The production of red blood cells is tightly regulated by erythropoietin (Epo). The phosphoinositide 3–kinase (PI 3-kinase) pathway was previously shown to be activated in response to Epo. We studied the role of this pathway in the control of Epo-induced survival and proliferation of primary human erythroid progenitors. We show that phosphoinositide 3 (PI 3)–kinase associates with 4 tyrosine-phosphorylated proteins in primary human erythroid progenitors, namely insulin receptor substrate–2 (IRS2), Src homology 2 domain–containing inositol 5′-phosphatase (SHIP), Grb2-associated binder–1 (Gab1), and the Epo receptor (EpoR). Using different in vitro systems, we demonstrate that 3 alternative pathways independently lead to Epo-induced activation of PI 3-kinase and phosphorylation of its downstream effectors, Akt, FKHRL1, and P70S6 kinase: through direct association of PI 3-kinase with the last tyrosine residue (Tyr479) of the Epo receptor (EpoR), through recruitment and phosphorylation of Gab proteins via either Tyr343 or Tyr401 of the EpoR, or through phosphorylation of IRS2 adaptor protein. The mitogen-activated protein (MAP) kinase pathway was also activated by Epo in erythroid progenitors, but we found that this process is independent of PI 3-kinase activation. In erythroid progenitors, the functional role of PI 3-kinase was both to prevent apoptosis and to stimulate cell proliferation in response to Epo stimulation. Finally, our results show that PI 3-kinase–mediated proliferation of erythroid progenitors in response to Epo occurs mainly through modulation of the E3 ligase SCFSKP2, which, in turn, down-regulates p27Kip1 cyclin-dependent kinase (CDK) inhibitor via proteasome degradation.
Introduction
The production of red blood cells is tightly regulated by the cytokine erythropoietin (Epo), which supports the survival and proliferation of erythroid progenitors.1 Epo binding to its cognate receptor activates the receptor-associated Janus kinase–2 (Jak2) tyrosine kinase.2The Epo receptor (EpoR) is tyrosine phosphorylated3 and recruits several Src homology–2 (SH2) domain–containing proteins, thereby leading to the activation of different intracellular signaling pathways.4 One of these pathways involves phosphoinositide 3–kinase (PI 3-kinase). PI 3-kinase products phosphoinositide 3,4 bisphosphate (PI(3,4)P2) and phosphoinositide 3,4,5-trisphosphate (PI(3,4,5)P3) are major intracellular second messengers acting as mediators between the plasma membrane and intracellular signaling molecules. The class IA family of PI 3-kinases consists of a regulatory subunit (p85) and a catalytic subunit (p110).5 PI 3-kinase plays a central role in controlling cell survival and cell cycle progression in different systems.6 In the erythroid lineage, PI 3-kinase is required for the protection of erythroid cells from apoptosis7 and is involved in Epo-induced mitogenic responses.8 We and others have previously shown that PI 3-kinase is associated through its SH2 domains with the activated EpoR.9-11 The last tyrosine residue (Tyr479) in the EpoR cytoplasmic domain was further shown to be a binding site for the p85 subunit of PI 3-kinase.12,13This cytosolic tyrosine residue was identified as the origin of a major signal transduction pathway leading to proliferation and differentiation of primary erythroid progenitors.14However, EpoR devoid of this tyrosine still activates PI 3-kinase, suggesting that alternative mechanisms for EpoR-induced PI 3-kinase activation exist.12,15 In fact, analyses of receptor forms with cytosolic truncations and deletions delineated the extended EpoR box 2 as a candidate subdomain for p85 binding.16 More recently, it was shown that the distal region and tyrosine residues of the EpoR were nonessential for in vivo erythropoiesis.17Thus, the molecular mechanisms of PI 3-kinase activation and their role in EpoR-mediated signaling are still unclear.
In this work, we studied PI 3-kinase activation in primary erythroid progenitors stimulated by Epo. We have found that alternative independent pathways lead to Epo-induced PI 3-kinase activation. Our data demonstrate that PI 3-kinase is activated by Epo through 3 mechanisms equally active in Epo-responsive cells; namely through direct association of PI 3-kinase with the EpoR, through phosphorylation of growth factor–bound protein (Grb)–associated binder (Gab), or through phosphorylation of insulin receptor substrate–2 (IRS2) adaptor proteins. Each of these mechanisms leads to the phosphorylation of PI 3-kinase downstream effectors Akt, FKHRL1, and P70S6-kinase. Our results also indicate that the mitogen-activated protein kinase (MAPK) pathway is activated by Epo independently of PI 3-kinase activation. Furthermore, we show that in human erythroid progenitors, the functional role of PI 3-kinase activation is both to protect cells from apoptosis and to mediate Epo-induced proliferation through down-regulation of p27Kip1 expression.
Materials and methods
DNA constructs and expression vectors
Ba/F3 cells expressing the wild-type or mutant form of the EpoR (Zero; Phe1-Phe2-Tyr3-8; Tyr1; Phe1-Tyr2-8; Tyr1-Phe2-Tyr3-8) have been described before.13
As described, 32D cells cultured in the presence of interleukin-3 (IL-3) were electroporated with pCDNA3 vectors encoding either wild-type or mutant forms of the EpoR (Tyr1; Tyr1-7; Phe1-Phe2-Tyr3-8).13
Cell culture and stimulation
UT7 cells were maintained in α-modified Eagle medium (MEM) containing 10% fetal calf serum and 1 U/mL Epo. Before each experiment, cells were washed 3 times in Iscove Dulbecco MEM (DMEM) containing 0.1% deionized bovine serum albumin (BSA) and 25 μg/mL iron-loaded human transferrin and deprived in the same medium overnight. Cells were stimulated in the same medium for 10 minutes at 37°C with 10 U/mL recombinant human Epo. Stimulation was terminated by diluting the cells with ice-cold phosphate-buffered saline (PBS) containing 50 μM sodium vanadate.
Ba/F3 cells and 32D cells expressing the normal EpoR or the different mutants of the EpoR were cultured in alpha MEM containing 10% fetal calf serum and 10% Wehi conditioned medium as a source of IL-3. Before each experiment, cells were washed 3 times in serum-free medium and deprived in the same medium for 4 hours, then stimulated with 10 U/mL Epo.
The proteasome inhibitor N-acetyl (N-Ac)–Leu-Leu-norleucinal (LLnL) was obtained from Sigma-Aldrich (St Quentin Fallavier, France).
Amplification and purification of normal human erythroid progenitors
Human erythroid progenitors were purified according to Freyssinier et al.18 Briefly, umbilical cord blood units (mean volume, 85 mL) from normal full-term deliveries were obtained, after informed consent of the mothers. Cord blood units were diluted with 50 mL phosphate-buffered saline containing 1% BSA (StemCell Technologies, Vancouver, BC, Canada) and submitted to Ficoll density gradient centrifugation. Low-density cells were recovered and CD34+ cells were separated by 2 cycles of positive selection by means of an immunomagnetic procedure (magnetic-activated cell separation [MACS], CD34 isolation kit; Miltenyi Biotech, Bergish Badgach, Germany). CD34+ cells were cultured in 5% CO2 in air at 37°C for 7 days in serum-free Iscove DMEM (Gibco-BRL Life Technologies, Paisley, Scotland), in the presence of 15% of a commercial mixture of BSA, insulin, and transferrin (BIT 9500; StemCell Technologies), 25 ng/mL stem cell factor (SCF) (AMGEN, Thousand Oaks, CA), 10 ng/mL IL-3, and 10 ng/mL IL-6. At day 7 (D7), CD36+ cells corresponding to a highly purified population of human erythroid progenitors cells were obtained by positive selection on CD36+ immunomagnetic beads, as previously reported.18 Day-7 CD36+ cells were then cultured in the presence of Epo at 2 U/mL for 1 day (D7 + 1), 2 days (D7 + 2), or 3 days (D7 + 3). For all stimulation studies reported, CD36+ cells were washed 3 times in BIT medium and deprived for 4 hours in the same medium. Erythroid cells were then stimulated for 10 minutes with 10 U/mL Epo.
Cell proliferation assays
DNA synthesis was measured by thymidine incorporation. Briefly, after 3 washes in PBS, UT7 cells were resuspended at 105/mL in Iscove DMEM containing 0.1% deionized BSA and 25 μg/mL iron-loaded human transferrin, and incubated on 96-well plates in the presence of 1 U/mL Epo for 48 hours. The [3H] thymidine was added for a final 4-hour pulse.
Cell proliferation assays were performed on human erythroid progenitors at D7 + 1 in the same conditions as described for UT7 cells except that they were cultured in serum-free Iscove DMEM (Gibco-BRL Life Technologies), in the presence of 15% of a commercial mixture of BSA, insulin, and transferrin (BIT 9500; StemCell Technologies) with 2 U/mL Epo.
Analysis of apoptosis
UT7 cells and D7 + 1 human erythroid progenitors were cultured at 105/mL without or with Epo at 1 or 2 U/mL, respectively, in the absence or presence of LY294002 at 50 μM. Apoptosis was quantified by fluorescent-activated cell sorter (FACS) analysis performed on an XL cytometer (Coultronics Beckman Coulter, Miami, FL). The percentage of annexin-V+–phycoerythrin-positive (annexin V+–PE+) cells was determined in the whole-cell population at 24 and 48 hours.
Antibodies
The production of the anti-Gab2 antibody has been described before.19 We used 2 anti–PI 3-kinase antibodies, produced by rabbit immunization with a mixture of recombinant proteins corresponding to the N- and C-terminal SH2 domains of p85 fused to glutathione-S-transferase (GST), and commercial anti–PI 3-kinase antibodies from Upstate Biotechnologies (UBI) (Lake Placid, NY) (catalog no. 06-195). Anti-Gab1 antibodies (catalog no. 06-579), anti-Jak2 antibodies (catalog no. 06-255), and anti-IRS2 antibodies (catalog no. 06-506) were from UBI. Antiphosphotyrosine antibodies (4G10) were generous gifts of Dr B. Drucker. Anti-EpoR antibodies were produced by immunizing rabbits with a fusion of the full intracellular domain of the murine EpoR to GST.20
Anti-pAkt (Ser473), anti-pP70S6K (Thr389), anti–p-extracellular signal regulated kinase-1 (anti-pERK1)/ERK2 were from Cell Signaling Technology (Beverly, MA). Anti-Akt, anti-P70S6K, anti-SCFSKP2, anti–cyclin D1, and anti–cyclin D3 antibodies were obtained from Santa Cruz Biotechnology (CA). Anti–caspase-3 antibody was from Pharmingen (San Diego, CA), whereas anti-pFKHRL1 (Thr32) and anti-FKHRL1 were from UBI. The anti-P27Kip1 antibody was from Transduction Laboratories (Lexington, KY).
LY294002 and rapamycin were purchased from Sigma (St Louis, MO) and PD98059 from New England Biolabs (Beverly, MA).
Preparation of cell extracts and Western blot analysis
Before each experiment, cells were washed 3 times in PBS and starved 18 hours for UT7 and 4 hours for human erythroid progenitors. Cells were then stimulated with Epo at 10 U/mL for 10 minutes. The experiment was terminated by diluting the cells with ice-cold PBS containing 50 μM sodium vanadate. Cells were solubilized with buffer A (10 mM Tris [tris(hydroxymethyl)aminomethane]/HCl, 150 mM NaCl, 5 mM EDTA [ethylene glycol tetraacetic acid], 10% glycerol, 1 mM sodium vanadate, 0.02% NaN3, and protease inhibitors from Roche [Meylan, France] [catalog no. 1873580]) containing 1% Brij 98. Protein extracts were quantified by means of commercial kit BCA protein assay (Pierce, New York, NY). Samples containing 50 to 100 μg protein were then boiled in Laemmli sample buffer, separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and analyzed by Western blot (WB) with the use of the appropriate antibodies. The chemoluminescence kit ECL (Amersham Pharmacia Biotech, Saclay, France) was used for development.
Results
Several tyrosine-phosphorylated proteins associate with PI 3-kinase in response to Epo in erythroid progenitors
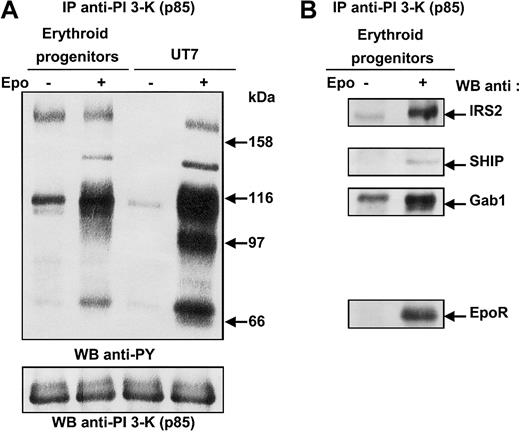
To investigate the pathways leading to PI 3-kinase activation in the erythroid lineage, we sought to identify the proteins associated with PI 3-kinase in primary human erythroid cells after Epo stimulation. To that end, erythroid progenitors isolated from human cord blood were deprived of growth factor and serum in culture medium complemented with BSA and transferrin (see “Materials and methods”), then stimulated for 10 minutes with a saturating concentration of Epo (10 U/mL). UT7 cells were used as Epo-responsive control cells. Cells were lysed under mild conditions, and cell lysates were immunoprecipitated with anti–PI 3-kinase antibodies, followed by SDS-PAGE analysis and immunoblotting with antiphosphotyrosine antibodies (Figure 1A). Four tyrosine-phosphorylated proteins of 180, 145, 116, and 70 kDa could be observed in Epo-stimulated erythroid progenitors. In Epo-stimulated UT7 cells, a fifth 97-kDa tyrosine-phosphorylated protein was detected. The blot was stripped and reprobed with various antibodies. As expected, the 180-kDa protein corresponded to IRS2; the 145- and 116-kDa proteins were Src homology 2 domain–containing inositol 5′-phosphatase (SHIP) and Gab1; and the 70-kDa protein was identified as the EpoR (Figure 1B).9,12,21,22 The 97-kDa protein detected only in UT7 cells was Gab2.8,19,23 Gab2 was not expressed in erythroid progenitors, as previously reported8 19 (Figure 1A). The phosphorylation of IRS2 and Gab1 proteins in unstimulated primary erythroid cells is probably due to the short period of deprivation. However, increasing the duration of deprivation led to strong apoptosis of the progenitor cells.
Phosphorylated proteins associated with PI 3-kinase in human erythroid progenitors and in UT7 cells stimulated with Epo.
(A) Anti-p85 immunoprecipitates were prepared from 20 × 106 human erythroid progenitors or 15 × 106 UT7 cells stimulated for 10 minutes with 10 U/mL Epo, and analyzed by Western blot with the use of antiphosphotyrosine antibodies (anti-PY). The blot was stripped and reprobed with anti-p85 antibodies. (B) The blot was reprobed with anti-IRS2, anti-SHIP, anti-Gab1 and anti-EpoR, successively.
Phosphorylated proteins associated with PI 3-kinase in human erythroid progenitors and in UT7 cells stimulated with Epo.
(A) Anti-p85 immunoprecipitates were prepared from 20 × 106 human erythroid progenitors or 15 × 106 UT7 cells stimulated for 10 minutes with 10 U/mL Epo, and analyzed by Western blot with the use of antiphosphotyrosine antibodies (anti-PY). The blot was stripped and reprobed with anti-p85 antibodies. (B) The blot was reprobed with anti-IRS2, anti-SHIP, anti-Gab1 and anti-EpoR, successively.
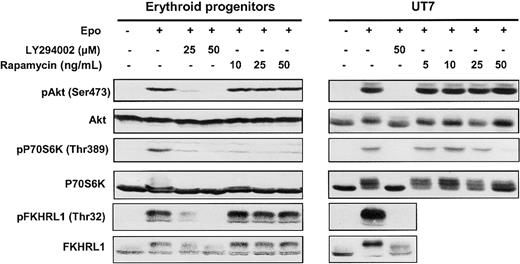
Two major PI 3-kinase downstream signaling pathways, which can be distinguished by their sensitivity to the drug rapamycin, have been described: namely, P70S6 kinase (P70S6K) and Akt/protein kinase B (PKB).24,25 P70S6K plays a role in regulating the translation machinery and is controlled by FK506-binding protein-12-rapamycin–associated protein (FRAP)/mammalian target of rapamycin (mTOR), the target of rapamycin. FKHRL1, a member of the transcription factor forkead family, is directly phosphorylated on threonine 32 by Epo-activated Akt kinase.26 To assess the activation of PI 3-kinase, we investigated the phosphorylation and activation of Akt, P70S6K, and FKHRL1 in Epo-stimulated erythroid progenitors and UT7 cells (Figure2).
Effect of LY294002 and rapamycin on phosphorylation.
LY294002 inhibits phosphorylation of Akt, P70S6K, and FKHRL1 proteins in human erythroid progenitors and UT7 cells whereas rapamycin inhibits only the mTOR/P70S6K pathway. Lysates were prepared from human erythroid progenitors or UT7 cells stimulated with Epo in the absence or presence of either LY294002 or rapamycin at different concentrations, and analyzed by Western blot with anti-pAkt (Ser473), anti-pP70S6K (Thr389), anti-pFKHRL1 (Thr32), anti-Akt, anti-P70S6K, and anti-FKHRL1 antibodies.
Effect of LY294002 and rapamycin on phosphorylation.
LY294002 inhibits phosphorylation of Akt, P70S6K, and FKHRL1 proteins in human erythroid progenitors and UT7 cells whereas rapamycin inhibits only the mTOR/P70S6K pathway. Lysates were prepared from human erythroid progenitors or UT7 cells stimulated with Epo in the absence or presence of either LY294002 or rapamycin at different concentrations, and analyzed by Western blot with anti-pAkt (Ser473), anti-pP70S6K (Thr389), anti-pFKHRL1 (Thr32), anti-Akt, anti-P70S6K, and anti-FKHRL1 antibodies.
In both cases, Epo induced the phosphorylation of Akt on Ser473 and of P70S6K on Thr389, thereby leading to enzyme activation.27Phosphorylation of FKHRL1 on Thr32 was also observed, confirming the activation of Akt. Pretreatment of cells with LY294002, a specific PI 3-kinase inhibitor, abolished the phosphorylation of Akt, P70S6K, and FKHRL1 whereas pretreatment with rapamycin inhibited only P70S6K activation. These results, obtained in primary erythroid progenitors, demonstrate that, after Epo stimulation, PI 3-kinase activation leads to downstream target Akt, P70S6 kinase, and FKHRL1 phosphorylation.
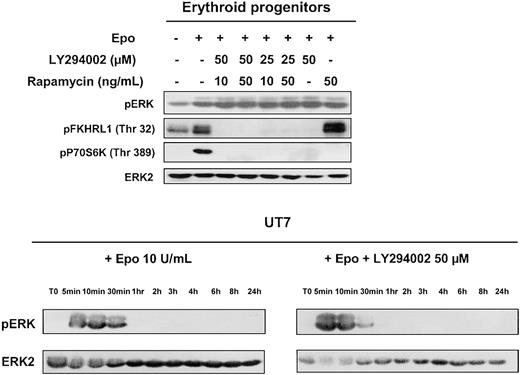
In addition to these main downstream targets, PI 3-kinase has also been implicated in the regulation of the MAPK cascade. In several cellular models, inhibition of PI 3-kinase leads to inhibition of IL-3–stimulated MAPK kinase (Mek), ERK1, and ERK2 activation, thereby showing the involvement of MAP kinase in PI 3-kinase–dependent regulation of IL-3–induced proliferation.28 Therefore, we tested the effect of LY294002 on Epo-mediated activation of this pathway in human erythroid progenitors and in UT7 cells. As can be seen in Figure 3, Epo indeed induced activation of ERK1 and ERK2; however, PI 3-kinase inhibition by LY294002 did not modify MAPK activation in either erythroid progenitors or UT7 cells. Rapamycin was tested on primary cells and did not affect ERK activation when used alone or in association with LY294002. The blot of erythroid progenitors was reprobed with antibodies directed against FKHRL1 and pP70S6K. Phosphorylation of both FKHRL1 and P70S6K was indeed inhibited by LY294002, whereas rapamycin inhibited only P70S6K. These results indicate that the MAPK pathway is activated in response to Epo stimulation independently of PI 3-kinase activation.
Effect of PI 3-kinase inhibition on MAP-kinase activation.
MAP-kinase activation observed in human erythroid progenitors and UT7 cells stimulated with Epo is independent of PI 3-kinase activation. Lysates were prepared from human erythroid progenitors stimulated 10 minutes with Epo at 10 U/mL, without or with LY294002 or rapamycin or both compounds, and analyzed by Western blot with the use of anti-pERK, anti-pFKHRL1, anti-p70S6K, and anti-ERK2 antibodies. Lysates obtained from UT7 cells stimulated at various times in the presence or absence of LY294002 at 50 μm were analyzed for MAPK activation with the use of an anti-pERK antibody. The blots were stripped and reprobed with an anti-ERK2 antibody.
Effect of PI 3-kinase inhibition on MAP-kinase activation.
MAP-kinase activation observed in human erythroid progenitors and UT7 cells stimulated with Epo is independent of PI 3-kinase activation. Lysates were prepared from human erythroid progenitors stimulated 10 minutes with Epo at 10 U/mL, without or with LY294002 or rapamycin or both compounds, and analyzed by Western blot with the use of anti-pERK, anti-pFKHRL1, anti-p70S6K, and anti-ERK2 antibodies. Lysates obtained from UT7 cells stimulated at various times in the presence or absence of LY294002 at 50 μm were analyzed for MAPK activation with the use of an anti-pERK antibody. The blots were stripped and reprobed with an anti-ERK2 antibody.
Specific tyrosines on EpoR mediate direct or indirect activation of PI 3-kinase
Following ligand binding, PI 3-kinase is directly recruited to the last tyrosine residue of the activated EpoR.12 This association occurs between the SH2 domains of p85 and tyrosine 479 of the EpoR, albeit Tyr479 is not surrounded by the consensus sequence for PI 3-kinase binding YpXXM.29 However, it has been reported that phosphatidylinositol-3,4,5-trisphosphate (PIP3) was generated in response to Epo in cells expressing either the wild-type EpoR or an EpoR mutant devoid of the last tyrosine. Moreover, cell proliferation strongly correlated with PI 3-kinase activation and was suppressed by PI 3-kinase inhibitors in both cases,12 15indicating that alternative pathways exist for Epo-dependent PI 3-kinase activation.
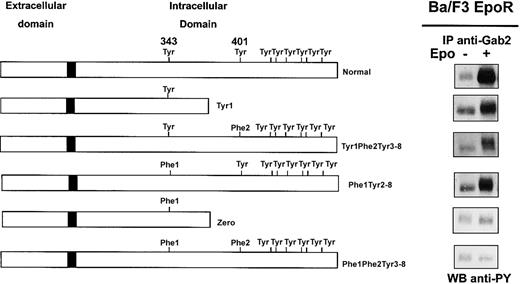
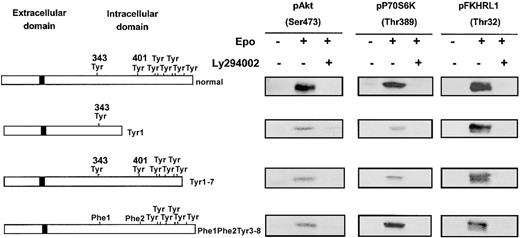
We and others reported phosphorylation of Gab proteins in response to Epo,8,22 and we show in Figure 1 that Epo stimulation strongly increased Gab1 association with PI 3-kinase in erythroid progenitors. To elucidate the mechanism of Gab protein engagement in response to Epo, we determined whether these proteins were directly associated with the EpoR. We did not detect EpoR in Gab immunoprecipitates, and conversely, no Gab proteins could be observed in EpoR immunoprecipitates after Epo stimulation (data not shown). Although no specific association was detected, we inquired whether specific tyrosine residues of the EpoR were required for Epo-induced Gab phosphorylation. To that end, Ba/F3 cells expressing EpoR mutants were stimulated with Epo for 10 minutes followed by Gab2 immunoprecipitation, since Gab1 protein is not expressed in Ba/F3 cells.30 Western blot analysis of immunoprecipitates showed that Gab2 was tyrosine phosphorylated in cells expressing normal EpoR and in cells expressing EpoR mutants carrying either Tyr343 (mutants Tyr1 and Tyr1-Phe2-Tyr3-8) or Tyr401 (mutant Phe1-Tyr2-8), but not in cells expressing mutants lacking both tyrosine residues (mutants Zero and Phe1-Phe2-Tyr3-8) (Figure 4). In the latter cells, Epo-induced cell stimulation was assessed by tyrosine phosphorylation of Jak2 (data not shown).
Phosphorylation of Gab2 protein requires either tyrosine 343 or tyrosine 401 of the EpoR.
Ba/F3 cells expressing either wild-type EpoR or mutants of the EpoR (Tyr1, Tyr1-Phe2-Tyr3-8, Phe1-Tyr2-8, Zero, Phe1-Phe2-Tyr3-8) were stimulated with Epo at 10 U/mL for 10 minutes. Lysates from 15 × 106 cells were immunoprecipitated with anti-Gab2 antibodies and analyzed by Western blot with the use of antiphosphotyrosine antibodies (anti-PY).
Phosphorylation of Gab2 protein requires either tyrosine 343 or tyrosine 401 of the EpoR.
Ba/F3 cells expressing either wild-type EpoR or mutants of the EpoR (Tyr1, Tyr1-Phe2-Tyr3-8, Phe1-Tyr2-8, Zero, Phe1-Phe2-Tyr3-8) were stimulated with Epo at 10 U/mL for 10 minutes. Lysates from 15 × 106 cells were immunoprecipitated with anti-Gab2 antibodies and analyzed by Western blot with the use of antiphosphotyrosine antibodies (anti-PY).
These results indicate that either Tyr343 or Tyr401 is able to independently mediate Epo-induced tyrosine phosphorylation of Gab2. Thus, the Gab1/2 docking proteins require proximal tyrosine residues Tyr1 or Tyr2 of the EpoR for their phosphorylation and subsequent PI 3-kinase activation.
PI 3-kinase is activated by 3 different mechanisms acting independently in Epo-stimulated cells
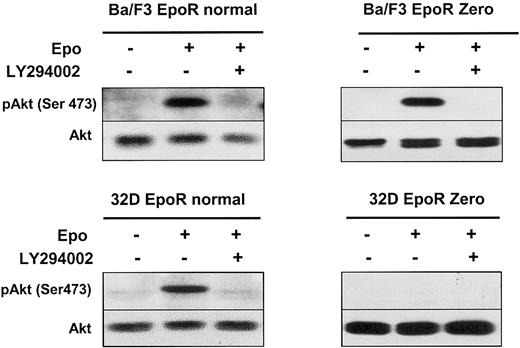
A 180-kDa protein is shown in Figure 1 to be associated with PI 3-kinase in erythroid progenitors. We previously showed that Epo induced tyrosine phosphorylation of the adaptor protein IRS2. Furthermore, we showed that neither the tyrosine residues nor the last 109 C-terminal amino acids of the EpoR were necessary for IRS2 phosphorylation in BA/F3 cells.21 The phosphorylation of IRS2 provides a third alternative pathway for ligand-induced PI 3-kinase activation. Therefore, Akt activation in Ba/F3 cells expressing a Zero EpoR mutant could be due to Epo-induced PI 3-kinase activation via phosphorylation of the IRS2 adaptor protein. As shown in Figure 5, activating phosphorylation on Ser473 of Akt was observed after Epo stimulation despite the lack of EpoR tyrosines. This phosphorylation was inhibited by addition of LY294002. These results, in conjunction with the results presented in Figure 4, suggest that distinct pathways function separately to activate PI 3-kinase in response to Epo stimulation.
An EpoR without tyrosine residues activates the PI 3-kinase/Akt pathway through IRS2.
Lysates from Ba/F3 or 32D cells expressing either a normal EpoR or a mutant EpoR Zero, stimulated with Epo at 10 U/mL for 10 minutes, in the presence or absence of LY294002 at 50 μM, were analyzed by Western blot with anti-pAkt (Ser473) and anti-Akt antibodies.
An EpoR without tyrosine residues activates the PI 3-kinase/Akt pathway through IRS2.
Lysates from Ba/F3 or 32D cells expressing either a normal EpoR or a mutant EpoR Zero, stimulated with Epo at 10 U/mL for 10 minutes, in the presence or absence of LY294002 at 50 μM, were analyzed by Western blot with anti-pAkt (Ser473) and anti-Akt antibodies.
To assess the functionality of each pathway of PI 3-kinase activation, we performed experiments in 32D murine cells that do not express IRS1 and IRS2 adaptor proteins.31 As shown in Figure 5, Epo induces the activation of Akt in 32D cells expressing a wild-type EpoR and not in cells expressing the Zero EpoR mutant. As a control, Epo induced the tyrosine phosphorylation of Jak2 in both cell lines, and experiments using 125I-Epo indicated that the transfected cells expressed roughly the same number of EpoRs at the cell surface (data not shown). These data show that no PI 3-kinase activation can be observed in the absence of the EpoR tyrosine residues in Epo-stimulated 32D cells.
The 32D cells are ideally suited to test PI 3-kinase activation through either Gab protein phosphorylation or its direct binding to EpoR because they lack the IRS2 adaptor protein. We transfected 32D cells with Tyr1 or Tyr1-7 EpoR mutants, able to stimulate Gab tyrosine phosphorylation but lacking the PI 3-kinase binding site (Tyr8) or, conversely, with the Phe1-Phe2-Tyr3-8 mutant, which does not allow Gab tyrosine phosphorylation but binds to PI 3-kinase via Tyr8. As shown in Figure 6, all these EpoR mutants stimulated the activation of PI 3-kinase downstream effectors: Akt, P70S6K, and FKHRL1. Moreover, in each case, phosphorylation of these proteins was inhibited by LY294002.
Independence of mechanisms of PI 3-K activation in response to Epo stimulation.
All mechanisms of PI 3-K activation in response to Epo stimulation are independently able to activate the PI 3-K/Akt/FKHRL1 and mTOR/P70S6K pathways. Lysates from 32D cells expressing either a normal EpoR or a mutant form of the receptor (EpoR Tyr1, Tyr1-7, Phe1-Phe2-Tyr3-8), stimulated with Epo at 10 U/mL for 10 minutes, in the absence or presence of LY294002 at 50 μM, were analyzed by Western blot with anti-pAkt (Ser473), anti-pP70S6K (Thr389), and anti-FKHRL1 (Thr32) antibodies.
Independence of mechanisms of PI 3-K activation in response to Epo stimulation.
All mechanisms of PI 3-K activation in response to Epo stimulation are independently able to activate the PI 3-K/Akt/FKHRL1 and mTOR/P70S6K pathways. Lysates from 32D cells expressing either a normal EpoR or a mutant form of the receptor (EpoR Tyr1, Tyr1-7, Phe1-Phe2-Tyr3-8), stimulated with Epo at 10 U/mL for 10 minutes, in the absence or presence of LY294002 at 50 μM, were analyzed by Western blot with anti-pAkt (Ser473), anti-pP70S6K (Thr389), and anti-FKHRL1 (Thr32) antibodies.
All together, these data indicate that 3 independent mechanisms of PI 3-kinase activation can be observed in Epo-responsive cells: (1) via direct binding of the enzyme to the last EpoR tyrosine; (2) via phosphorylation of Gab adaptor proteins, which requires tyrosine 343 or 401 of the EpoR; and (3) via the adaptor IRS2.
The PI 3-kinase inhibitor LY294002 induces apoptosis in erythroid progenitors but not in UT7 cells, whereas inhibition of proliferation is observed in both cells
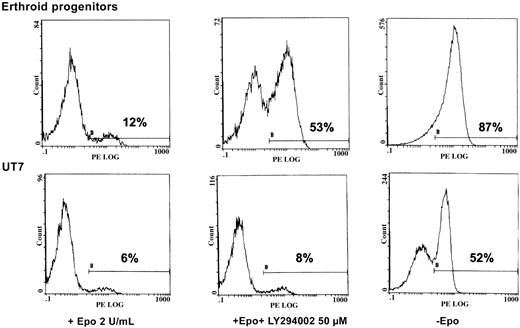
We sought to determine the physiologic role of PI 3-kinase in Epo-induced cell activation. To that end, we tested the viability of both UT7 cells and human erythroid progenitors in 3 experimental conditions: with 2 U/mL Epo, with Epo plus LY294002, or under Epo deprivation. After 2 days of treatment, apoptosis of these cells was measured by flow cytometry analysis of annexin V binding. As seen in Figure 7, massive apoptosis was observed after 2 days of Epo deprivation in both situations. Addition of LY294002 reproducibly led to apoptosis in erythroid progenitors, albeit to a lesser degree than Epo deprivation. In contrast, no apoptosis was observed when a similar concentration of PI 3-kinase inhibitor was added to UT7 cells (Figure 7).
LY294002 induces apoptosis in human erythroid progenitors but not in UT7 cells.
Inhibition of PI 3-K by LY294002 induces apoptosis in human erythroid progenitors but not in UT7 cells. D7 + 1 human erythroid progenitors and UT7 cells were plated at 105/mL with 2 U/mL Epo in the absence or presence of LY294002 at 50 μM. Four separate experiments were performed, giving similar results. Results are given for one experiment at 48 hours of incubation with the inhibitor. Controls were obtained from cells cultured in the absence of Epo. Apoptosis was quantified by flow cytometry analysis of the percentage of annexin-V+–PE+ cells from the whole population.
LY294002 induces apoptosis in human erythroid progenitors but not in UT7 cells.
Inhibition of PI 3-K by LY294002 induces apoptosis in human erythroid progenitors but not in UT7 cells. D7 + 1 human erythroid progenitors and UT7 cells were plated at 105/mL with 2 U/mL Epo in the absence or presence of LY294002 at 50 μM. Four separate experiments were performed, giving similar results. Results are given for one experiment at 48 hours of incubation with the inhibitor. Controls were obtained from cells cultured in the absence of Epo. Apoptosis was quantified by flow cytometry analysis of the percentage of annexin-V+–PE+ cells from the whole population.
The apoptosis observed in erythroid progenitors, occurring either in the presence of LY294002 or after Epo deprivation, was caspase dependent as assessed by the appearance of caspase 3 degradation bands at day 2 of the experiment (data not shown).
To test the role of PI 3-kinase in cell proliferation, Epo-stimulated erythroid progenitors and UT7 cells were cultured with 50 μM LY294002 to inhibit PI 3-kinase, or 50 ng/mL rapamycin to block P70S6K activation (Figure 8). Cell proliferation tested by [3H] thymidine incorporation was totally blocked by LY294002, whereas the inhibition of P70S6K by rapamycin did not significantly modify cell growth.
Effect of LY294002 on proliferation of human erythroid progenitors and UT7 cells.
Inhibition of PI 3-K/Akt pathway by LY294002 dramatically inhibits proliferation of both human erythroid progenitors and UT7 cells. D7 + 1 human erythroid progenitors and UT7 cells were cultured at 105/mL in serum-free medium with Epo at 2 U/mL, without or with LY294002 (at 50 μM) or rapamycin (at 50 ng/mL) for 48 hours. DNA synthesis was assessed by [3H] thymidine incorporation after 4 hours. Analysis is given as the mean of 3 separate experiments.
Effect of LY294002 on proliferation of human erythroid progenitors and UT7 cells.
Inhibition of PI 3-K/Akt pathway by LY294002 dramatically inhibits proliferation of both human erythroid progenitors and UT7 cells. D7 + 1 human erythroid progenitors and UT7 cells were cultured at 105/mL in serum-free medium with Epo at 2 U/mL, without or with LY294002 (at 50 μM) or rapamycin (at 50 ng/mL) for 48 hours. DNA synthesis was assessed by [3H] thymidine incorporation after 4 hours. Analysis is given as the mean of 3 separate experiments.
These results indicate that upon Epo signaling, PI 3-kinase is a permanent inductor of cell proliferation whereas its antiapoptotic properties differ according to the cellular type (UT7 versus primary erythroid cells).
Addition of LY294002 induced accumulation of p27Kip1 in erythroid progenitors
We next explored the mechanisms underlying the blockage of cell proliferation in the presence of the PI 3-kinase inhibitor LY294002. Progression through the cell cycle is regulated by cyclins, cyclin-dependent kinases (CDKs), and cyclin-dependent kinase inhibitors (CKIs).32
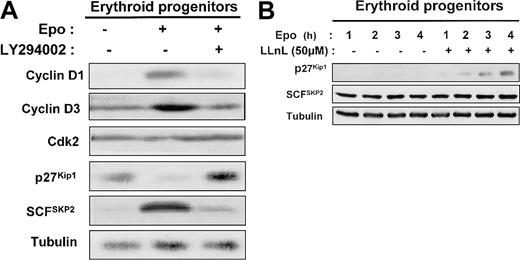
Epo stimulation enhanced the level of cyclins D1 and D3 in human erythroid progenitors, which returned to basal expression after addition of LY294002 (Figure 9A). The level of CDK2 was not modified under these conditions. The blockage of PI 3-kinase activity by addition of LY294002 for 1 day in Epo-stimulated erythroid progenitors induced a strong increase of p27Kip1 level (Figure 9A). SCFSKP2 is a critical component in the PI 3-kinase pathway for the regulation of cell proliferation.33 Indeed, the expression of the p27Kip1 protein is primarily regulated posttranscriptionally through the ubiquitin E3 ligase SCFSKP2 complex that mediates p27Kip1ubiquitin-dependent proteolysis.34 35 Therefore, we determined the level of SCFSKP2 and showed that the levels of SCFSKP2 inversely correlated with those of p27Kip1 (Figure 9A). We also observed that LLnL, a proteasome inhibitor, induces a time-dependent p27Kip1accumulation in human erythroid progenitors cultured in the presence of Epo. In contrast, SCFSKP2 level was unchanged (Figure9B).
Mechanism of PI 3-kinase regulation by p27Kip1 protein expression in human erythroid progenitors.
In human erythroid progenitors, PI 3-kinase regulates p27Kip1 protein expression via proteasome degradation by the E3 ligase SCFSKP2. (A) D7 + 1 human erythroid progenitors were cultured in serum-free medium for 24 hours in the absence or presence of 2 U/mL Epo and in the absence or presence of LY294002 at 50 μM. Lysates were prepared and analyzed by Western blot with the use of anti–cyclin D1, anti–cyclin D3, anti-Cdk2, anti-p27Kip1, anti-SCFSKP2, and antitubulin antibodies. (B) D7 + 1 progenitors were cultured in the presence of 2 U/mL Epo without or with LLnL at 50 μM. Cell lysates were analyzed at indicated times with anti-p27Kip1, anti-SCFSKP2, and antitubulin antibodies.
Mechanism of PI 3-kinase regulation by p27Kip1 protein expression in human erythroid progenitors.
In human erythroid progenitors, PI 3-kinase regulates p27Kip1 protein expression via proteasome degradation by the E3 ligase SCFSKP2. (A) D7 + 1 human erythroid progenitors were cultured in serum-free medium for 24 hours in the absence or presence of 2 U/mL Epo and in the absence or presence of LY294002 at 50 μM. Lysates were prepared and analyzed by Western blot with the use of anti–cyclin D1, anti–cyclin D3, anti-Cdk2, anti-p27Kip1, anti-SCFSKP2, and antitubulin antibodies. (B) D7 + 1 progenitors were cultured in the presence of 2 U/mL Epo without or with LLnL at 50 μM. Cell lysates were analyzed at indicated times with anti-p27Kip1, anti-SCFSKP2, and antitubulin antibodies.
These results strongly suggest that, in primary erythroid cells, PI 3-kinase mediates cell proliferation through p27Kip1down-regulation via proteasome degradation by the E3 ligase SCFSKP2.
Discussion
In this report, we investigated the mechanisms of activation of PI 3-kinase in Epo-stimulated erythroid progenitors. We show that several tyrosine-phosphorylated proteins, IRS2, SHIP, Gab1, and the EpoR, were associated with PI 3-kinase in these cells following Epo stimulation (Figure 1). In contrast to a previous report, we did not detect Vav or Cbl in anti–PI 3-kinase immunoprecipitates.36 Src has been recently described as associated with PI 3-kinase in response to Epo in K562 cells. It was suggested that Src lies upstream of PI 3-kinase in the signal transduction of erythroid differentiation induced by Epo.37 Using either monoclonal anti-Src or polyclonal anti–pan Src antibodies, we did not detect an association between Src and the PI 3-kinase in the different erythroid cells used throughout this study. Moreover, no association between Src and PI 3-kinase was detected in primary human erythroid cells after Epo stimulation (data not shown).
The activation of PI 3-kinase in response to Epo stimulation leads to phosphorylation of its downstream targets Akt, FKHRL1, and P70S6 kinase (Figure 2). Epo-dependent activation of MAPK was also observed; however, we show that the MAPK pathway was engaged independently from PI 3-kinase (Figure 3). This is a matter of debate. Some authors have reported that activation of PI 3-kinase by the EpoR can support activation of MAPK, further blocked by pretreatment of cells with wortmannin.14 On the other hand, it has been reported that PI 3-kinase activity is not required for activation of MAPK by cytokines38 and, moreover, that wortmannin inhibits MAPK activation through a mechanism independent of PI 3-kinase.39
The major goal of this study was to elucidate the mechanisms of PI 3-kinase activation in Epo-stimulated erythroid cells and to study whether these different pathways can function independently. First, we and others previously described that PI 3-kinase is associated through its SH2 domains with the activated EpoR.9-11 Second, we used Ba/F3 cells expressing a Zero EpoR mutant to ensure that PI 3-kinase is activated by several mechanisms acting independently in Epo-stimulated cells. Under these conditions, we showed that a downstream target of PI 3-kinase, Akt, was activated as a result of IRS2 phosphorylation (Figure 5). In contrast, no Akt activation was observed in 32D cells devoid of IRS1 and IRS2 adaptor proteins and expressing a Zero EpoR (Figure 5). A well-established consequence of IRS2 activation is the recruitment and activation of the PI 3-kinase in insulin signaling.40 We previously described such an activation in UT7 cells stimulated by Epo and showed formation of a complex containing IRS2, PI 3-kinase, and SHIP.21 The ability of EpoR devoid of tyrosine residues to activate PI 3-kinase through IRS2 can explain why such truncated receptors remain able to sustain erythropoiesis,17 since PI 3-kinase activation is absolutely required for cell proliferation and protection of cells from apoptosis. Third, we showed that the EpoR tyrosine residues Tyr1 or Tyr2 were able to independently mediate Epo-induced phosphorylation of Gab proteins (Figure 6). These data differ from results of Wickrema et al,8 who reported that both tyrosines Tyr1 and Tyr2 were required for engagement of Gab2 in Epo signaling.
Activation of the PI 3-kinase/Akt pathway by the IL-3 receptor, whose β common chain lacks direct binding sites for the SH2 domains of the p85 subunit of PI 3-kinase, has been reported.41 These authors showed that the Shc binding site (Tyr577) on the β common chain was the major site required for IL-3–induced Gab2 tyrosine phosphorylation. Gab2 was recruited to activated IL-3 receptor by means of an Shc-Grb2 complex. The resultant Shc-Grb2-Gab2 complex is responsible for PI 3-kinase activation. Interestingly, the sequences surrounding the Tyr1 (YLVL) and Tyr2 (YTIL) EpoR tyrosine residues necessary for Gab2 phosphosphorylation correspond to consensus sequences for Shc SH2 binding.29In fact, we found that Shc was bound to these 2 EpoR tyrosine residues and that Epo-induced phosphorylation of Shc is mediated by them (P.M., unpublished data, November 1996). Therefore, an attractive possibility is that, upon Epo stimulation, Shc similarly binds to phosphorylated tyrosine residues of the EpoR and becomes phosphorylated, thereby allowing the sequential binding of Grb2, Gab2, and PI 3-kinase to occur.
The inhibition of PI 3-kinase activation by LY294002 led to apoptosis in human erythroid progenitors, whereas no effect was observed in UT7 cells (Figure 7). A similar result has been reported by Haseyama et al7 in human erythroid precursors. However, the authors suggested that the LY294002-sensitive pathway involved in the protection from apoptosis in primary erythroid cells might not be directly related to the presence of Epo, but possibly to stem cell factor (SCF). In this report, we clearly show that apoptosis of erythroid progenitors occurred during Epo deprivation, as well as during the addition of 50 μM LY294002, (Figure 7). Thus, PI 3-kinase activation plays a major, essential antiapoptotic role in Epo signaling. This apoptosis is caspase dependent as assessed by the appearance of caspase 3 degradation bands at day 2 of the experiment (data not shown). In contrast, the inhibition of PI 3-kinase activation by the LY294002 compound did not induce any apoptotic feature in UT7 cells. This result highlights the differences between primary erythroid progenitors and in vitro–established erythroleukemia cell lines. We have shown that activation of both signal transducer and activator of transcription–5 (Stat5) and PI 3-kinase by IL-3 were tightly connected and cooperated to mediate IL-3–dependent suppression of apoptosis in Ba/F3 cells.42 Presumably, additional signaling pathways, caused for example by strong activation of Stat5 in response to Epo stimulation,15 allow the resistance to apoptosis in UT7 cells.
In erythroid progenitors as well as in UT7 cells, the blockage of PI 3-kinase activation by addition of LY294000 induced a strong inhibition of cell proliferation (Figure 8), as already reported in primary murine embryo fibroblasts43 or satellite cells.44 We sought to examine the cellular mechanisms of growth arrest that follow interruption of Epo-induced PI 3-kinase signaling by addition of LY294002 in primary erythroid progenitors. By immunoblot analysis of cell lysates, we showed that inhibition of PI 3-kinase activation led to a strong decrease of both cyclin D1 and cyclin D3 expression, whereas the level of Cdk2 was not modified (Figure 9). In growth factor–stimulated fibroblasts, PI 3-kinase is required for cell cycle progression by increasing both mRNA and protein levels of cyclin D1.45 On the other hand, the PI 3-kinase/Akt pathway also prevents cyclin D1 degradation by regulating the activity of the cyclin D1 kinase, glycogen synthase kinase-3β (GSK3β).46
We also measured the levels of the CDK inhibitors p21Cip1 and p27Kip1. We did not find any expression of p21Cip1 in human erythroid progenitors under our experimental conditions (data not shown). The p21Cip1 is expressed at later stages of erythroid differentiation.47 The addition of LY294002 induced the accumulation of p27Kip1 and a strong decrease of its regulating ubiquitin E3 ligase SCFSKP2 (Figure 9A). The CDK inhibitor p27Kip1 plays a central role in the regulation of growth factor–dependent proliferation of hematopoietic cells, as shown in other cells.48-50 Part of the p27Kip1-positive regulation may take place at the transcriptional level, and recent data suggest that even a mild increase in transcription of the p27Kip1 gene may be sufficient to increase the half-life of the protein and to induce cell cycle arrest.51 It was shown that IL-3 inhibits the expression of p27Kip1 through modulation of the transcription factor FKHRL1 in a PI 3-kinase–dependent manner.52 This may be also true for Epo signaling. On the other hand, posttranscriptional regulation of p27Kip1 is also important; PTEN, the phosphatase for PIP3, negatively regulates the expression of the ubiquitin E3 ligase SCFSKP2that mediates p27Kip1 ubiquitin-dependent proteolysis.33 Our results do suggest that Epo mediates proliferation of primary human erythroid progenitors by p27Kip1 down-regulation and stimulation of its degradation by the E3 ligase SCFSKP2.
We showed that PI 3-kinase signaling plays a major role in Epo-induced proliferation of human erythroid progenitors. This pathway is overactivated in a wide range of human cancers.53Further studies are needed to identify possible deregulation of the PI 3-kinase/Akt pathway in human erythroid disorders.
We thank F. Verdier for critical review of the manuscript.
Prepublished online as Blood First Edition Paper, December 27, 2002; DOI 10.1182/blood-2002-07-2332.
Supported by grants from Institut National de la Santé et de la Recherche Médicale (INSERM) and from the Comité de Paris de La Ligue Nationale contre le Cancer (Associate Laboratory 4).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Catherine Lacombe, Département d'Hématologie, Institut Cochin, INSERM U567, 27 rue du Faubourg Saint Jacques, 75014 Paris, France; e-mail:lacombe@cochin.inserm.fr.

![Fig. 8. Effect of LY294002 on proliferation of human erythroid progenitors and UT7 cells. / Inhibition of PI 3-K/Akt pathway by LY294002 dramatically inhibits proliferation of both human erythroid progenitors and UT7 cells. D7 + 1 human erythroid progenitors and UT7 cells were cultured at 105/mL in serum-free medium with Epo at 2 U/mL, without or with LY294002 (at 50 μM) or rapamycin (at 50 ng/mL) for 48 hours. DNA synthesis was assessed by [3H] thymidine incorporation after 4 hours. Analysis is given as the mean of 3 separate experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/9/10.1182_blood-2002-07-2332/4/m_h80934214008.jpeg?Expires=1767821518&Signature=g-yrkB7t6ZaQjj9LYhsv~wzpweLA96apsiRLFcKB8Jm5P0oaZXxlkQnkjdIQs5kOAluCFI2C8ucqIeOevl48gnS4ZeHEKiasd2bIA3un0ma73vye2pEcNDdgbrUrjs8yqn1FpomZFJ1nqUdROTz8QPq7RS5leH1BnLaSR1nqS3y3UR2H-4fxnG5rYAXQ4i60TFae5dJ1l1Ys3ekV06GwoqzLKjztu8EVitMUedzD1jmGDL5G5GX~bcgJbFUhQTFYv74uNqYCI2YY1pSdMf4oKnD5x9UFK1L-flv8j-HL1~eNAwhi5-wqHiY~6-ykDZa0kxhKOyfIQSHLw3-mSsMpLg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal