Interleukin-25 (IL-25) is a recently described T helper 2 (TH2) cell–derived cytokine that belongs to the IL-17 family and induces the production of IL-4, IL-5, and IL-13 from an unidentified non–T-cell population. Here, we show that mast cells are also potent IL-25–producing cells. When bone marrow–derived mast cells were stimulated by immunoglobulin E cross-linking, IL-25 mRNA was induced within 30 minutes in a calcineurin-dependent manner, and the levels of IL-25 mRNA were comparable with those of activated TH2 cells. Production of IL-25 by mast cells was also detected at protein levels by immunoblotting. These results suggest that mast cells may enhance TH2-type immune response by producing IL-25.
Introduction
Recently, cytokines homologous to interleukin (IL)–17 have been identified by database searching. There have been 5 new family members identified, namely IL-17B, IL-17C, IL-17D, IL-17E/IL-25, and IL-17F, which possess 20% to 30% homology to IL-17.1,2 Among IL-17 family cytokines, it has been shown that the in vivo and in vitro biologic activities of IL-25 are markedly different from those described for IL-17 and other IL-17 family cytokines.2-7 The expression of IL-25 results in the expansion of eosinophils through the production of IL-5 from an unidentified non–T-cell population,2-4 whereas other IL-17 family cytokines induce the expansion of neutrophils.5-7 In addition, IL-25 induces elevated gene expression of IL-4 and IL-13 in multiple tissues and the resultant T helper 2 (TH2)–type immune responses (increased serum immunoglobulin E [IgE] levels and pathologic changes in the lungs and digestive tract with eosinophilic infiltrates, increased mucus production, and epithelial cell hyperplasia,2-4), indicating that IL-25 is capable of amplifying allergic inflammation.
Although it has been shown that IL-25 mRNA is exclusively expressed in polarized TH2 cells,4quantitative reverse transcriptase–polymerase chain reaction (RT-PCR) analysis shows that IL-25 mRNA is detected in multiple tissues, including colon, uterus, stomach, small intestine, kidney, and lung,2-4 suggesting that, in addition to TH2 cells, other cell types may produce IL-25. We show here that primary bone marrow–derived mast cells (BMMCs) produce IL-25 upon IgE cross-linking, suggesting that, in addition to TH2 cells, mast cells are potent IL-25 producers and mast cell–derived IL-25 may be involved in the augmentation of TH2-type immune response.
Study design
Cell culture
BMMCs were prepared and cultured as described previously.8 More than 98% of cells obtained after 4 weeks of culture were positive for c-kit expression. CFTL-15 cells9 were cultured in RPMI 1640 medium containing IL-3. For stimulation of BMMCs via Fcε receptors, BMMCs were first incubated with mouse anti-dinitrophenol (DNP) IgE (1 μg/mL, YAMASA, Tokyo, Japan) at 37°C for 2 hours, washed twice with RPMI 1640 medium, and then incubated with DNP-HSA (human serum albumin; 50 ng/mL, Sigma, St Louis, MO) at 37°C for 1 hour. In some experiments, BMMCs were stimulated with IL-3 (10 ng/mL, R&D Systems, Minneapolis, MN), stem cell factor (SCF; 10 ng/mL, a gift from Kirin Brewery, Takasaki, Gunma, Japan), lipopolysaccharide (LPS, 1 μg/mL; Sigma), A23187 (500 ng/mL, Sigma), and phorbol myristate acetate (PMA; 100 ng/mL, Sigma) at 37°C for 1 hour. TH0 cells and TH2 cells were prepared from DO11.10 T-cell receptor transgenic mice and stimulated as described previously.10 Splenic B cells and M12.4.5 cells were stimulated with LPS as described elsewhere.11
RT-PCR assay
Total cellular RNA was prepared and RT-PCR analysis was performed as described previously.11 The following primer pairs were used for PCR: IL-25 (ATGTACCAGGCTGTTGCATTCTTG and CTAAGCCATGACCCGGGGCC), IL-17 (AGGCCCTCAGACTACCTCAACC and GCCTCTGAATCCACATTCCTTG), IL-17B (CTGACTTGGTGGGATGGACTG and ATTCACGCAACCCAAACATAGG), and tumor necrosis factor α (TNF-α) (ATGAGCACAGAAAGCATGATCC and GAAGACTCCTCCCAGGTATATG). Primer pairs for IL-17C, IL-17D, and IL-17F were described elsewhere.2 RT-PCR for β-actin was performed as a control. All PCR amplifications were performed at least 3 times with multiple sets of experimental RNAs.
Taqman PCR analysis
Expression of IL-25 mRNA was determined by real-time Taqman PCR using standard protocol on ABI PRISM 7000 instrument (Applied Biosystems, Foster City, CA). The following PCR primers and a fluorogenic probe were used: sense primer CACACTGCGTCAGCCTACAGA, antisense primer TGTGGTAAAGTGGGACGGAGTT, and probe FAM CTCCCACATGGACCCGCTGGG TAMARA. Taqman PCR for TNF-α was performed as described previously.3 The levels of IL-25 or TNF-α mRNA were normalized to the levels of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA (Applied Biosystems).
Immunoblotting
After BMMCs were stimulated with A23187 + PMA at 37°C for 3 hours, culture supernatant was collected by centrifugation, concentrated with Microcon (Millipore, Billerica, MA), and separated on 12% sodium dodecyl sulfate (SDS) gel. Rabbit antisera to murine IL-25 were produced using synthetic peptide (CPSKEQEPPEEW) as an antigen according to the standard protocol.12 The antigenic peptide did not exhibit any significant similarity to other IL-17 family members. Immunoblotting was performed as described previously.13
Results and discussion
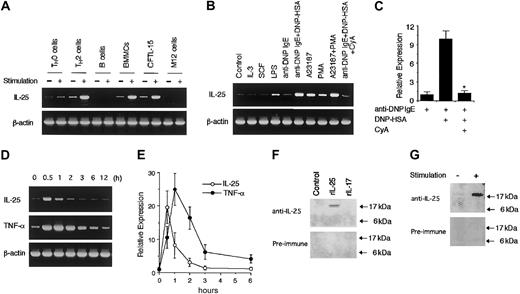
To determine whether mast cells produce IL-25 upon activation, we first examined the expression of IL-25 mRNA in BMMCs by RT-PCR analysis. When BMMCs were stimulated with IgE cross-linking by anti-DNP IgE + DNP-HSA, BMMCs produced IL-25 mRNA at levels comparable with those of activated TH2 cells (Figure1A). We also obtained similar results using sorted c-kit–positive BMMCs (data not shown). CFTL-15 cells, a mast cell line, also expressed IL-25 mRNA upon activation with A23187 + PMA (Figure 1A). In addition, we confirmed the expression of IL-25 mRNA by sequencing. By contrast, neither splenic B cells nor a B-cell line (M12 cells) expressed IL-25 mRNA upon activation with LPS (Figure 1A). Among various conditions we tested, the best stimulation for IL-25 mRNA expression in BMMCs was IgE cross-linking (anti-DNP IgE + DNP-HSA) or A23187 + PMA (Figure 1B). A23187 alone or PMA alone induced IL-25 mRNA expression at lower levels (Figure 1B). LPS also weakly induced IL-25 mRNA expression (Figure 1B). On the other hand, neither IL-3 nor SCF induced IL-25 mRNA expression in BMMCs (Figure 1B). Interestingly, cyclosporin A inhibited IL-25 mRNA expression induced by IgE cross-linking (Figure 1B). Real-time PCR analysis revealed that cyclosporin A significantly decreased the IL-25 mRNA expression by 88% (n = 4, P < .01) (Figure 1C). These results suggest that a calcineurin-dependent pathway is essential for IL-25 mRNA expression in IgE-stimulated mast cells.
Mast cells produce IL-25 upon activation via IgE receptors.
(A) Mast cells express IL-25 mRNA upon activation. TH0 cells and TH2 cells were stimulated with plate-bound anti-CD3 antibody at 37°C for one hour. Splenic B cells and M12.4.5 cells were stimulated with LPS (1 μg/mL) for one hour. BMMCs were incubated with anti-DNP IgE for 2 hours and then surface IgE was cross-linked with DNP-HSA for 1 hour. CFTL-15 cells were stimulated with A23187 (500 ng/mL) + PMA (100 ng/mL) for one hour. Total cellular RNA was prepared from these cells, and RT-PCR for IL-25 and β-actin (as a control) mRNA was performed. Preliminary experiments revealed that the peak of IL-25 mRNA expression in anti-CD3–stimulated TH2 cells was one hour after stimulation. Shown are representative data from 5 independent experiments. (B) Cyclosporin A inhibits IL-25 mRNA induction in mast cells. BMMCs were stimulated with the following stimuli for one hour: IL-3 (10 ng/mL), SCF (10 ng/mL), LPS (1 μg/mL), anti-DNP IgE, anti-DNP IgE + DNP-HSA, A23187 (500 ng/mL), PMA (100 ng/mL), and A23187 + PMA. Where indicated, cyclosporin A (CyA) was added at 1 μg/mL. RT-PCR analysis for IL-25 and β-actin mRNA was performed as described in “Study design.” Shown are representative data from 4 independent experiments. (C) Real-time PCR analysis for the effect of cyclosporin A. BMMCs were incubated with anti-DNP IgE alone or anti-DNP IgE + DNP-HSA for one hour in the presence or absence of cyclosporin A (1 μg/mL). Real-time PCR analysis for IL-25 as well as GADPH (as a control) mRNA was performed, and the levels of IL-25 mRNA were normalized to the levels of GADPH mRNA. Data are means ± SD from 4 experiments. *Significantly different from the mean value of control responses (without cyclosporin A); P < .01. (D) Kinetics of IL-25 mRNA expression upon activation. BMMCs were incubated with anti-DNP IgE and then surface IgE was cross-linked with DNP-HSA. At indicated times after IgE cross-linking, total cellular RNA was prepared and RT-PCR for IL-25, TNF-α, and β-actin mRNA was performed. Shown are representative data from 4 independent experiments. (E) Real-time PCR analysis of IL-25 and TNF-α mRNA expression. Similar to panel D, BMMCs were stimulated with anti-DNP IgE + DNP-HAS, and total cellular RNA was prepared at indicated times after stimulation. Real-time PCR analysis for IL-25 (○) and TNF-α (●) as well as for GADPH (as a control) mRNA was performed. The levels of IL-25 or TNF-α mRNA were normalized to the levels of GAPDH mRNA. Data are means ± SD from 4 experiments. (F) Detection of IL-25 at protein levels. COS7 cells were transiently transfected with IL-25 expression vector (pME18S–IL-25) or control vector (pME18S), and these cell lysates as well as recombinant IL-17 (0.5 μg, Endogen, Woburn, MA) were subjected to immunoblotting with rabbit antisera to murine IL-25 or preimmune rabbit serum. (G) Mast cells release IL-25 upon activation. BMMCs were stimulated with and without A23187 + PMA for 3 hours. Culture supernatant was collected by centrifugation, concentrated with Microcon, separated on 12% SDS gel, and blotted with rabbit antisera to IL-25 or with preimmune rabbit serum. Shown is a representative blot from 4 independent experiments.
Mast cells produce IL-25 upon activation via IgE receptors.
(A) Mast cells express IL-25 mRNA upon activation. TH0 cells and TH2 cells were stimulated with plate-bound anti-CD3 antibody at 37°C for one hour. Splenic B cells and M12.4.5 cells were stimulated with LPS (1 μg/mL) for one hour. BMMCs were incubated with anti-DNP IgE for 2 hours and then surface IgE was cross-linked with DNP-HSA for 1 hour. CFTL-15 cells were stimulated with A23187 (500 ng/mL) + PMA (100 ng/mL) for one hour. Total cellular RNA was prepared from these cells, and RT-PCR for IL-25 and β-actin (as a control) mRNA was performed. Preliminary experiments revealed that the peak of IL-25 mRNA expression in anti-CD3–stimulated TH2 cells was one hour after stimulation. Shown are representative data from 5 independent experiments. (B) Cyclosporin A inhibits IL-25 mRNA induction in mast cells. BMMCs were stimulated with the following stimuli for one hour: IL-3 (10 ng/mL), SCF (10 ng/mL), LPS (1 μg/mL), anti-DNP IgE, anti-DNP IgE + DNP-HSA, A23187 (500 ng/mL), PMA (100 ng/mL), and A23187 + PMA. Where indicated, cyclosporin A (CyA) was added at 1 μg/mL. RT-PCR analysis for IL-25 and β-actin mRNA was performed as described in “Study design.” Shown are representative data from 4 independent experiments. (C) Real-time PCR analysis for the effect of cyclosporin A. BMMCs were incubated with anti-DNP IgE alone or anti-DNP IgE + DNP-HSA for one hour in the presence or absence of cyclosporin A (1 μg/mL). Real-time PCR analysis for IL-25 as well as GADPH (as a control) mRNA was performed, and the levels of IL-25 mRNA were normalized to the levels of GADPH mRNA. Data are means ± SD from 4 experiments. *Significantly different from the mean value of control responses (without cyclosporin A); P < .01. (D) Kinetics of IL-25 mRNA expression upon activation. BMMCs were incubated with anti-DNP IgE and then surface IgE was cross-linked with DNP-HSA. At indicated times after IgE cross-linking, total cellular RNA was prepared and RT-PCR for IL-25, TNF-α, and β-actin mRNA was performed. Shown are representative data from 4 independent experiments. (E) Real-time PCR analysis of IL-25 and TNF-α mRNA expression. Similar to panel D, BMMCs were stimulated with anti-DNP IgE + DNP-HAS, and total cellular RNA was prepared at indicated times after stimulation. Real-time PCR analysis for IL-25 (○) and TNF-α (●) as well as for GADPH (as a control) mRNA was performed. The levels of IL-25 or TNF-α mRNA were normalized to the levels of GAPDH mRNA. Data are means ± SD from 4 experiments. (F) Detection of IL-25 at protein levels. COS7 cells were transiently transfected with IL-25 expression vector (pME18S–IL-25) or control vector (pME18S), and these cell lysates as well as recombinant IL-17 (0.5 μg, Endogen, Woburn, MA) were subjected to immunoblotting with rabbit antisera to murine IL-25 or preimmune rabbit serum. (G) Mast cells release IL-25 upon activation. BMMCs were stimulated with and without A23187 + PMA for 3 hours. Culture supernatant was collected by centrifugation, concentrated with Microcon, separated on 12% SDS gel, and blotted with rabbit antisera to IL-25 or with preimmune rabbit serum. Shown is a representative blot from 4 independent experiments.
We next examined the kinetics of IL-25 mRNA expression in mast cells. As shown in Figure 1D, IL-25 mRNA expression reached a peak within 1 hour after stimulation and decreased to the baseline levels at 3 hours. The peak of IL-25 production was faster than that of TNF-α production (Figure 1D). In addition, analogous kinetics of IL-25 as well as TNF-α production was observed by real-time PCR analysis (Figure 1E). Moreover, we found that a 513–base pair (bp) fragment of the 5′ region of IL-25 gene exhibited the responsiveness to A23187 + PMA stimulation in CFTL-15 cells (data not shown), suggesting that IL-25 mRNA is induced by the direct activation of the IL-25 promoter.
To examine IL-25 production at protein levels, we generated antisera to murine IL-25. The antisera could detect recombinant murine IL-25 but did not react with IL-17 (Figure 1F). When BMMCs were stimulated with A23187 + PMA for 3 hours and culture supernatant was subjected to immunoblotting, a specific band was detected at approximately 17 kDa with antisera to murine IL-25 but not with preimmune serum (Figure 1G). These results suggest that BMMCs release IL-25 protein upon activation.
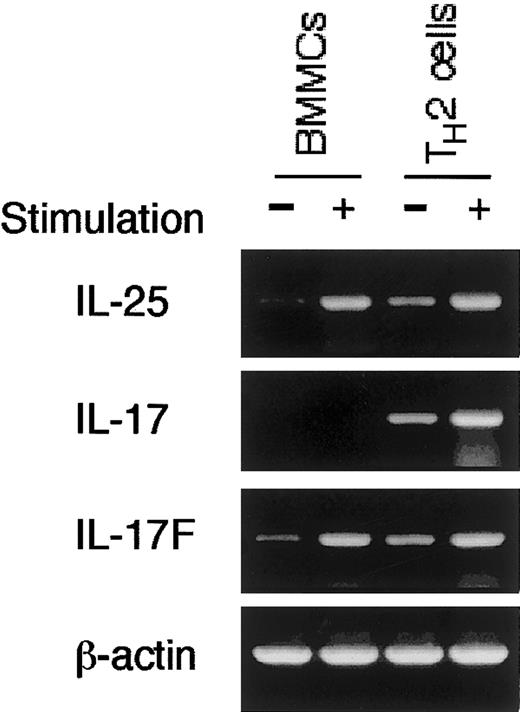
Finally, to determine whether mast cells selectively produce IL-25, we performed RT-PCR analysis for other IL-17 family cytokines (IL-17, IL-17B, IL-17C, IL-17D, or IL-17F). Interestingly, in addition to IL-25 mRNA, BMMCs expressed IL-17F mRNA upon IgE cross-linking (Figure2). In contrast, BMMCs did not express mRNA for IL-17, IL-17B, IL-17C, or IL-17D (Figure 2 and data not shown). On the other hand, activated TH2 cells expressed mRNA not only for IL-25 and IL-17F but also for IL-17 (Figure 2), suggesting that the expression of IL-17 family cytokines is differently regulated between mast cells and TH2 cells.
Mast cells produce IL-25 and IL-17F but not IL-17.
BMMCs were stimulated with anti-DNP IgE + DNP-HSA and TH2 cells were stimulated with anti-CD3 antibody as described in Figure 1A. RT-PCR analysis for IL-25, IL-17, and IL-17F mRNA was performed. Shown are representative data from 4 independent experiments.
Mast cells produce IL-25 and IL-17F but not IL-17.
BMMCs were stimulated with anti-DNP IgE + DNP-HSA and TH2 cells were stimulated with anti-CD3 antibody as described in Figure 1A. RT-PCR analysis for IL-25, IL-17, and IL-17F mRNA was performed. Shown are representative data from 4 independent experiments.
In summary, we show that, upon IgE cross-linking, mast cells produce IL-25. Because IL-25 induces IgE production and eosinophilic inflammation in multiple tissues through the expression of IL-4, IL-5, and IL-13, our findings suggest that mast cell–derived IL-25 may play a pivotal role in IgE-dependent atopic diseases.
We thank Dr K. M. Murphy for DO11.10 mice and Dr M. A. Brown for CFTL-15 cells.
Prepublished online as Blood First Edition Paper, January 2, 2003; DOI 10.1182/blood-2002-09-2817.
Supported in part by grants from the Ministry of Education, Science and Culture, Japan, and Health Science Research Grants, Japan.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Hiroshi Nakajima, Department of Internal Medicine II, Chiba University School of Medicine, 1-8-1 Inohana, Chiba City, Chiba 260-8670, Japan; e-mail:nakajimh@intmed02.m.chiba-u.ac.jp.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal