed with a 20- to 50-fold molar excess of the cold competitor oligonucleotide. DNA–protein complexes are numbered. (B) The probe was incubated with specific antibodies against ATF1, ATF3, ATF4 (CREB-2), or CREB-1. Anti-Sp1 antibody was used as a negative control. Specific DNA–protein complexes are indicated by roman numerals. NS indicates nonspecific complex; SS, supershifted band; and CRE-con, CRE-consensus.
Binding of transcription factors of the CREB/ATF family to the CRE-like sequence in the Fuc-T VII promoter.
Electrophoretic mobility shift assays were conducted with Jurkat T cell nuclear extracts, as described in “Materials and methods.” (A) A 27-bp oligonucleotide containing the CRE-like sequence was used as a probe in the absence and presence of competitor oligonucleotides. Complexes were resolved on a 4% nondenaturing gel. Sequence-specific binding species obtained were competed with a 20- to 50-fold molar excess of the cold competitor oligonucleotide. DNA–protein complexes are numbered. (B) The probe was incubated with specific antibodies against ATF1, ATF3, ATF4 (CREB-2), or CREB-1. Anti-Sp1 antibody was used as a negative control. Specific DNA–protein complexes are indicated by roman numerals. NS indicates nonspecific complex; SS, supershifted band; and CRE-con, CRE-consensus.
Association of Tax with CRE-like DNA-protein complexes containing CREB and CBP/P300
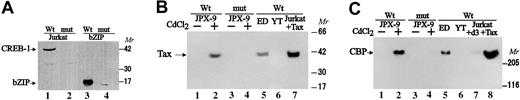
Tax itself is not able to bind DNA,29 but it reportedly interacts with CBP to stabilize the assembly of a multiprotein complex containing CBP/P300, Tax, and CREB/ATF on viral CRE.47 To demonstrate an indirect association of Tax with the CRE-like element, we used a DNAP assay. Biotinylated DNA probes consisting of repeats of the Fuc-T VII CRE-like element were incubated with nuclear extracts from Jurkat T cells, from ED40515-N cells expressing Tax and Fuc-T VII, from YT cells expressing Fuc-T VII but not Tax, and from JPX-9 cells, a Jurkat T-cell subline expressing Tax on incubation with CdCl2. The DNA-protein complexes formed were isolated and examined by immunoblotting for CREB-1, for Tax, and for CBP/P300 (Figure 5). The CRE-like element specifically formed a complex with CREB-1 (molecular mass, 43 kDa) and other undefined CREB family proteins (Figure 5A, lanes 1 and 2). Examination of whether an exogenous bZIP domain, which is conserved among the CREB/ATF family, can associate with the CRE-like element of the Fuc-T VII promoter demonstrated efficient binding to the wild-type, but not to the mutant, element, indicating that the bZIP domain is sufficient for specific DNA binding (Figure 5A, lanes 3 and 4). When the wild-type CRE-like element was used, Tax and CBP/P300 were detected in the CRE-like DNA-protein complex in the nuclear extracts of Tax-expressing cells (Figure 5B-C, lanes 2 and 5) but not with use of the mutated oligonucleotide (Figure 5B-C, lanes 3 and 4), indicating that association of these proteins on the CRE-like element includes the endogenous Tax protein. As with endogenous Tax protein, histidine-Tax was detected in the CRE-like element–protein complex (Figure 5B, lane 7), and CBP/P300 was associated with exogenous Tax (Figure 5C, lane 8). However, the mutant Tax, d3, did not associate with the CRE-like element in the Fuc-T VII promoter (Figure5C, lane 7), strongly indicating that complex formation of Tax–nuclear protein with the CRE-like element is dependent on binding to the CREB.28
Demonstration of complex formation of Tax protein and CBP/P300 coactivator in nuclear extract with the Fuc-T VIICRE-like element.
Nuclear extracts were analyzed in DNAP assay. (A) Detection of CREB-1 protein and the bZIP domain peptide in the Fuc-T VIICRE-like element protein complex. Nuclear extracts from Jurkat T cells (lanes 1-2) or the bZIP domain peptide (lanes 3-4) were incubated with the biotinylated wild-type oligonucleotide (lanes 1 and 3) or the mutated one (lanes 2 and 4), blotted with the anti–CREB-1 antibody reacting to the bZIP domain specifically. (B) Detection of Tax protein in the Fuc-T VII CRE-like element–protein complex. JPX-9 cells were treated with CdCl2 (15 ng/mL) for 24 hours to induce Tax protein in vivo (lanes 2 and 4) and without CdCl2 (lanes 1 and 3). Nuclear extracts were incubated with the biotinylated wild-type oligonucleotide (lanes 1-2 and 5-7) or the mutated oligonucleotide (lanes 3 and 4) of the CRE-like element, blotted with the anti-Tax antibody. Nuclear factor–dependent association of exogenous Tax was detected in the presence of histidine-Tax (lane 7). (C) Association of CBP/P300 coactivator in the nuclear protein–complex formation with Tax. Nuclear extracts of Jurkat T cells were incubated with the CRE-like element in the presence of inactive Tax mutant histidine–d3 (lane 7) or histidine–Tax (lane 8). DNA-protein complexes were analyzed by immunoblotting with anti-CBP. Mr indicates kilodaltons of molecular size markers; and ED, ED40515-N.
Demonstration of complex formation of Tax protein and CBP/P300 coactivator in nuclear extract with the Fuc-T VIICRE-like element.
Nuclear extracts were analyzed in DNAP assay. (A) Detection of CREB-1 protein and the bZIP domain peptide in the Fuc-T VIICRE-like element protein complex. Nuclear extracts from Jurkat T cells (lanes 1-2) or the bZIP domain peptide (lanes 3-4) were incubated with the biotinylated wild-type oligonucleotide (lanes 1 and 3) or the mutated one (lanes 2 and 4), blotted with the anti–CREB-1 antibody reacting to the bZIP domain specifically. (B) Detection of Tax protein in the Fuc-T VII CRE-like element–protein complex. JPX-9 cells were treated with CdCl2 (15 ng/mL) for 24 hours to induce Tax protein in vivo (lanes 2 and 4) and without CdCl2 (lanes 1 and 3). Nuclear extracts were incubated with the biotinylated wild-type oligonucleotide (lanes 1-2 and 5-7) or the mutated oligonucleotide (lanes 3 and 4) of the CRE-like element, blotted with the anti-Tax antibody. Nuclear factor–dependent association of exogenous Tax was detected in the presence of histidine-Tax (lane 7). (C) Association of CBP/P300 coactivator in the nuclear protein–complex formation with Tax. Nuclear extracts of Jurkat T cells were incubated with the CRE-like element in the presence of inactive Tax mutant histidine–d3 (lane 7) or histidine–Tax (lane 8). DNA-protein complexes were analyzed by immunoblotting with anti-CBP. Mr indicates kilodaltons of molecular size markers; and ED, ED40515-N.
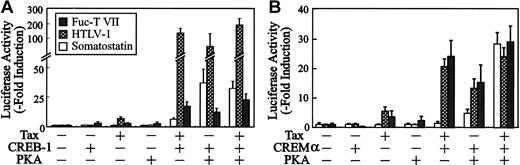
CRE-like element in the Fuc-T VII promoter resembles HTLV-1 LTR viral CRE
Surveying the reported sequences that can be transactivated by Tax, we found a similarity with the CRE-like element. Alignment of all three 21-bp repeats in the HTLV-1 LTR, containing viral CREs, revealed the consensus sequence, GGCNNTGACGNNNNCCCC, with a half-palindromic CRE motif (CGTCA) flanked by G+C–rich sequences. In the CRE-like element, .........GGGGGCGTCATATT........... GCCC, in the Fuc-T VII promoter, the nonpalindromic/half-palindromic CRE motif (underlined) is flanked by the G+C–rich sequences (dotted-underlined), the same as the 21-bp repeats in the HTLV-1 LTR. This G+C–rich flanking sequence in the HTLV-1 LTR reportedly contributes to protein kinase A (PKA)–independent activation of HTLV-1 LTR by Tax.30 To examine whether the G+C–rich sequences of the CRE-like element in theFuc-T VII promoter contribute to phosphorylation-independent Tax transactivation, we measured expression of the Fuc-T VIIreporter gene in F9 teratocarcinoma cells in the presence or absence of PKA and Tax, of the somatostatin-Luc reporter gene for cellular CRE, and of the HTLV-1 LTR reporter gene for viral CRE.31 F9 cells were chosen for these studies because they lack functional levels of CREB and PKA.48 They were cotransfected with Tax and CREB-1 in the absence of PKA. The results documented in Figure6A indicate that in the presence of Tax, CREB-1 activated the Fuc-T VII promoter to more than 20-fold the basal level, as well as the HTLV-1 LTR promoter through the viral CRE. Control transfections indicated no activation in the presence of exogenous CREB-1 alone, suggesting that phosphorylation of CREB-1 is not essential for Tax activation of the Fuc-T VIIpromoter. To confirm this differential requirement for phosphorylation in Tax-mediated activation of Fuc-T VII, viral CRE, and other Tax-activated cellular CREs, we used CRE modulator α (CREMα), which lacks the glutamine-rich domains present in CREB and generally represses CRE-mediated transactivation.49 F9 cells were cotransfected with Tax and CREMα in the absence of PKA. The results in Figure 6B indicate that in the presence of Tax, CREMα activated the Fuc-T VII promoter 15-fold as well as the HTLV-1 LTR promoter through the viral CRE. However, cellular CRE required the presence of PKA for Tax-transactivation with CREMα. These results revealed that the glutamine-rich regions (lacking in CREMα) are not essential for Tax activation of the Fuc-T VII promoter. Laurance et al30 have provided a model that CBP/P300 should be recruited to the viral CRE through a direct interaction between Tax and the conserved bZIP domain of this isoform through different domains of the CREB/ATF factors, depending on the particular promoter context.
Activation of the HTLV-1 and the Fuc-T VII promoters by CREB-1 or CREMα.
F9 teratocarcinoma cells were transfected with 4 μg reporter constructs, somatostatin-Luc (■), pU3R-Luc (░), or Fuc-T VII promoter (p(−791) (▪), and 0 to 4 μg pCGTax, together with 0 to 4 μg RSV-CREB (A) or 0 to 4 μg RSV-CREMα (B) and 4 μg RSV-PKA, as indicated. Resultant activities are plotted as fold induction normalized for CAT activity over that in the absence of Tax. Error bars depict the standard deviations from 3 independent experiments.
Activation of the HTLV-1 and the Fuc-T VII promoters by CREB-1 or CREMα.
F9 teratocarcinoma cells were transfected with 4 μg reporter constructs, somatostatin-Luc (■), pU3R-Luc (░), or Fuc-T VII promoter (p(−791) (▪), and 0 to 4 μg pCGTax, together with 0 to 4 μg RSV-CREB (A) or 0 to 4 μg RSV-CREMα (B) and 4 μg RSV-PKA, as indicated. Resultant activities are plotted as fold induction normalized for CAT activity over that in the absence of Tax. Error bars depict the standard deviations from 3 independent experiments.
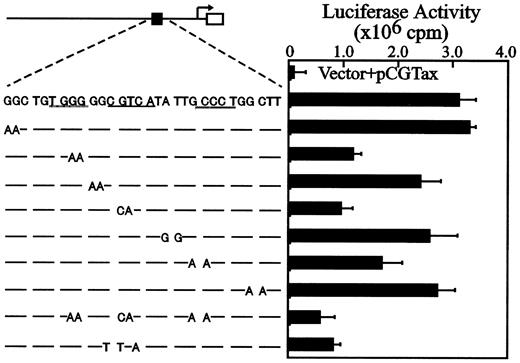
G+C–rich flanking sequences in the CRE-like element are required for Tax-mediated activation of the Fuc-T VIIpromoter
The functional resemblance of the CRE-like element in theFuc-T VII promoter to viral CRE in the HTLV-1 LTR suggests that these G+C–rich flanking sequences have functional importance in transactivation by Tax. Mutations were, therefore, introduced into the relevant regions of the CRE-like element, including the half-palindromic motif, and the effects on transactivation were analyzed. As shown in Figure 7, substitutions in the 5′ G+C–rich sequence (TGGGG→TGAAG) remarkably reduced reporter activity, but not to the same extent as when the mutant had a substitution in the half-palindromic CRE motif. The latter nearly abolished transactivation. A requirement of the flanking G+C–rich sequences, especially the 5′ G+C–rich sequence, in transactivation by Tax has also been found for HTLV-1 LTR.29
Tax activation of the Fuc-T VII promoter is dependent on the CRE-like element and on its flanking G+C–rich sequences.
(A) The CRE-like element and the luciferase reporter construct are represented by closed and open boxes, respectively. The Fuc-T VIIpromoter from nucleotide −791 to +104 with the CRE-like element underlined. Specific mutations in each reporter construct are shown below. (B) Cells were transfected with 4 μg mutant reporter construct alone or in combination with 4 μg pCGTax. Results are expressed as relative light units normalized for CAT activity. Error bars depict the standard deviations from 3 independent experiments.
Tax activation of the Fuc-T VII promoter is dependent on the CRE-like element and on its flanking G+C–rich sequences.
(A) The CRE-like element and the luciferase reporter construct are represented by closed and open boxes, respectively. The Fuc-T VIIpromoter from nucleotide −791 to +104 with the CRE-like element underlined. Specific mutations in each reporter construct are shown below. (B) Cells were transfected with 4 μg mutant reporter construct alone or in combination with 4 μg pCGTax. Results are expressed as relative light units normalized for CAT activity. Error bars depict the standard deviations from 3 independent experiments.
Discussion
We previously reported that Tax transactivates the Fuc-T VII promoter, which results in the appearance of sialyl Lewis X, the selectin-ligand on leukocytes.22 Although Tax contains a transcriptional activation domain, it is unable to bind DNA directly.29,50 Thus, it has been proposed that the transactivation function of Tax requires its interaction with cellular DNA-binding proteins. In the present study, we documented the results of EMSA experiments and reporter assays indicating that the CRE-like element enhances transactivation by Tax in association with CREB/ATF transcription factors. In particular, DNAP experiments showed the formation of a complex with CREB-1, Tax, and CBP mediated by the CRE-like element in the Fuc-T VII promoter. Results of the reporter assays with mutants of Tax also indicated the involvement of CRE in Tax transactivation of the Fuc-T VII promoter. Previously we described that Tax mutant d3 did not form a complex with either CREB or CREM but rather with the NF-κB factors.51The inability to form the complex and to transactivate the Fuc-T VII promoter also indicates that association with transcription factors of the CREB/ATF family occurs in a Tax sequence-specific manner.
We proposed here that Tax activates the CRE-like element of theFuc-T VII promoter through a mechanism similar to that for viral CRE in HTLV-1 LTR. Laurance et al30 earlier reported that Tax activates cellular CREs and viral CREs in HTLV-1 LTR through mechanisms that are differentially dependent on phosphorylation. With the use of CREMα, which is unable to transactivate cellular CREs even in the presence of Tax, we confirmed the results of Laurance et al30 and also found that in the presence of Tax, these normally repressive nuclear factors of the CREB/ATF family activate theFuc-T VII promoter and the viral CRE-like element in HTLV-1 LTR in a phosphorylation-independent manner, in contrast to the other cellular CREs. This is in line with the report that the activation of the HTLV-1 promoter through Tax and CREB/ATF nuclear factors is PKA independent.52 We conclude that activation of the viral promoter occurs through the recruitment of CBP/P300 and requires, in addition to Tax, only the bZIP domain that is conserved among CREB and CREMα. In contrast, activation of the cellular CREs by Tax appears to require the presence of PKA and an isoform of CREB or CREM that contains the kinase-inducible domain (KID). Thus, our results indicate that activation of the Fuc-T VII CRE-like element by Tax is attributed to the ability of Tax, interacting with the minimal bZIP domain, to recruit the coactivator CBP/P300 to the promoter even without phosphorylation. Regarding the transactivation of HTLV-1 LTR by Tax, it has been demonstrated that the G+C–rich sequences flanking the viral CREs are essential for Tax-enhanced binding of CREB/ATF transcription factors to the half-palindromic CRE-like element.53-55 We also reported previously that another G+C–rich sequence, TRE2S, adjacent to the 21-bp enhancer in LTR, can influence the 21-bp enhancer activity through the novel binding factor Tax-helping protein-1 (THP-1) and Gli2 protein.43,56,57 We could detect a similar effect of THP-1 and Gli2 protein on the Fuc-T VII promoter (T.Y. et al, unpublished results, September 2002) as that on the 21-bp enhancer of HTLV-1. With viral CRE, G+C–rich flanking sequences are critical for Tax-CREB/ATF-DNA ternary complex assembly and transactivation.58-60 Furthermore, it is known that Tax enhances binding of the factors to the CRE-like element but not the palindromic CREs, such as somatostatin promoter cellular CRE,61 providing support for the idea that the occupancy of CREB over a particular CRE site is influenced by flanking sequences. As here shown in Figure 8, Tax may provide a bridge between the coactivator CBP/P300 and CREB/ATF nuclear factor(s) assembled on the Fuc-T VII CRE-like element in a phosphorylation-independent manner. In view of the homology to TRE2S and the findings with undefined proteins in the EMSA shown in Figure 4, other transcriptional factors, such as THP-1 and Gli2, may be involved in cooperation with the CRE-like element in responding to Tax transactivation.43 62
Model depicting a suggested mechanism by which Tax could increase the cooperative effect of CBP on the Fuc-T VII CRE-like element.
CREB/ATF refers to CREB/ATF transcription factor(s). Tax, in association with CREB/ATF and the CRE-like element, creates a binding site that anchors CBP/P300 to the promoter. The initiator consensus sequence (Inr) is described in “Results” and in Figure1.
Model depicting a suggested mechanism by which Tax could increase the cooperative effect of CBP on the Fuc-T VII CRE-like element.
CREB/ATF refers to CREB/ATF transcription factor(s). Tax, in association with CREB/ATF and the CRE-like element, creates a binding site that anchors CBP/P300 to the promoter. The initiator consensus sequence (Inr) is described in “Results” and in Figure1.
Recently, Fimia et al46 reported the existence of a novel nuclear factor that can associate with CRE elements, bypassing the classic requirement of activation by CREB and CREM. That is, it does not require their phosphorylation by PKA. They suggested the possibility of other phosphorylation-independent CREB activators, which might contribute to the mechanism of transactivation through CRE-like element in the Fuc-T VII promoter. The observed mode of transactivation might suggest the presence of a new class of cofactors whose function does not require the phosphorylation of CREB/ATF transcription factors.
We thank Dr R. H. Goodman and Dr J. F. Habener for providing plasmid DNAs and Dr K. Ohtani and Professor M. Nakamura for the gift of JPX-9 cells.
Prepublished online as Blood First Edition Paper, December 27, 2002; DOI 10.1182/blood-2002-07-2301.
Supported in part by the Ministry of Education, Culture, Sports, Science and Technology, Japan (grants-in-aid 13680732,13670582, and on priority areas 14030092) and grants-in-aid for the Second Term Comprehensive 10-Year Strategy for Cancer Control from the Ministry of Health, Labor and Welfare, Japan.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Reiji Kannagi, Division of Molecular Pathology, Aichi Cancer Center, Chikusa-ku, Nagoya, 464-8681, Japan; e-mail: rkannagi@aichi-cc.jp.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal