Abstract
To obtain insight into the mechanism(s) of posttransplantation humoral immunodeficiency, we evaluated factors affecting serum antibody levels against polio, tetanus, Haemophilus influenzae, andStreptococcus pneumoniae in 87 patients. Patients with hematologic malignancies were randomized to receive marrow versus blood stem cells, which contain approximately 10 times more lymphocytes than marrow. Blood stem cell recipients did not have higher antibody levels than marrow recipients. Recipient pretransplantation antibody levels were correlated with the posttransplantation levels, especially in the first 6 months after transplantation when the correlation coefficients typically exceeded 0.6. Donor pretransplantation antibody levels had less of a correlation with posttransplantation levels in the recipient. Patient or donor age, total body irradiation, and graft-versus-host disease or its treatment appeared to have no effect. In conclusion, antibody levels in the first year after transplantation are affected primarily by pretransplantation antibody levels in the recipient and, to a lesser degree, in the donor. These findings suggest that immunization of the recipient and the donor before transplantation may be more effective in improving antibody immunity after transplantation than manipulating graft-versus-host disease, changing conditioning, or increasing the number of lymphocytes in the graft.
Introduction
Allogeneic hematopoietic cell transplantation (HCT) is a recognized treatment for certain hematologic malignancies, aplastic anemia, and inborn errors of cells originating from hematopoietic stem cells.1,2 Immune deficiency, involving both cellular and antibody immunity, follows transplantation and lasts for longer than one year.3,4 Deficient antibody immunity predisposes transplant recipients to infections, primarily those due to encapsulated bacteria (eg, Streptococcus pneumoniae or Haemophilus influenzae).5-8
The primary purpose of this study was to determine whether peripheral blood stem cell (PBSC) recipients have higher antibody levels to recall antigens than marrow recipients, as PBSC recipients have more B cells and helper T cells, probably due to the higher B and T cell numbers in the PBSC grafts.9-11 The secondary goal of this study was to evaluate whether the following factors might influence the posttransplantation antibody levels: (1) pretransplantation antibody levels in the recipient or the donor, as infection- or vaccination-induced antibodies to a given antigen in the donor or the recipient before transplantation have been associated with a higher likelihood of detection of antibodies to that antigen after transplantation12-16; (2) patient age, as in one study antibody responses to vaccination were more robust in young versus older individuals17; (3) conditioning regimen, as in a mouse model 9.5 Gy total body irradiation (TBI) was associated with lower antibody responses to immunization than 8.0 Gy18; and (4) graft-versus-host disease (GVHD) or its treatment, as patients with GVHD have shown more deficient antibody responses to immunization compared with patients without GVHD.13,19 20
Patients and methods
Patients
Of 140 patients randomized to either marrow or PBSC allografting and undergoing transplantation at the Fred Hutchinson Cancer Research Center between May 28, 1996, and January 1, 2000, 116 patients participated in the study of antibody immunity. This was a mechanistic study added to the clinical study of Bensinger et al.21 Both studies were approved by the institutional review board and signing of 2 separate consent forms was required. The randomization to PBSC versus marrow was done using stratification for age (≤ 30 versus > 30 years) and disease/disease stage (good versus poor risk).21 Of the 116 patients, 1 patient declined participation after randomization to marrow versus PBSC, and 28 patients died or relapsed by day 80 (18 marrow and 10 PBSC recipients). Thus 87 patients were available for the testing of antibody levels on or after day 80. Of the 87 patients, 50 participated also in a study of the effect of peritransplantation immunization of donors or recipients with tetanus, Haemophilus influenzae, andStreptococcus pneumoniae vaccines. Because the peritransplantation immunization may have had a significant effect on the posttransplantation antibody levels, only the 37 patients not participating in that study (19 marrow and 18 PBSC recipients) were evaluated for antibody levels against tetanus, H influenzae,and S pneumoniae. All 87 patients (40 marrow and 47 PBSC recipients) were evaluated for antibody levels against polio. The evaluated patients or their donors were not vaccinated against polio, tetanus, H influenzae, or S pneumoniae around the time of transplantation or after transplantation prior to day 365. The demographic and clinical characteristics of the evaluated patients are displayed in Table 1. As shown, the PBSC recipients did not significantly differ from the marrow recipients. The marrow recipients evaluated for antibodies to tetanus, H influenzae, and S pneumoniae(n = 19) were not significantly different from the total marrow recipients evaluated for antibodies to polio (n = 40), except possibly in the percentage of patients with good risk disease/disease stage and patients conditioned with total body irradiation (TBI). The PBSC recipients evaluated for antibodies to tetanus,H influenzae, and S pneumoniae (n = 18) were not significantly different from the total PBSC recipients evaluated for antibodies to polio (n = 47), except possibly in the percentage of patients dying without relapse between day 80 and 365 (Table 1, footnote *).
Patient characteristics
| Patients evaluated for antibody levels against . | Polio . | Tetanus, H influenzae, and S pneumoniae . | ||||
|---|---|---|---|---|---|---|
| Source of stem cells . | Marrow, n = 40 . | PBSC, n = 47 . | Significance of difference* . | Marrow, n = 19 . | PBSC, n = 18 . | Significance of difference* . |
| Patient age at transplantation, y, median (range) | 43 (22-59) | 43 (18-61) | NS | 43 (22-59) | 43 (25-54) | NS |
| Donor age at transplantation, y, median (range) | 45 (24-65) | 40 (13-62) | NS | 40 (24-65) | 41 (13-62) | NS |
| Patient sex (%) | ||||||
| Males | 23 (58) | 30 (64) | NS | 10 (53) | 13 (72) | NS |
| Females | 17 (42) | 17 (36) | NS | 9 (47) | 5 (28) | NS |
| Donor-patient histocompatibility (%) | ||||||
| HLA-A, B, and DR-matched sibling | 40 (100) | 46 (98) | NS | 19 (100) | 17 (94) | NS |
| HLA-A, B, and DR-matched child | 0 (0) | 1 (2) | NS | 0 (0) | 1 (6) | NS |
| Disease/disease stage at transplantation†(%) | ||||||
| Good risk | 26 (65) | 26 (55) | NS | 9 (47) | 8 (44) | NS |
| Poor risk | 14 (35) | 21 (45) | NS | 10 (53) | 10 (56) | NS |
| First transplantation (%) | 40 (100) | 47 (100) | — | 19 (100) | 18 (100) | — |
| Cytomegalovirus (CMV) serostatus before transplantation (%) | ||||||
| Donor + and recipient + | 17 (43) | 13 (28) | NS | 7 (37) | 5 (28) | NS |
| Donor − and recipient + | 7 (18) | 11 (23) | NS | 3 (16) | 4 (22) | NS |
| Donor + and recipient − | 5 (13) | 11 (23) | NS | 3 (16) | 4 (22) | NS |
| Donor − and recipient − | 11 (28) | 11 (23) | NS | 6 (32) | 5 (28) | NS |
| Unknown or equivocal | 0 (0) | 1 (2) | NS | 0 (0) | 0 (0) | NS |
| Splenectomized patients (%) | 2 (5) | 1 (2) | NS | 0 (0) | 1 (6) | NS |
| Conditioning‡ (%) | ||||||
| Chemotherapy only | 24 (60) | 25 (53) | NS | 7 (37) | 9 (50) | NS |
| Chemotherapy plus TBI | 16 (40) | 22 (47) | NS | 12 (63) | 9 (50) | NS |
| GVHD prophylaxis with methotrexate and cyclosporine22(%) | 40 (100) | 47 (100) | — | 19 (100) | 18 (100) | — |
| Acute GVHD (%) | ||||||
| Grade 0-1 | 12 (30) | 15 (32) | NS | 5 (26) | 6 (33) | NS |
| Grade 2-41-153 | 28 (70) | 32 (68) | NS | 14 (74) | 12 (67) | NS |
| Chronic GVHD diagnosed by day 365 (%) | ||||||
| None or subclinical | 13 (33) | 6 (13) | NS1-164 | 6 (32) | 2 (11) | NS |
| Clinical limited | 8 (20) | 10 (21) | NS | 5 (26) | 4 (22) | NS |
| Clinical extensive1-155 | 19 (48) | 31 (66) | NS | 8 (42) | 12 (67) | NS |
| Chimerism status (%) | ||||||
| Full chimera1-154 | 39 (97) | 43 (87) | NS | 18 (95) | 17 (94) | NS |
| Unknown | 1 (3) | 4 (13) | NS | 1 (5) | 1 (6) | NS |
| Relapse between day 80 and 365# (%) | 2 (5) | 4 (9) | NS | 0 (0) | 1 (6) | NS |
| Death without relapse between day 80 and 365 (%) | 8 (20) | 3 (6) | NS | 3 (16) | 3 (17) | NS |
| Glucocorticoid treatment between day 50 and 365 (%) | 33 (83) | 38 (81) | NS | 16 (84) | 14 (78) | NS |
| IVIG administration between day 50 and 3651-160(%) | 2 (5) | 5 (11) | NS | 0 (0) | 1 (6) | NS |
| Patients evaluated for antibody levels against . | Polio . | Tetanus, H influenzae, and S pneumoniae . | ||||
|---|---|---|---|---|---|---|
| Source of stem cells . | Marrow, n = 40 . | PBSC, n = 47 . | Significance of difference* . | Marrow, n = 19 . | PBSC, n = 18 . | Significance of difference* . |
| Patient age at transplantation, y, median (range) | 43 (22-59) | 43 (18-61) | NS | 43 (22-59) | 43 (25-54) | NS |
| Donor age at transplantation, y, median (range) | 45 (24-65) | 40 (13-62) | NS | 40 (24-65) | 41 (13-62) | NS |
| Patient sex (%) | ||||||
| Males | 23 (58) | 30 (64) | NS | 10 (53) | 13 (72) | NS |
| Females | 17 (42) | 17 (36) | NS | 9 (47) | 5 (28) | NS |
| Donor-patient histocompatibility (%) | ||||||
| HLA-A, B, and DR-matched sibling | 40 (100) | 46 (98) | NS | 19 (100) | 17 (94) | NS |
| HLA-A, B, and DR-matched child | 0 (0) | 1 (2) | NS | 0 (0) | 1 (6) | NS |
| Disease/disease stage at transplantation†(%) | ||||||
| Good risk | 26 (65) | 26 (55) | NS | 9 (47) | 8 (44) | NS |
| Poor risk | 14 (35) | 21 (45) | NS | 10 (53) | 10 (56) | NS |
| First transplantation (%) | 40 (100) | 47 (100) | — | 19 (100) | 18 (100) | — |
| Cytomegalovirus (CMV) serostatus before transplantation (%) | ||||||
| Donor + and recipient + | 17 (43) | 13 (28) | NS | 7 (37) | 5 (28) | NS |
| Donor − and recipient + | 7 (18) | 11 (23) | NS | 3 (16) | 4 (22) | NS |
| Donor + and recipient − | 5 (13) | 11 (23) | NS | 3 (16) | 4 (22) | NS |
| Donor − and recipient − | 11 (28) | 11 (23) | NS | 6 (32) | 5 (28) | NS |
| Unknown or equivocal | 0 (0) | 1 (2) | NS | 0 (0) | 0 (0) | NS |
| Splenectomized patients (%) | 2 (5) | 1 (2) | NS | 0 (0) | 1 (6) | NS |
| Conditioning‡ (%) | ||||||
| Chemotherapy only | 24 (60) | 25 (53) | NS | 7 (37) | 9 (50) | NS |
| Chemotherapy plus TBI | 16 (40) | 22 (47) | NS | 12 (63) | 9 (50) | NS |
| GVHD prophylaxis with methotrexate and cyclosporine22(%) | 40 (100) | 47 (100) | — | 19 (100) | 18 (100) | — |
| Acute GVHD (%) | ||||||
| Grade 0-1 | 12 (30) | 15 (32) | NS | 5 (26) | 6 (33) | NS |
| Grade 2-41-153 | 28 (70) | 32 (68) | NS | 14 (74) | 12 (67) | NS |
| Chronic GVHD diagnosed by day 365 (%) | ||||||
| None or subclinical | 13 (33) | 6 (13) | NS1-164 | 6 (32) | 2 (11) | NS |
| Clinical limited | 8 (20) | 10 (21) | NS | 5 (26) | 4 (22) | NS |
| Clinical extensive1-155 | 19 (48) | 31 (66) | NS | 8 (42) | 12 (67) | NS |
| Chimerism status (%) | ||||||
| Full chimera1-154 | 39 (97) | 43 (87) | NS | 18 (95) | 17 (94) | NS |
| Unknown | 1 (3) | 4 (13) | NS | 1 (5) | 1 (6) | NS |
| Relapse between day 80 and 365# (%) | 2 (5) | 4 (9) | NS | 0 (0) | 1 (6) | NS |
| Death without relapse between day 80 and 365 (%) | 8 (20) | 3 (6) | NS | 3 (16) | 3 (17) | NS |
| Glucocorticoid treatment between day 50 and 365 (%) | 33 (83) | 38 (81) | NS | 16 (84) | 14 (78) | NS |
| IVIG administration between day 50 and 3651-160(%) | 2 (5) | 5 (11) | NS | 0 (0) | 1 (6) | NS |
— indicates not applicable.
Significance of difference between marrow and PBSC recipients. This was determined by Mann-Whitney-Wilcoxon signed rank test for patient and donor age. For all other characteristics, the significance was determined by χ2 test and, when appropriate, also by Fisher exact test. NS denotes not significant(P > .05, by both χ2 test and Fisher exact test when appropriate). The significance of differences between the marrow group evaluated for polio antibodies (n = 40) and the marrow subgroup evaluated for tetanus, H influenzae, and S pneumoniae antibodies (n = 19) and between the PBSC group evaluated for polio antibodies (n = 47) and the PBSC subgroup evaluated for tetanus,H influenzae, and S pneumoniaeantibodies (n = 18) was tested by the same statistical tests; for all the characteristics the differences were not significant (not shown in the table). More rigorous analyses, comparing the marrow subgroup evaluated for tetanus, H influenzae,and S pneumoniae (n = 19) with the marrow subgroup not evaluated for tetanus, H influenzae, andS pneumoniae (n = 21) disclosed a near-significant difference in the proportion of patients with good-risk disease/disease stage (P = .06 by χ2 test and P = .05 by Fisher exact test) and a significant difference in the proportion of patients conditioned with TBI (P = .01 by χ2 test and P = .01 by Fisher exact test). The comparison of the PBSC subgroup evaluated for tetanus, H influenzae, and S pneumoniae (n =18) with the PBSC subgroup not evaluated for tetanus, H influenzae, andS pneumoniae (n = 29) disclosed a significant difference in the proportion of patients dying without relapse between days 80 and 365 (P = .04 by χ2test and P = .03 by Fisher exact test).
Good risk: chronic myelogenous leukemia in first chronic or accelerated phase, acute leukemia in first remission, refractory anemia, and myelofibrosis. Poor risk: chronic myelogenous leukemia in blast crisis, acute leukemia beyond first remission, refractory anemia with excess blasts, lymphoma, and multiple myeloma.
All conditioning regimens were myeloablative. The most frequent chemotherapy only regimen was IV cyclophosphamide (120 mg/kg) with PO busulfan (approximately 16 mg/kg). The most frequent chemotherapy plus TBI regimen was cyclophosphamide (120 mg/kg) with fractionated TBI (12.0-13.2 Gy). Four patients from the “chemotherapy plus TBI” group received fractionated total marrow irradiation (9.0 Gy, similar to upper mantle and inverted Y) instead of TBI (1 marrow recipient and 3 PBSC recipients).
Typically treated with prednisone 1-2 mg/kg/d PO for 10 to 14 days with subsequent taper over 50 days.
Typically treated with prednisone (0.5-1.0 mg/kg every other day PO) plus cyclosporine (approximately 6 mg/kg every other day PO) for at least 9 months.
At least 90% marrow cells of donor origin by day 80.
#For chronic myelogenous leukemia, the detection of bcr/abl transcript by PCR in the absence of cytogenetic or hematologic relapse was not considered a relapse. Because the goal of the study was to evaluate transplantation-associated immune deficiency and not malignancy-associated immune deficiency, antibody levels of patients who relapsed after transplantation were evaluated only before the diagnosis of relapse.
Intravenous immunoglobulin (IVIG), typically 200 mg/kg weekly before day 100, and 500 mg/kg monthly after day 100 until preinfusion level of 400 mg/dL has been achieved. Antibody levels in sera obtained within 2 months after the administration of intravenous immunoglobulin were not evaluated.
Significant by χ2 test for linear trend with 1 degree of freedom (P = .03). Similarly, if the groups with limited and extensive clinical chronic GVHD are collapsed into a group of any clinical chronic GVHD, then the percent PBSC recipients with any clinical chronic GVHD may be higher than the percent marrow recipients with any clinical chronic GVHD (P = .05, χ2 test; P = .04, Fisher exact test).
Antibody levels
Blood was drawn for the determination of specific antibody levels in the donors before transplantation, and in the patients before transplantation and on approximately days 80, 180, and 365.
Serum levels of antibodies against poliovirus 1 were determined by a virus-binding inhibition assay as described.23 Briefly, formaldehyde-inactivated poliovirus 1 was incubated with serially diluted patient serum in a 96-well cell culture plate. A 96-well enzyme-linked immunosorbent assay (ELISA) plate was coated with bovine anti–poliovirus 1 hyperimmune serum. The patient serum-virus mixtures were transferred from the cell culture plate to the coated ELISA plate. The amount of virus bound to each ELISA plate well, which is inversely proportional to the amount of antibody in patient serum, was determined using mouse anti–poliovirus 1 immunoglobulin G (IgG), goat anti–mouse IgG antibody conjugated to alkaline phosphatase, p-nitrophenylphosphate, and a multichannel spectrophotometer set at 405 nm. The optical density (OD) of a “no virus control” (0.5% bovine serum albumin [BSA]/phosphate buffered saline [PBS]–based diluent was transferred into an anti–polio 1–coated ELISA plate well instead of the serum-virus mixture) was always lower than 0.2. Typically, the plot of OD over serum dilution yielded a sigmoid curve starting at an OD of lower than 0.5 and ending at approximately 1.0. The reciprocal of the first serum dilution that showed 50% or higher OD of the OD of a “no virus-binding inhibition control” (in the cell culture plate, the virus was incubated with PBS instead of serum; typically, the OD was approximately 1.0) was taken as the titer of the serum sample. Occasionally, the lowest point of the curve had an OD value 50% or higher of the OD value of the “no virus-binding inhibition control.” Such sera were considered negative and, for statistical evaluation, were assigned an inverse titer of 1 (ie, one half of the lowest inverse titer among all the sera that yielded typical curves). In each assay, a standard serum was included. Among assays, the titer of the standard serum differed by a maximum of one dilution. In some assays, serum from a person that had never been vaccinated was included. This serum was always negative by the above criteria.
Levels of IgG specific for tetanus toxoid, Haemophilus influenzae capsular polysaccharide, or for a mixture of 23 common pneumococcal polysaccharide serotypes were determined by ELISA, using kits purchased from The Binding Site (Birmingham, United Kingdom). These kits contain sera of known IgG levels or their dilutions as standards. The lower detection limits (the lowest standards) are 0.01 IU/mL for tetanus, 0.1 mg/L for H influenzae, and 3 mg/L forS pneumoniae. For statistical evaluations, IgG levels in sera studied that were below the lower detection limit (antibody concentration of the least concentrated standard) were assigned values equaling the lower detection limit divided by 2. Levels of antibodies above the upper detection limit (the highest standards) were remeasured using 10- and 100-times higher serum dilutions. On the rare occasion when even with the 100-times higher serum dilution the reading was higher than the upper detection limit (antibody concentration of the most concentrated standard × 100), the level was assigned a value equaling the upper detection limit multiplied by 2.
Statistics
Significance of differences in specific antibody levels between patient groups at each time point was tested by Mann-Whitney-Wilcoxon rank sum test. Significance of differences in specific antibody levels between patient groups at all 3 posttransplantation time points together was tested by repeated measures analysis of variance (ANOVA) using ranked antibody levels. Associations between antibody levels before transplantation and at each time point after transplantation were tested by Spearman rank order correlation test. Because of the correlation among the analyses presented, it is difficult to provide an accurate adjustment for multiple comparisons; however, to minimize the number of spurious findings, the stringent significance level of 0.01 was used as the cutoff for declaring statistical significance. Given are 2-tailed Pvalues. Power calculations were performed using nQuery Advisor 4.0 (Statistical Solutions, Saugus, MA).
Results
Effect of marrow versus PBSCs
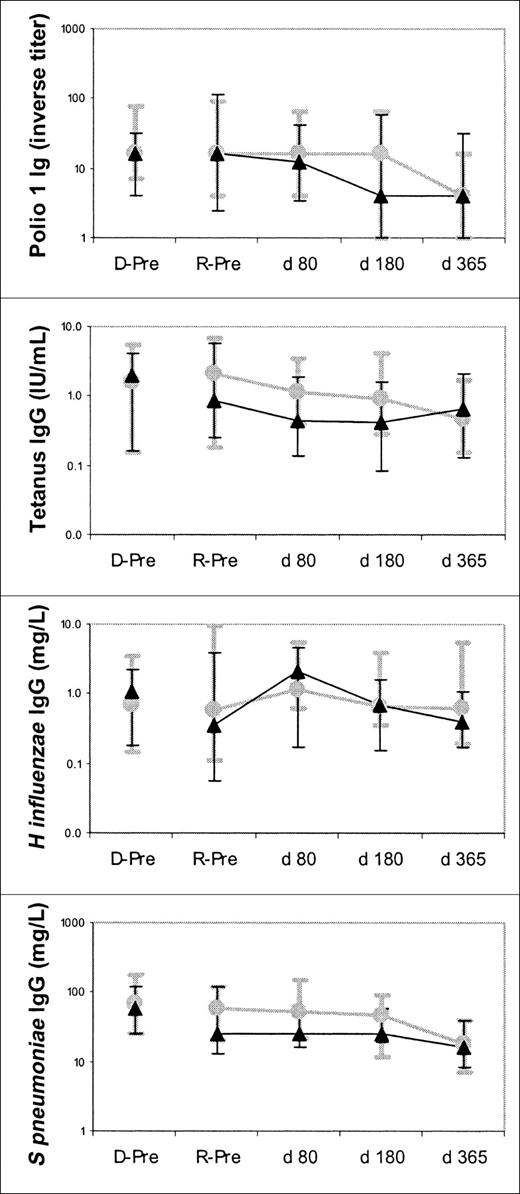
PBSC recipients did not have significantly higher antibody levels to polio, tetanus, H influenzae, or S pneumoniaeat any one of the posttransplantation time points (Figure1). On the contrary, there was a trend toward higher tetanus and S pneumoniae IgG levels after marrow grafting (significant for tetanus on day 80,P = .03; and for S pneumoniae on day 80,P = .05), which might be related to the trend toward higher tetanus and S pneumoniae IgG levels in the marrow recipients before transplantation (Figure 1). When antibody levels at all 3 posttransplantation time points were analyzed together using the repeated measures ANOVA (avoiding spurious results at some time points), there was no significant difference between PBSC and marrow recipients for polio, tetanus, H influenzae, or S pneumoniae. Likely, the lack of significant difference was not due to small sample size as the power to detect a 4-fold difference in antibody levels was estimated at 90% for polio and 65% for tetanus,H influenzae, and S pneumoniae. The fact that PBSC recipients did not have higher levels (as hypothesized) cannot be attributed to differences in pretransplantation donor levels, as they were almost identical (Figure 1). Likewise, it cannot be attributed to differences in pretransplantation recipient antibody levels for polio and H influenzae, as they were also similar (Figure1). PBSC and marrow recipients were also not significantly different in the following factors that could theoretically affect the antibody levels: patient age, donor age, histocompatibility, cytomegalovirus (CMV) serostatus, splenectomy, conditioning, GVHD prophylaxis, incidence of grades 2-4 acute GVHD, relapse rate, and the administration of glucocorticoids (Table 1). The incidence of clinical chronic GVHD (limited + extensive) was higher in the PBSC recipients—27 of 40 marrow versus 41 of 47 PBSC recipients developed chronic GVHD by day 365 (Table 1, footnote ††). However, the similarity of antibody levels (as opposed to the anticipated higher levels in PBSC recipients) cannot be attributed to the higher incidence of chronic GVHD in the PBSC recipients, as a subset analysis of patients without clinical chronic GVHD showed that PBSC recipients without clinical chronic GVHD had similar antibody levels on days 80, 180, and 365 compared with marrow recipients without clinical chronic GVHD (data not shown).
Similarity of median Ig levels in the recipients of marrow and blood stem cells before transplantation and on approximately days 80, 180, and 365 after transplantation.
Recipients of marrow, ; and blood stem cells, ▴. R-Pre denotes recipients before transplantation, and D-Pre denotes donors before transplantation. Error bars denote the 10th to 90th percentiles. The numbers of marrow and blood stem cell donors studied were 39 and 45, respectively. The numbers of marrow and blood stem cell recipients studied were 37 and 42 before transplantation, 35 and 37 on day 80, 28 and 36 on day 180, and 27 and 31 on day 365, respectively, for polio. For tetanus, H influenzae and S pneumoniae, the numbers of donors studied were 19 and 18, and recipients studied were 18 and 18 before transplantation, 16 and 12 on day 80, 15 and 11 on day 180, and 14 and 9 on day 365, respectively.
; and blood stem cells, ▴. R-Pre denotes recipients before transplantation, and D-Pre denotes donors before transplantation. Error bars denote the 10th to 90th percentiles. The numbers of marrow and blood stem cell donors studied were 39 and 45, respectively. The numbers of marrow and blood stem cell recipients studied were 37 and 42 before transplantation, 35 and 37 on day 80, 28 and 36 on day 180, and 27 and 31 on day 365, respectively, for polio. For tetanus, H influenzae and S pneumoniae, the numbers of donors studied were 19 and 18, and recipients studied were 18 and 18 before transplantation, 16 and 12 on day 80, 15 and 11 on day 180, and 14 and 9 on day 365, respectively.
Similarity of median Ig levels in the recipients of marrow and blood stem cells before transplantation and on approximately days 80, 180, and 365 after transplantation.
Recipients of marrow, ; and blood stem cells, ▴. R-Pre denotes recipients before transplantation, and D-Pre denotes donors before transplantation. Error bars denote the 10th to 90th percentiles. The numbers of marrow and blood stem cell donors studied were 39 and 45, respectively. The numbers of marrow and blood stem cell recipients studied were 37 and 42 before transplantation, 35 and 37 on day 80, 28 and 36 on day 180, and 27 and 31 on day 365, respectively, for polio. For tetanus, H influenzae and S pneumoniae, the numbers of donors studied were 19 and 18, and recipients studied were 18 and 18 before transplantation, 16 and 12 on day 80, 15 and 11 on day 180, and 14 and 9 on day 365, respectively.
; and blood stem cells, ▴. R-Pre denotes recipients before transplantation, and D-Pre denotes donors before transplantation. Error bars denote the 10th to 90th percentiles. The numbers of marrow and blood stem cell donors studied were 39 and 45, respectively. The numbers of marrow and blood stem cell recipients studied were 37 and 42 before transplantation, 35 and 37 on day 80, 28 and 36 on day 180, and 27 and 31 on day 365, respectively, for polio. For tetanus, H influenzae and S pneumoniae, the numbers of donors studied were 19 and 18, and recipients studied were 18 and 18 before transplantation, 16 and 12 on day 80, 15 and 11 on day 180, and 14 and 9 on day 365, respectively.
Effect of donor/recipient antibody levels before transplantation
For polio, there was a strong correlation between recipient antibody levels before and after transplantation, and a weak correlation or a trend toward a correlation between donor levels before transplantation and recipient levels after transplantation (Table2). A correlation or a trend toward a correlation between recipient antibody levels before and after transplantation was also observed for tetanus, H influenzae,and S pneumoniae. However, for these antigens there was no significant correlation between donor antibody levels before transplantation and recipient levels after transplantation. The correlation between recipient levels before and after transplantation appeared to be stronger in the first 6 months than at 1 year after grafting.
Association between recipient/donor antibody levels before transplantation and recipient antibody levels on days 80, 180, or 365 after transplantation
| Variable tested for correlation with recipient level after transplantation . | Day 80 . | Day 180 . | Day 365 . |
|---|---|---|---|
| Polio 1 Ig | |||
| Recipient level before transplantation | |||
| r | .80 | .67 | .48 |
| P | <.001 | <.001 | <.001 |
| Donor level before transplantation | |||
| r | .23 | .38 | .34 |
| P | NS | .002 | .008 |
| Tetanus IgG | |||
| Recipient level before transplantation | |||
| r | .76 | .89 | .50 |
| P | <.001 | <.001 | NS |
| Donor level before transplantation | |||
| r | −.13 | −.18 | −.18 |
| P | NS | NS | NS |
| Haemophilus influenzae IgG | |||
| Recipient level before transplantation | |||
| r | .29 | .75 | .30 |
| P | NS | <.001 | NS |
| Donor level before transplantation | |||
| r | .28 | .04 | −.16 |
| P | NS | NS | NS |
| Streptococcus pneumoniae IgG | |||
| Recipient level before transplantation | |||
| r | .80 | .73 | .27 |
| P | <.001 | <.001 | NS |
| Donor level before transplantation | |||
| r | .08 | .12 | .01 |
| P | NS | NS | NS |
| Variable tested for correlation with recipient level after transplantation . | Day 80 . | Day 180 . | Day 365 . |
|---|---|---|---|
| Polio 1 Ig | |||
| Recipient level before transplantation | |||
| r | .80 | .67 | .48 |
| P | <.001 | <.001 | <.001 |
| Donor level before transplantation | |||
| r | .23 | .38 | .34 |
| P | NS | .002 | .008 |
| Tetanus IgG | |||
| Recipient level before transplantation | |||
| r | .76 | .89 | .50 |
| P | <.001 | <.001 | NS |
| Donor level before transplantation | |||
| r | −.13 | −.18 | −.18 |
| P | NS | NS | NS |
| Haemophilus influenzae IgG | |||
| Recipient level before transplantation | |||
| r | .29 | .75 | .30 |
| P | NS | <.001 | NS |
| Donor level before transplantation | |||
| r | .28 | .04 | −.16 |
| P | NS | NS | NS |
| Streptococcus pneumoniae IgG | |||
| Recipient level before transplantation | |||
| r | .80 | .73 | .27 |
| P | <.001 | <.001 | NS |
| Donor level before transplantation | |||
| r | .08 | .12 | .01 |
| P | NS | NS | NS |
r indicates Spearman correlation coefficient; and NS, not significant (P > .01).
For polio (but not for tetanus, H influenzae, orS pneumoniae), there was a significant correlation between recipient antibody levels before transplantation and donor levels before transplantation (r = .31,P = .007). Therefore, the correlation between recipient levels before transplantation and recipient levels after transplantation could in actuality be due to the correlation between donor levels before transplantation and recipient levels before transplantation, and vice versa. This was not the case as shown by bivariate analyses (Table 3). Collectively, the results presented in Tables 2 and 3 indicate that the antibody levels in the first 6 months are strongly influenced by the pretransplantation levels of the recipient, whereas the levels at 6 to 12 months after grafting might be influenced by pretransplantation levels of both the donor and the recipient.
Association between recipient polio 1 Ig levels before transplantation and recipient levels on days 80, 180, or 365 after transplantation, adjusted for donor levels before transplantation, and between donor levels before transplantation and recipient levels after transplantation, adjusted for recipient levels before transplantation
| Variable tested for correlation with recipient polio 1 antibody level after transplantation . | Day 80 . | Day 180 . | Day 365 . |
|---|---|---|---|
| Recipient polio 1 Ig level before transplantation | |||
| r | .77 | .62 | .43 |
| P | <.001 | <.001 | .002 |
| Donor polio 1 Ig level before transplantation | |||
| r | .04 | .35 | .37 |
| P | NS | .01 | .007 |
| Variable tested for correlation with recipient polio 1 antibody level after transplantation . | Day 80 . | Day 180 . | Day 365 . |
|---|---|---|---|
| Recipient polio 1 Ig level before transplantation | |||
| r | .77 | .62 | .43 |
| P | <.001 | <.001 | .002 |
| Donor polio 1 Ig level before transplantation | |||
| r | .04 | .35 | .37 |
| P | NS | .01 | .007 |
r indicates Spearman correlation coefficient; and NS, not significant (P > .01).
Effects of irradiation, GVHD, and age
Other factors had no or minor effect on the specific antibody levels after transplantation. There was no significant difference in the polio, tetanus, H influenzae, or S pneumoniae antibody levels on days 80, 180, or 365 between patients conditioned with TBI versus without TBI.
There was no significant difference in the antibody levels on days 80, 180, or 365 between patients who developed grades 0-1 acute GVHD versus grades 2-4 acute GVHD. Similarly, there was no significant difference between patients treated versus not treated with a glucocorticoid between days 30 and 100. Also, there was no significant difference between patients who developed no clinical chronic GVHD versus any clinical chronic GVHD (limited or extensive), no or clinical limited chronic GVHD versus clinical extensive chronic GVHD, or patients treated versus not treated with a glucocorticoid between days 100 and 365.
No significant correlation was found between the antibody levels at all posttransplantation time points and patient or donor age, except for a correlation between donor age and H influenzae IgG on day 180 (r = .54, P = .004).
Multivariate analysis
Multivariate analysis was performed for polio to further evaluate the potential association between the above variables and the posttransplantation antibody levels. (It was not performed for tetanus,H influenzae, and S pneumoniae as the sample size was small for meaningful multivariate analyses.) Antibody levels at all 3 posttransplantation time points were analyzed together as repeated measures. The following variables were included into the model: graft (PBSC vs marrow), recipient levels before transplantation, donor levels before transplantation, conditioning (with vs without TBI), acute GVHD (grades 0-1 vs 2-4), chronic GVHD (none or limited vs extensive), donor age, and patient age. Glucocorticoid treatment was not included as it was highly correlated with GVHD. Of all the variables, only donor levels and recipient levels before transplantation were significantly associated with the posttransplantation levels.
Discussion
This study is the first to compare antigen-specific antibody levels after allogeneic PBSC versus marrow transplantation. Despite the fact that PBSC recipients have more B and CD4 T cells and fewer postengraftment infections,9,24 we failed to find any evidence for higher antibody levels. This study also shows that the levels of antibodies in the recipient before transplantation and perhaps also the donor before transplantation, likely reflecting the exposure to the antigen before transplantation, influence the levels of antibodies to that antigen after transplantation. Transfer of antibody immunity from the donor to the recipient as well as persistence of pretransplantation recipient antibody immunity into the posttransplantation period were previously documented.12-16 Our results confirm that the persistence of the pretransplantation recipient antibody production is important particularly in the first 6 months after transplantation, as high antibody levels in the recipient before transplantation are strongly associated with high levels in the first 6 months after transplantation. It is also shown that donor or patient age, TBI, and GVHD or its treatment do not influence posttransplantation antibody levels to a great extent. A small influence of these factors cannot be ruled out, given the limited sample size in our study. The practical consequence of our study is that attempts to increase posttransplantation antibody levels should focus on strategies increasing the pretransplantation antibody levels and not on strategies changing the number of lymphocytes in the graft, conditioning, GVHD, or pharmacologic immunosuppression. For example, repetitive vaccination of the donor and the recipient before transplantation should be studied.
The surprising lack of difference in antibody immunity between marrow and PBSC recipients remains unexplained. Possible explanations include the following: (1) The differences in B-cell counts (6-fold higher in PBSC recipients on day 30, 3-fold higher on day 80, and similar on days 180 and 365) and CD4 T-cell counts (2- to 3-fold higher in PBSC recipients throughout the first posttransplantation year)9may not be large enough to impact the generation of antibodies. (2) Other cells important for the generation of antibodies may be equally low in quantity after marrow and PBSC grafting. For example, follicular dendritic cells are scarce after marrow grafting25 and could theoretically be equally scarce after PBSC grafting, as follicular dendritic cells probably do not originate from hematolymphopoietic cells.26-28 (3) The B cells and T cells, though higher in quantity in PBSC grafts, may be qualitatively deficient.29 (4) The numbers of memory B cells may be similar in PBSC and marrow grafts, possibly because memory B cells may not circulate. However, this is unlikely, as compared with marrow grafts PBSC grafts contain approximately 10-fold more IgD−B cells, which are enriched for somatically mutated (presumed memory) B cells.30,31 (5) PBSC inoculum contains fewer plasma cells than marrow inoculum.9 If the grafted plasma cells contribute to antibody production in the recipient, the lower plasma cell content of the PBSC inoculum could explain why antibody levels are not higher in PBSC recipients. (6) Antibody levels may be primarily determined by the number of residual host plasma cells, which should be similar after PBSC and marrow grafting. This hypothesis is supported by the strong correlation between recipient antibody levels before transplantation and after transplantation, especially in the first 6 months (Tables 2-3). (7) The 18 of 58 marrow recipients who relapsed or died before day 80 (not evaluated in this study) might have had substantially lower antibody levels than the 10 of 57 PBSC recipients who relapsed or died before day 80 (also not evalutated). If this were true and if these patients survived to day 80 or longer, the median levels at day 80 or longer in the PBSC recipients could have been higher than in the marrow recipients (as hypothesized). Also, other selection biases may have influenced the result, as at each time point sera were not available on all 87 evaluated patients (Figure 1 legend).
The correlations between recipient or donor pretransplantation antibody levels and recipient posttransplantation antibody levels suggest that both host and donor antibody immunity before transplantation contribute to the recipient antibody immunity after transplantation. As B and T lymphocytes after myeloablative and T-replete grafting are typically of donor origin32-35 (consistent with that, all our patients became complete chimeras by day 80; Table 1), persisting host B or T cells probably contribute little, if at all, to the antibody immunity after transplantation. The persisting antigen-specific antibody production after transplantation could be attributed to the persistence of the antigen in the host (eg, on follicular dendritic cells) or the persistence of host preplasma cells or plasma cells. The role of the antigen is supported by the fact that administration of a recall protein antigen to the recipient during or within several days after conditioning (when generation of new plasma cells from host B cells is unlikely) improves posttransplantation antibody levels.15In this setting the antigen probably stimulates donor B cell to plasma cell differentiation. The role of the persistent host plasma cells is also likely as plasma cells are relatively radio/chemoresistant36 and host-type antibodies are produced for months to years after grafting.37-44 Donor contribution to recipient-specific antibody immunity after transplantation can be attributed to the transfer of specific helper T cells, B cells, or plasma cells. The plasma cells of the donor are probably nonessential as antibody immunity can be transferred through lymphocytes.45 The T cells of the donor may also be nonessential as antibody immunity can be transferred through T-cell–depleted marrow.14-16 46 Thus, donor B cells may be essential. Perhaps, the strong correlation between recipient antibody levels before transplantation and recipient levels in the first 6 months after grafting (Tables 2-3) reflects antibody production by recipient plasma cells, whereas the weak correlation between donor levels before transplantation and recipient levels after transplantation reflects antibody production by plasma cells originating from a small number of engrafted donor B cells.
In summary, specific antibody levels in the recipient before transplantation and in the donor before transplantation appear to impact the levels in the first year after transplantation. Therefore, strategies aiming to achieve high antibody levels in the recipient and the donor before transplantation should be explored (eg, repetitive vaccination of both the donor and the recipient before transplantation). A 10-fold increase in the lymphocyte content of the graft does not appear to boost the antibody immunity.
We thank Terri Cunningham (study nurse), the staff of the FHCRC Long-Term Follow-Up Department, and the patients who agreed to participate in the study. We also thank Drs Frederick Appelbaum, Rainer Storb, and Paul Martin for reviewing the manuscript.
Prepublished online as Blood First Edition Paper, December 27, 2002; DOI 10.1182/blood-2002-05-1376.
Supported by National Institutes of Health grants nos. CA68496, AI46108, HL69710, CA18221, CA18029, HL36444, and CA15704.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Jan Storek, FHCRC, D1-100, 1100 Fairview Ave N, Seattle, WA 98109-1024; e-mail: jstorek@fhcrc.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal