We read with great interest the recent article by Heyworth et al,1 which describes the ratio between the p47phox pseudogenes (ψNCF1) and the p47phox gene (NCF1). Gene duplication has prevented elucidation of the genomic sequence at 7q11.23, although the ψNCF1/NCF1 ratio had been assumed2-4 to be 2:1. Specifically, the location and quantity of ψNCF1pseudogenes is unknown. Heyworth et al1 demonstrated the ratio to be 1:1 and 1:2 in 13% and 4% of healthy individuals, respectively, the rest being 2:1. Using a family study, they elegantly showed that variability in the ratio probably occurred following DNA exchange by recombination or, conceivably, gene conversion betweenNCF1 and ψNCF1, to produce a gene hybrid (type II ψNCF1). Similar to NCF1, type II ψNCF1 contains a GT repeat (GTGT) at the start of exon 2, and therefore its transcription product encodes a full-length protein that is homologous to NCF1. ψNCF1, however, contains a dinucleotide deletion at this allele (ΔGT) that results in a premature stop codon. The functional significance of type II ψNCF1 remains unknown.
We adapted a Genescan method5 to try to identifyNCF1 heterozygotes using the ΔGT/GTGT ratio in 138 patients with inflammatory bowel disease (IBD) and 37 healthy individuals. Several findings suggested that NCF1haploinsufficiency could be a susceptibility factor for IBD. First, chronic bowel inflammation is a feature of chronic granulomatous disease (CGD) caused by p47phox deficiency. Second, as with CGD, defects in innate immunity are found in Crohn disease (CD).6,7 Third, significant linkage has been demonstrated in IBD8 to microsatellite markers spanning a 22-centimorgan (cM) region of chromosome 7, which encompasses the NCF1 locus. Finally, p47phox heterozygotes have reduced neutrophil oxygen consumption in response to phorbol myristate acetate but have normal neutrophil oxygen consumption in response to opsonized zymosan,9 and we noted the same phenomenon in a minority of patients with CD (M.H., unpublished observation, March 2002).
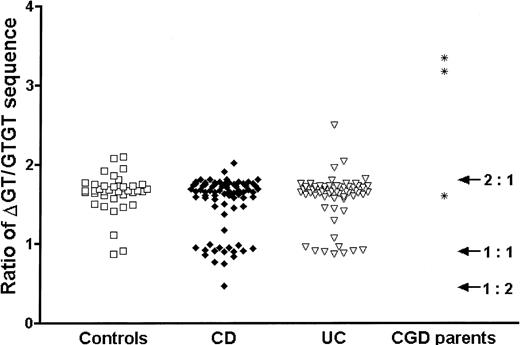
We were surprised to find an excess of IBD patients with a ΔGT/GTGT ratio of approximately 1:1, which was greater in patients with CD (CD, 22.4%; control, 8.1%; Fisher exact test, P < .05, odds ratio 3.3) than in those with ulcerative colitis (UC) (UC, 14.1%;P = .28) (Figure1). This suggested that type II ψNCF1 might be a susceptibility factor for CD. Therefore, we assessed the effect of the ΔGT/GTGT ratio on cellular migration into an acute inflammatory cantharidin blister.10 In patients with a ratio lower than 1.2 (n = 10; 5 CD, 3 healthy subjects) there was a significant increase in blister cell number compared to those with a ratio higher than 1.2 (n = 62; 28 CD, 26 healthy subjects), comprising a geometric mean 3.27 × 106 cells/mL, compared with 1.33 × 106 cells/mL (t test,P = .04). This finding applied both to neutrophils (1.52 × 106 cells/mL compared with 0.60 × 106 cells/mL) and macrophages (0.32 × 106 cells/mL compared with 0.16 × 106 cells/mL). The mechanism is unknown.
Ratio of ΔGT/GTGT sequence in patients with CD and UC and 3 parents of CGD patients.
Three ratio populations were apparent, approximating to 2:1, 1:1, and 1:2. There was a significant excess of the 1:1 ratio in CD (P < .05), implying an excess of the type II ψNCF1 pseudogene. Each point represents the mean of triplicate measurements.
Ratio of ΔGT/GTGT sequence in patients with CD and UC and 3 parents of CGD patients.
Three ratio populations were apparent, approximating to 2:1, 1:1, and 1:2. There was a significant excess of the 1:1 ratio in CD (P < .05), implying an excess of the type II ψNCF1 pseudogene. Each point represents the mean of triplicate measurements.
Three parents of confirmed p47phox CGD patients, presumed heterozygous for mutant NCF1, had ratios of 3.4, 3.2, and 1.6 (Figure 1). The ratio of 1.6 indicates either that the type II ψNCF1 pseudogene was present or that spontaneous mutation had occurred in NCF1 to cause CGD.
We agree with the conclusion of Heyworth et al that determining the ΔGT/GTGT ratio cannot always detect NCF1heterozygosity. As such, we were unable to conclusively refute our original hypothesis, although it appears unlikely that p47phox haploinsufficiency is a susceptibility factor for IBD. However, it is conceivable that the presence of type II ψNCF1 exaggerates the inflammatory response and so predisposes to IBD. This needs to be confirmed in a replicative association study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal