We were intrigued by the article by Mount et al1 on the reversal of hemophilia B following the intraportal injection of an adeno-associated viral vector encoded with the coagulation factor IX gene into hemophiliac dogs. Mount et al reported substantial correction of hemophilia B in 3 of the 4 animals. However, we think that the fourth dog, Beech, is more interesting. He obtained only temporary reversal and died. Necropsy revealed early cirrhosis thought to be associated with iron overload secondary to concurrent pyruvate kinase deficiency.1 Furthermore, at 12 years of age, Beech was the oldest dog studied.
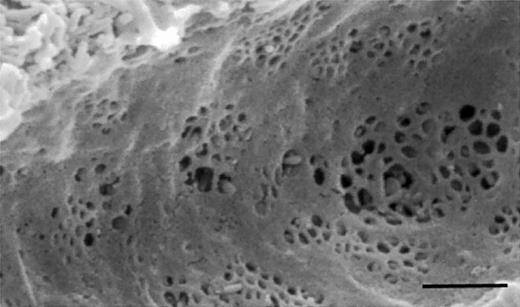
Cirrhosis and old age both are associated with loss of the liver sieve.2,3 The “liver sieve” is a term used to describe the endothelial cells that line the hepatic sinusoids. These cells are perforated by multiple fenestrae of approximately 100-nm diameters, within very thin cytoplasmic extensions (Figure1). Lacking a basal lamina, sinusoidal fenestrae are truly discontinuous and thus allow unimpeded passage of macromolecules up to those with a diameter of about 100 nm including, potentially, viral vectors such as those involved in gene therapy. Indeed, we suggested 25 years ago that the normal liver sieve allows circulating viruses smaller than 100 nm to contact hepatocytes,4 and this has largely been substantiated since.5,6 The adeno-associated virus used by Mount et al has a diameter of about 20 nm.7
Scanning electron micrograph of the liver sieve, demonstrating fenestrations clustered into liver sieve plates. The scale bar indicates 1 μm.
Scanning electron micrograph of the liver sieve, demonstrating fenestrations clustered into liver sieve plates. The scale bar indicates 1 μm.
Mount and colleagues suggested that in Beech's case the usual hepatic tolerance of the viral vector and gene was decreased secondary to a change of the normal tolerance-inducing liver endothelial cell phenotype that might occur in cirrhosis. We agree, and posit that loss of fenestrations hinders contact between hepatocytes and circulating lymphocytes, which also is known to induce tolerance.8 We therefore suggest that loss of fenestrations in the hepatic sinusoids, by hindering interactions between both the lymphocytes and the viral vector in the portal blood with the hepatocytes, decreased the uptake, expression, and tolerance of the introduced viral vector and its gene in Beech.
Conversely, it is of interest that interventions undertaken to improve success of gene therapy, such as partial hepatectomy,9 are associated with increased fenestration of the liver sieve.10 Therefore, modulation of the liver sieve may prove to have an important role in the outcome of gene therapy, either through influencing hepatocyte-lymphocyte interactions or, alternatively, the physical uptake of the viral vector.
AAV-mediated gene transfer to liver
Fraser et al raise the interesting possibility that loss of fenestrations in the endothelial cell lining of the hepatic sinusoids might, in animals with cirrhosis, result in decreased uptake and expression of vector administered via portal vein and poor induction of tolerance to the transgene product on that basis. While we agree that the role of the “liver sieve”—and indeed the role of portal vein versus hepatic artery administration in induction of tolerance to the transgene product—has not been thoroughly studied yet, we do not concur that our data support a hypothesis based on reduced transduction in the case of the animal with early cirrhotic changes. As noted in Figure 1 of Mount et al,1-1 this animal initially showed a rapid rise in circulating levels of factor IX, reaching a level of 600 ng/mL at 3 weeks after vector administration and 2500 ng/mL at 4 weeks. Note that these levels are 4-fold to 10-fold higher than levels seen at the same time point in dogs receiving a 3-fold lower dose. Thus, uptake and expression of vector were excellent in the cirrhotic animal initially. Subsequently, this animal developed an inhibitory antibody; although we cannot exclude reduced interaction between lymphocytes and hepatocytes as a factor in failure of tolerance induction, the role of such an interaction is entirely speculative. We suspect that other factors besides this anatomic one are involved in the difference in immune response to the transgene product seen in the cirrhotic versus the normal animals. Differences in the hepatic microenvironment in the cirrhotic liver (eg, local cytokine concentrations and number and identity of antigen-presenting cells) and/or differences in the level of antigen (factor IX) produced and presented (since the cirrhotic animal received a 3-fold higher dose) are both potential factors in the different immune response to the transgene product in the cirrhotic animal versus animals with normal livers.
In the ongoing clinical trial based on these findings,1-2vector is delivered via the hepatic artery rather than the portal vein (since the former can be accessed via percutaneous cannulation of the femoral artery). In preclinical studies in dogs with hemophilia B caused by a missense mutation,1-3 levels of transgene expression are equivalent using these 2 routes of administration (K.A.H., V.A., unpublished data, May 2002), and neither route results in inhibitory antibody formation at doses up to 1 × 1012 vector genomes (vg)/kg. Mingozzi et al1-4 have shown that portal vein administration of AAV-F.IX vector in hemophilic mice induces tolerance through the induction of antigen-specific CD4+ T regulatory cells and that such tolerance can be adoptively transferred. Whether a similar mechanism accounts for lack of inhibitor development in hemophilic dogs and humans is currently under investigation. If the mechanism of tolerance induction is similar whether vector is administered via the portal vein or the hepatic artery, this would argue against a role for the “liver sieve” in this phenomenon, since the fenestrated sinusoidal endothelium is a characteristic of the portal circulation but not of the hepatic arterial vasculature.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal