Abstract
A mitochondrial half-type ATP-binding cassette (ABC) protein, ABC7, plays a role in iron homeostasis in mitochondria, and defects in human ABC7 were shown to be responsible for the inherited disease X-linked sideroblastic anemia/ataxia. We examined the role of ABC7 in the biosynthesis of heme in erythroid cells where hemoglobin is a major product of iron-containing compounds. RNA blots showed that the amount of ABC7 mRNA in dimethylsulfoxide (Me2SO)-treated mouse erythroleukemia (MEL) cells increased markedly in parallel with the induction of the mRNA expression of ferrochelatase, the last enzyme in the pathway to synthesize heme. The transfection of the antisense oligonucleotide to mouse ABC7 mRNA into Me2SO-treated MEL cells led to a decrease of heme production, as compared with sense oligonucleotide–transfected cells. ABC7 protein was shown to be colocalized with ferrochelatase in mitochondria, as assessed by immunostaining. Furthermore, in vitro and in vivo pull-down assays revealed that ABC7 protein is interacted with the carboxy-terminal region containing the iron-sulfur cluster of ferrochelatase. The transient expression of ABC7 in mouse embryo liver BNL-CL2 cells resulted in an increase in the activity and level of ferrochelatase and thioredoxin, a cytosolic protein containing iron-sulfur. These increases were also observed in MEL cells stably expressing ABC7. When ABC7 transfectants were treated with Me2SO, an increase in cellular heme concomitant with a marked induction of the expression of ferrochelatase was observed. The extent of these increases was 3-fold greater than in control cells. The results indicated that ABC7 positively regulates not only the expression of extramitochondrial thioredoxin but also that of an intramitochondrial iron-sulfur–containing protein, ferrochelatase. Then, the expression of ABC7 contributes to the production of heme during the differentiation of erythroid cells.
Introduction
As the last step in the pathway to synthesize heme, ferrochelatase catalyzes the insertion of ferrous ions into protoporphyrin IX to form protoheme, and the eukaryotic enzyme is located in the inner membrane facing the matrix of the mitochondrion.1 The mammalian enzyme is nuclear-encoded as a precursor and translocated into the mitochondrion where the enzyme is proteolytically processed to its mature form of 41 to 42 kDa.2 The mammalian enzyme contains a cluster of [2Fe-2S] molecules at the carboxy-terminal.3,4 Human ferrochelatase mutated in this region was reported to be inactive.3-5 Thus, the iron-sulfur cluster is essential for the enzyme activity, although the cluster itself is not involved in the binding of ferrous ions as the substrate.4,5Furthermore, the expression of ferrochelatase was regulated by intracellular levels of iron via the cluster.6 However, the biogenesis of the iron-sulfur cluster in the mammalian ferrochelatase is not well understood.
Iron is not only a substrate for the biosynthesis of heme but also utilized in the formation of non-heme iron proteins in the mitochondria, where the main prosthetic group of enzyme subunits for respiratory chain complexes contains an iron-sulfur cluster. Recently, 3 gene products of a half-type ATP-binding cassette (ABC) transporter, MTABC3, ABC7, and ABC-me, located in mammalian mitochondria have been characterized.7-10 A yeast homolog of the mitochondrial transporter, Atm1p, was shown to promote the mitochondrial iron metabolism and to be involved in the biosynthesis of cytosolic iron-sulfur–containing proteins.11,12 Atm1p-deficient strains have been shown to accumulate iron in mitochondria,12,13 and ABC7 and MTABC3 proteins were able to complement the deficiency in yeast.14,15 In human beings, defects in ABC7 cause the mitochondrial accumulation of iron in the inherited disorder sideroplastic anemia, an X-linked recessive disease.9,15 ABC-me is highly expressed in tissues related to hematopoiesis. Its induction was observed during erythroid maturation, and its overexpression in mouse erythroleukemia (MEL) cells led to an enhanced biosynthesis of hemoglobin.10 Another half-type ABC transporter, MTABC3, has been isolated recently and when expressed in yeast ABC3 complemented the accumulation of iron in mitochondria caused by the defect in Atm1p.7 It has been demonstrated that the import of iron into yeast mitochondria to be utilized as a substrate for ferrochelatase was dependent on membrane potential as an energy source.16 It is unclear, however, whether the iron transporter supplying ferrochelatase is also involved in importing iron into the mitochondrial matrix for the biogenesis of the iron-sulfur cluster. Moreover, the roles of mitochondrial ABC transporters in heme biosynthesis have received little attention. We focused on the involvement of ABC7 in the biosynthesis of heme in erythroid cells where hemoglobin is the predominant iron-containing product. We report here the induction of ABC7 mRNA expression during dimethylsulfoxide (Me2SO)-induced differentiation of MEL cells. The importance of the interaction of ABC7 with ferrochelatase, and the subsequent increase in ferrochelatase activity, to the regulation of heme biosynthesis is also shown.
Materials and methods
Materials
α-[32P]Deoxycytidine triphosphate ([α-32P]dCTP) and nylon membranes were purchased from Amersham-Pharmacia (Buckinghamshire, United Kingdom). Restriction endonucleases and DNA-modifying enzymes were obtained from Takara (Kyoto, Japan) and Toyobo (Tokyo, Japan). Mesoporphyrin IX was produced by Porphyrin Products (Logan, UT). Anti–c-Myc (clone 9E10) and antithioredoxin antibodies were purchased from Sigma (St Louis, MO) and IBL (Gunma, Japan), respectively. Antibodies for ferrochelatase were as previously described.6 17Anticatalase was kindly provided by Dr T. Osumi, Himeji Institute of Technology. All other chemicals were of analytical grade.
DNA probes
DNA probes were generated by polymerase chain reaction (PCR) from the cDNA of MEL cells using as primers (listed 5′ to 3′) ATGGCACCTCCCCTTCAA and GACAACAAAAAGAGAGA for mouse ABC7; AGTTCATTCTACTCCGAG and GCTCTGTTTCTGTCTGT for mouse ABC-me; and CATAAGGGCCGGATTGA and GCCGTGTCCTGAGGTTT for mouse MTABC3 cDNA. The cDNA for mouse ferrochelatase was as described.18
Plasmids
The full-length cDNA of mouse ABC7 was isolated by PCR using an Me2SO-induced MEL cell cDNA library.19 The primers used were AAGAATTCATGGCGTGCCGATA and AAAAGCTTGGGCAGGAACAATTTCCAC. The DNA fragment was digested withEcoRI and HindIII and ligated intoEcoRI-HindIII–digested pcDNA3.1(–)/Myc-His B. The resulting plasmid (pcDNA3(c-Myc)-ABC7) was transformed intoEscherichia coli XL1-Blue. pCD-MF carrying the entire mouse ferrochelatase cDNA was as described.18 To construct glutathione-S-transferase (GST) fusion protein expression plasmids, portions of mouse ABC7 cDNA were amplified by PCR and the resulting fragments were ligated into pGEX-4T vector (Amersham-Pharmacia). The plasmids thus constructed were pGEX/ABCG1188-2087, pGEX/ABCG1388-2087, and pGEX/ABCG1608-2087 (numbers indicate the base position of mouse ABC7 cDNA),19 which encoded the ABC7 subfragment fused with GST designated as GST-ABC7 (458-752), GST-ABC7 (521-752), and GST-ABC7 (596-752), respectively (numbers indicate the amino acid positions of mouse ABC7 protein). These proteins were expressed inE coli (strain: DH-5α) with 0.3 mM isopropyl-1-thio-β-D-galactoside at 25°C for 12 hours. Mouse ferrochelatase was also expressed in E coli as follows: the vector pAR20 linearized by digestion withNdeI and EcoRI was ligated with a whole insert derived from mouse ferrochelatase cDNA,18 and the resulting plasmid was designated pAR-MF, which expresses the mouse ferrochelatase lacking a putative leader peptide in the E coli expression system.18,20 To make pAR-MF-N395Δ, which lacks the C-terminal 25 amino acids corresponding to the region of the iron-sulfur cluster of mouse ferrochelatase,6 18 2 primers, 5′-AACATATGACCACCAAACCCCAAGC-3′ and 5′-ATGAATTCTCACAGCTTGTTGGATGTGGA-3′, were synthesized. pAR-MF was digested with EcoRI and used as a template. The nucleotide sequence of each construct was verified by DNA sequencing.
Partial purification of ferrochelatase
Ferrochelatase (wild-type and N395Δ) expressed in E coli was partially purified using Blue-Sepharose beads.20 The proteins were dialyzed against 10 mM Tris (tris(hydroxymethyl)aminomethane)-HCl (pH 8.0) containing 20% glycerol, 0.2% Tween 20, 1 mM dithiothreitol (DTT), and 2 mM EDTA (ethylenediaminetetraacetic acid).
Preparation of protein extracts and binding assay
GST fusion proteins were expressed in E coli. The cells were resuspended in lysis buffer (50 mM Tris-HCl [pH 7.5], 150 mM NaCl, 0.5% Tween 20, 10% glycerol, 1 mM DTT, and 0.1 mM phenylmethylsulfonyl fluoride [PMSF]) and then lysed by brief sonication. The GST fusion proteins were purified with glutathione (GSH)-Sepharose 4B beads in accordance with the manufacturer's instructions. For binding assays, beads (30 to 100 μL) bound to GST fusion protein were used. The amount of protein used was estimated by staining the polyacrylamide gel with Coomassie blue. Binding assays were performed in a total volume of 400 μL containing partially purified ferrochelatase. The mixture was rotated on a wheel at 4°C for 6 hours. The beads were pelleted by centrifugation at 2000 rpm and washed 5 times with ice-cold lysis buffer. After the final wash, the pellets were boiled in SDS-PAGE gel loading buffer for 1 minute and Western blotting was performed.
Cell cultures
Mouse erythroleukemia (MEL) cells, mouse embryonic liver BNL-CL2 cells, and monkey kidney Cos7 cells were grown in Dulbecco modified Eagle medium (DMEM) supplemented with 10% fetal calf serum (FCS) and antibiotics. BNL-CL2 and Cos7 cells were transfected with 2 μg of the plasmid using the transfection reagent Lipofectamine Plus (Invitrogen Life Technologies, Tokyo, Japan) for 4 to 5 hours according to the manufacturer's recommendation. The cells were then incubated first in DMEM supplemented with 10% fetal calf serum for 5 hours and subsequently in the absence or presence of desferrioxamine for 16 hours. The amounts of heme and porphyrin in the cells were determined as previously described.6
Transfection of oligonucleotides
Phosphorothioate sense and antisense oligonucleotides corresponding to mouse ABC7 (nucleotide position: 20-40),19 MTABC3 (22-42),7 and ABC-me (1-21)10 were synthesized and purified. MEL cells cultured in the presence of 2% Me2SO for 30 hours were collected and rinsed twice with serum-free DMEM. Phosphothioate antisense or sense oligonucleotides (10 μM) were transfected into the cells using a DOTAP transfection reagent (Roche Molecular Biochemicals, Mannheim, Germany). After 6 hours of incubation, the medium was changed to DMEM containing 7% FCS and antibiotics. The cells were further treated with 2% Me2SO for 18 hours and then collected.
Immunostaining
For immunostaining, cultured cells were fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS) containing 1 mM CaCl2 and 0.5 mM MgCl2 (PBS [+]) for 20 minutes and permeabilized with 0.1% Triton X-100 in PBS (+) for 30 minutes. The cells were incubated with anti–c-Myc as described.21 After a wash with PBS (+), they were incubated with a fluorolink cyanine 2 (Cy2)–labeled antimouse immunoglobulin G (IgG) (Amersham-Pharmacia). The cells were then thoroughly washed and incubated with antiferrocheratase followed by a fluorolink Cy3-labeled antirabbit IgG (Amersham-Pharmacia). Fluorescence microscopy was performed with an Olympus microscope (Tokyo, Japan).
Stable transfection of MEL cells
pcDNA(c-Myc)-ABC7 (10 μg) or pcDNA3 (10 μg) was electroporated into MEL cells as described previously.22For the selection, G418 at a final concentration of 500 μg/mL was added to the culture medium. After 7 days, the G418-resistant cells were diluted, seeded in a 96-well tissue-culture plate, and cultured in the medium containing 500 μg/mL G418. Individual clones were isolated and tested for the expression of mouse ABC7 by immunoblotting using a monoclonal antibody for c-Myc-tag (clone 9E10). Five ABC7-overexpressing clones were obtained, mixed to avoid clonal variation, and maintained in DMEM medium containing 10% FCS and antibiotics.
RNA blots
Total RNA was isolated from MEL cells by the guanidium isothiocyanate method.6 The RNA (20 μg) was applied to a 1% agarose gel, electrophoresed, and subsequently transferred onto a nylon membrane (Hybond N+; Amersham-Pharmacia) for hybridization with DNA probes. The filters were hybridized, washed, and exposed to an x-ray film as described.6 18
Immunoblotting and immunoprecipitation
The lysates from BNL-CL2 cells and Cos7 cells transfected with the empty vector pcDNA3, pcDNA(c-Myc)-ABC7, or pCD-MF were also subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and electroblotted onto polyvinylidene difluoride (PVDF) membrane. Immunoblotting was done with antiferrochelatase, anti–c-Myc-tag, anticatalase, and antithioredoxin antibodies as the primary antibodies.6,22 Immunoprecipitation was performed as described23 using antiferrochelatase or anti–c-Myc (9E10) antibodies.
Enzyme assays
Results
Expression of ABC-type transporter mRNA in MEL cells treated with Me2SO
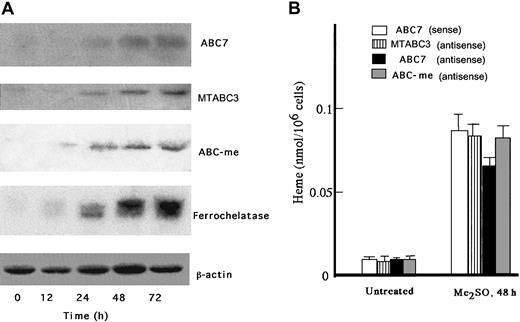
When treated with 2% Me2SO, MEL cells undergo erythroid differentiation and the production of heme is markedly induced. ABC-me was isolated in Me2SO-treated MEL cells, and its overexpression increased the amounts of hemoglobin produced.10 MTABC3 and ABC7 are known to be involved in iron homeostasis in mitochondria. To clarify whether MTABC3, ABC7, and ABC-me are produced in response to Me2SO treatment, RNA blotting was performed. As shown in Figure1A, ABC7 mRNA was markedly expressed, in parallel with ferrochelatase mRNA. The induction of MTABC3 and ABC-me expression was also observed but to less of an extent than that of ABC7. Although mitochondrial ABC transporters may be involved in the export of iron to cytoplasm rather than the transport of heme, the major-iron containing product in erythroid cells is hemoglobin. Then the above results may suggest that ABC transporters play an important role in the synthesis of hemoprotein. To examine which ABC transporters affect the differentiation of erythroid cells, MEL cells treated with Me2SO for 30 hours were transfected with antisense oligonucleotide to MTABC3, ABC7, or ABC-me, and the the intracellular level of heme was compared with that in cells treated with the sense oligonucleotide. As shown in Figure 1B, the amount of heme in uninduced MEL cells was similar among all the treated cells. The heme content of Me2SO-exposed MEL cells treated with antisense oligonucleotide to ABC7 mRNA decreased to 75%, as compared with that in cells treated with sense oligonucleotide to ABC7 mRNA. When the cells were treated with antisense oligonucleotides to MTABC3 or ABC-me mRNA, the heme content also decreased but to a lesser extent. These results indicated that ABC7 is tightly associated with synthesis of heme in MEL cells induced by Me2SO.
Expression of ABC7, MTABC3, and ABC-me during the differentiation of MEL cells.
(A) Northern blots. MEL cells were cultured for the period indicated in the presence of 2% Me2SO. Total RNA was collected, separated by electrophoresis, transferred onto a membrane, and hybridized with radiolabeled probes specific for ABC7, MTABC3, ABC-me, ferrochelatase, and β-actin. (B) Effect of antisense phosphorothioate oligonucleotides to mouse ABC7, MTABC3, and ABC-me mRNAs on induction of heme biosynthesis in MEL cells. Cells treated with or without 2% Me2SO were incubated for 30 hours. They were then transfected with sense oligonucleotide (10 μM) to ABC7 mRNA or antisense oligonucleotide (10 μM) to ABC7, MTABC3, or ABC-me mRNA. The cells were further incubated with 2% Me2SO for 18 hours, after which they were collected and the amount of heme was estimated by fluorescence spectrophotometry. Bars represent SD (n = 4).
Expression of ABC7, MTABC3, and ABC-me during the differentiation of MEL cells.
(A) Northern blots. MEL cells were cultured for the period indicated in the presence of 2% Me2SO. Total RNA was collected, separated by electrophoresis, transferred onto a membrane, and hybridized with radiolabeled probes specific for ABC7, MTABC3, ABC-me, ferrochelatase, and β-actin. (B) Effect of antisense phosphorothioate oligonucleotides to mouse ABC7, MTABC3, and ABC-me mRNAs on induction of heme biosynthesis in MEL cells. Cells treated with or without 2% Me2SO were incubated for 30 hours. They were then transfected with sense oligonucleotide (10 μM) to ABC7 mRNA or antisense oligonucleotide (10 μM) to ABC7, MTABC3, or ABC-me mRNA. The cells were further incubated with 2% Me2SO for 18 hours, after which they were collected and the amount of heme was estimated by fluorescence spectrophotometry. Bars represent SD (n = 4).
Interaction of ABC7 with ferrochelatase in vitro
To determine whether ABC7 protein can interact with ferrochelatase, GST fusion protein with the entirely soluble C-terminal half of ABC7, containing the adenosine triphosphate (ATP)-binding domain and presumably expanding in the matrix of mitochondria, was coupled to glutathione-Sepharose beads and incubated with a partially purified recombinant mouse ferrochelatase. As shown in Figure 2A, the GST-ABC (458-752) fusion protein was found to associate with ferrochelatase. A weak interaction was observed with GST-ABC (521-752), but deletion of part of the ATP-binding domain (GST-ABC [596-752]) led to a complete loss of interaction. No interaction was observed with GST alone either. Mammalian ferrochelatase contains an iron-sulfur cluster at the carboxy-terminal. When a truncated form of ferrochelatase (N395Δ) whose carboxy-terminal region was deleted was expressed and incubated with the GST-ABC7 (458-752) fusion protein, no interaction was observed (Figure 2B). These results indicated that ABC7 interacted with ferrochelatase at the carboxy-terminus.
Binding of ABC7 to ferrochelatase.
(A) Effect of deletions in GST-ABC7 on binding to ferrochelatase. GST, GST-ABC7 (458-752), GST-ABC7 (521-752), and GST-ABC7 (596-752) were immobilized on glutathione beads, after which they were incubated with partially purified ferrochelatase. Bound proteins were analyzed by SDS-PAGE, followed by immunoblotting with antiferrochelatase. Input: A Coomassie blue staining of GST-ABC7 fusion proteins bound to glutathione beads. (B) Effect of deletion of ferrochelatase (FECH) on binding to GST-ABC7 (458-752). Partially purified ferrochelatase (wild-type) and a mutant (N395Δ) expressed in E coli were incubated with GST-ABC7 (458-752) immobilized on the beads. Bound proteins were analyzed by SDS-PAGE, followed by immunoblotting with antiferrochelatase. Input: Coomassie blue staining of partially purified ferrochelatase.
Binding of ABC7 to ferrochelatase.
(A) Effect of deletions in GST-ABC7 on binding to ferrochelatase. GST, GST-ABC7 (458-752), GST-ABC7 (521-752), and GST-ABC7 (596-752) were immobilized on glutathione beads, after which they were incubated with partially purified ferrochelatase. Bound proteins were analyzed by SDS-PAGE, followed by immunoblotting with antiferrochelatase. Input: A Coomassie blue staining of GST-ABC7 fusion proteins bound to glutathione beads. (B) Effect of deletion of ferrochelatase (FECH) on binding to GST-ABC7 (458-752). Partially purified ferrochelatase (wild-type) and a mutant (N395Δ) expressed in E coli were incubated with GST-ABC7 (458-752) immobilized on the beads. Bound proteins were analyzed by SDS-PAGE, followed by immunoblotting with antiferrochelatase. Input: Coomassie blue staining of partially purified ferrochelatase.
Interaction of ABC7 with ferrochelatase in vivo
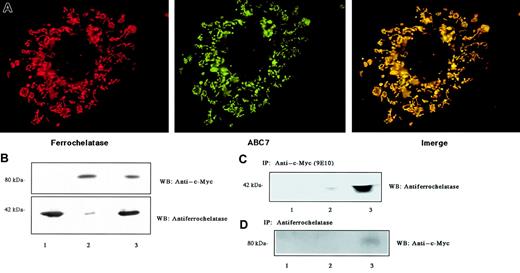
To examine the localization of ABC7 and ferrochelatase, both their cDNAs were cotransfected into Cos7 cells. The proteins expressed were then stained with antibodies. Ferrochelatase is known to be distributed in the inner membrane of mitochondria. Immunofluorescence analysis with antiferrochelatase indicated that the mouse ferrochelatase appeared predominantly in the mitochondria of Cos7 cells (Figure 3A). Protein extracts prepared from cells expressing ferrochelatase and/or c-Myc–tagged ABC7 were analyzed by immunoblotting (Figure 3B). The expression of mouse ferrochelatase with a molecular mass of 42 kDa markedly increased on transfection with pCD-MF. Endogenous ferrochelatase was detected as a faint band of immunoprecipitates only in the cells transfected with ABC7 cDNA. When c-Myc–tagged ABC7 was expressed, the monoclonal antibody for c-Myc reacted only with a protein in the mitochondria (Figure 3A). A protein of about 80 kDa was detected by immunoblotting (Figure 3B). The merged pattern showed that the 2 stainings matched well, indicating that the proteins have a similar location. To demonstrate that ferrochelatase could interact with ABC7 protein, coimmunoprecipitation analysis was performed. Immunoprecipitation of the cell extracts with monoclonal antibody for c-Myc, followed by immunoblot analysis using antiferrochelatase, revealed that ferrochelatase was present in c-Myc immunoprecipitates (Figure 3C). Conversely, immunoprecipitation with antiferrochelatase, followed by immunoblot analysis using anti–c-Myc, revealed that ABC7 was present in ferrochelatase immunoprecipitates from cells transfected with ABC7 cDNA (Figure 3D). These results confirmed the association between ferrochelatase and ABC7.
Interaction of ABC7 with ferrochelatase.
(A) Mitochondrial localization. Cos7 cells were cotransfected with pcDNA3(c-myc)-ABC7 and pCD-MF and incubated for 44 hours. They were then fixed, permeabilized, and reacted simultaneously with anti–c-Myc and antiferrochelatase to show colocalization of ABC7 (green) and ferrochelatase (red). The merged exposure (yellow) confirms that the dots colocalize. (B) Immunoblots of extracts from Cos7 cells transfected with pcDNA3(c-myc)-ABC7 and pCD-MF. The cells transfected with pCD-MF (lanes 1 and 3) or pcDNA3(c-myc)-ABC7 (lanes 2 and 3) were cultured for 24 hours. Immunoblots were performed with anti–c-Myc (top panel) and antiferrochelatase (bottom panel). (C) Extracts from panel B were subjected to immunoprecipitation with anti–c-Myc, followed by immunoblotting with antiferrochelatase. (D) Extracts from panel B were subjected to immunoprecipitation with antiferrochelatase, followed by immunoblotting with anti–c-Myc.
Interaction of ABC7 with ferrochelatase.
(A) Mitochondrial localization. Cos7 cells were cotransfected with pcDNA3(c-myc)-ABC7 and pCD-MF and incubated for 44 hours. They were then fixed, permeabilized, and reacted simultaneously with anti–c-Myc and antiferrochelatase to show colocalization of ABC7 (green) and ferrochelatase (red). The merged exposure (yellow) confirms that the dots colocalize. (B) Immunoblots of extracts from Cos7 cells transfected with pcDNA3(c-myc)-ABC7 and pCD-MF. The cells transfected with pCD-MF (lanes 1 and 3) or pcDNA3(c-myc)-ABC7 (lanes 2 and 3) were cultured for 24 hours. Immunoblots were performed with anti–c-Myc (top panel) and antiferrochelatase (bottom panel). (C) Extracts from panel B were subjected to immunoprecipitation with anti–c-Myc, followed by immunoblotting with antiferrochelatase. (D) Extracts from panel B were subjected to immunoprecipitation with antiferrochelatase, followed by immunoblotting with anti–c-Myc.
Positive role for ABC7 in heme biosynthesis in nonerythroid and erythroid cells
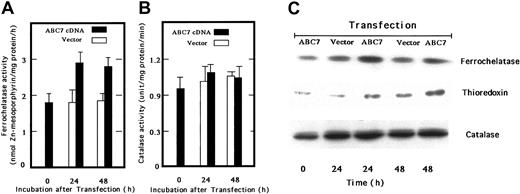
We next transfected ABC7 cDNA into mouse embryo liver BNL-CL2 cells and examined the ferrochelatase activity. The activity in cells transiently expressing ABC7 increased, as compared with that in empty vector–transfected cells (Figure 4A). Catalase activity was also examined, but the activity was not changed by the expression of ABC7 (Figure 4B). Immunoblot analysis confirmed that the amount of ferrochelatase in transfected cells also increased (Figure 4C). Bekri et al reported that expression of ABC7 in Δatm1 yeast restores the formation of extramitochondrial iron-sulfur cluster proteins.9 Then we examined the level of thioredoxin, a cytosolic iron-sulfur cluster protein in BNL-CL2 cells transiently expressing ABC7. As shown in Figure 4C, the amount of thioredoxin increased in the transfected cells as compared with the controls, whereas that of catalase did not change on the expression of ABC7.
Activities and levels of ferrochelatase, catalase, and iron-sulfur–containing proteis in ABC7 cDNA-transfected BNL-CL2 cells.
(A) Ferrochelatase activity. Mouse embryo liver BNL-CL2 cells were transfected with empty vector (■) or pcDNA(c-Myc)-ABC7 plasmids (▪) and incubated for 24 and 48 hours. The cells were then collected, washed twice with PBS, and homogenized. The homogenates were centrifuged at 900g for 10 minutes. The assay of ferrochelatase activity was as described. (B) Catalase activity. Catalase activity was measured using homogenates as above. Data in panels 4A-B are expressed as the means ± SDs of triplicate experiments. (C) Immunoblot analysis. Cells transfected with empty vector or pcDNA3-ABC7 were cultured. The cellular proteins were analyzed by SDS-PAGE, transferred onto PVDF membrane, and immunoblotted using antiferrochelatase, anticatalase, and antithioredoxin.
Activities and levels of ferrochelatase, catalase, and iron-sulfur–containing proteis in ABC7 cDNA-transfected BNL-CL2 cells.
(A) Ferrochelatase activity. Mouse embryo liver BNL-CL2 cells were transfected with empty vector (■) or pcDNA(c-Myc)-ABC7 plasmids (▪) and incubated for 24 and 48 hours. The cells were then collected, washed twice with PBS, and homogenized. The homogenates were centrifuged at 900g for 10 minutes. The assay of ferrochelatase activity was as described. (B) Catalase activity. Catalase activity was measured using homogenates as above. Data in panels 4A-B are expressed as the means ± SDs of triplicate experiments. (C) Immunoblot analysis. Cells transfected with empty vector or pcDNA3-ABC7 were cultured. The cellular proteins were analyzed by SDS-PAGE, transferred onto PVDF membrane, and immunoblotted using antiferrochelatase, anticatalase, and antithioredoxin.
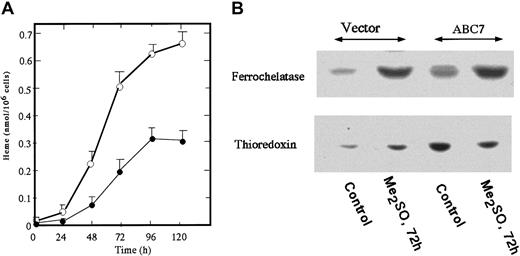
To generate MEL cells stably expressing mouse ABC7, pcDNA(c-Myc)-ABC was transfected and G418-resistant cells were selected. Five clones stably expressing ABC7 were isolated and mixed, and the expression of ABC7 was examined by immunoblotting. A specific band reacting with anti–c-Myc-tag in ABC7 cDNA-transfected cells was detected (data not shown). Figure 5A shows the amount of heme in ABC7-expressing MEL cells. The amount in uninduced ABC7-transfectants was about 2.5-fold higher than that in empty vector–transfected cells. The growth rate of MEL cells expressing ABC7 was similar to that of control cells. To cause the MEL cells to differentiate, they were treated with 2%Me2SO. The amount of heme in the ABC7 transfectants increased sharply with time, whereas the production of heme in control MEL cells treated with Me2SO started slowly. At 120 hours after the induction, the level of heme in ABC7 transfectants was about 2.5-fold that in the control cells. When the amounts of ferrochelatase and thioredoxin were compared between ABC7-expressing and control cells, both were found to be higher in the former (Figure 5B). On treatment with Me2SO, the amounts of ferrochelatase and thioredoxin increased in control cells. In ABC7 transfectants, treatment with Me2SO increased the expression of ferrochelatase while decreasing that of thioredoxin. Thus, the expression of ABC7 led to an increase in the production of heme in nonerythroid and erythroid cells and to an accelerated induction of differentiation of MEL cells.
The amount of heme and levels of ferrochelatase and thioredoxin in Me2SO-induced ABC7-expressing MEL cells.
Cells expressing ABC7 and empty vector (5 × 105/mL each) were incubated with 2% Me2SO. (A) At the times indicated, the cells expressing ABC7 (○) and control (●) cells were collected and the amount of heme was measured. Data are expressed as the means ± SDs of triplicate experiments. (B) Immunoblot analysis. Immunoblotting was performed using antiferrochelatase and antithioredoxin. The transfected and control cells were separately cultured with 2% Me2SO for 0 hours and 72 hours.
The amount of heme and levels of ferrochelatase and thioredoxin in Me2SO-induced ABC7-expressing MEL cells.
Cells expressing ABC7 and empty vector (5 × 105/mL each) were incubated with 2% Me2SO. (A) At the times indicated, the cells expressing ABC7 (○) and control (●) cells were collected and the amount of heme was measured. Data are expressed as the means ± SDs of triplicate experiments. (B) Immunoblot analysis. Immunoblotting was performed using antiferrochelatase and antithioredoxin. The transfected and control cells were separately cultured with 2% Me2SO for 0 hours and 72 hours.
Discussion
The present study has demonstrated the involvement of ABC7 in the biosynthsis of heme via interaction with ferrochelatase. Comparison of the mRNA levels of 3 known ABC transporters during the differentiation of MEL cells revealed that MTABC3, ABC7, and ABC-me mRNAs were all induced to express, and the extent of induction was greatest for ABC7 mRNA. Furthermore, the expression of ABC7 mRNA during the cellular differentiation paralleled that of ferrochelatase. The importance of ABC7 in the iron-dependent regulation of ferrochelatase led us to examine its involvement in the biosynthesis of heme in erythroid cells where heme is a major product of iron metabolism. Antisense oligonucleotide to ABC7 mRNA caused inhibition of the biosynthesis of heme in Me2SO-treated MEL cells, and MEL cells overexpressing ABC7 contained 2.5-fold more heme than control MEL cells.
The induction of ABC7 during the differentiation of MEL cells roughly parallels that of ABC-me, MTABC3, and the heme-biosynthetic enzymes. The low level of expression of the ABC-me transcript in GATA-1–negative erythroid colonies was explained by the GATA-1–controlled expression of ABC-me.10 Because several putative binding sequences for factors involved in erythroid cell differentiation, including GATA-1 and E-box, were found in the promoter region of the human ABC7 gene,9 the transcription of ABC7 can be controlled by GATA-1 and an erythroid-specific E-box–activating factor, Tal1.
Mitochondria play roles in many essential and specialized functions during erythroid differentiation, including the synthesis of hemoglobin, production of energy, integration of apoptotic signals, and gradual self-elimination. Although the site and role of ABC7 involvement in iron homeostasis is unclear, the effects of the expression of ABC7 in MEL cells favor a role in the production of hemoglobin. Because ABC7 and ABC-me do not induce the spontaneous differentiation of MEL cells,10 the effects of these ABC transporters differed from those of other proteins related to transcription, including Tal-126 and HERF-1,27 which triggered spontaneous differentiation of MEL cells. Thus, these transporters enhance the functions of the hemoglobin synthesis machinery coordinately activated during Me2SO-induced erythroid differentiation.
In vitro and in vivo protein-protein interaction experiments revealed that ABC7 binds specifically to ferrochelatase. The importance of the carboxy-terminal region that contains the iron-sulfur cluster of ferrochelatase is highlighted by the inability of a truncated ferrochelatase (N395Δ) to bind GST-ABC7 (458-752). Based on the observations that the iron of the iron-sulfur cluster of ferrochelatase is not the substrate for the enzyme reaction,28 ABC7 would be involved in the formation and maintenance of the iron-sulfur cluster. On the other hand, GST-ABC7 (458-752) corresponded to the entire ATP-binding region, which expanded in the matrix of mitochondria bound to ferrochelatase. GST-ABC7 (521-752) or GST-ABC7 (589-752) missing in the ATP-binding site of ABC7 was not able to interact with ferrochelatase. Thus, there is interaction between the ATP-binding domain of ABC7 and the iron-sulfur cluster region of ferrochelatase to support normal iron homeostasis in mitochondria.
It is reported that yeast mutants lacking Atm1p, a yeast homolog of ABC7, synthesize heme normally12,13 and heme trafficking is not compromised by the absence,29 suggesting that yeast ferrochelatase is expressed normally in Δatm1 cells. This is inconsistent with the present findings. This discrepancy is explained by the different properties of yeast and mammalian ferrochelatase because the mammalian enzyme contains an iron-sulfur cluster at the carboxy-terminus, whereas the yeast enzyme does not.4Moreover, the mammalian ferrochelatase is shown to be dimerized through the iron-sulfur cluster, while the enzyme lacking the cluster presents as a monomer.30 Our previous findings that the amount of mouse ferrochelatase overexpressed in Cos7 cells markedly decreased under iron depletion,6 whereas bacterial ferrochelatase lacking the iron-sulfur cluster was not affected, indicated that the expression of mammalian ferrochelatase was definitely regulated by the level of intracellular iron. Considering that the amount of ferrochelatase was dependent on the expression of ABC7, a novel mechanism regulating heme biosynthesis at the terminal step must exist in mammals. Because the syndrome of ABC7 defects in humans is characterized by an elevated level of free protoporphyrin as well as an absence of excessive iron deposition,9 causing the uncoupling of iron from the metabolism of porphyrin, the interaction of ferrochelatase with ABC7 is required for the positive regulation of heme biosynthesis in mammals.
The import of iron into mitochondria must be tightly regulated because little accumulation of iron is observed in the mutants defective in heme biosynthesis,31 but the mechanism of iron metabolism in mitochondria is unclear. Recently, Arabidopsis at ABC1 was shown to be involved in the synthesis of chlorophyll and required for the transport and correct distribution of protoporphyrin.32 Defects in Atm1p in yeast cause a marked increase in the mitochondrial iron content, and ABC7 expression complemented to a large extent the failure of Δatm1 cells.9,15 Although the substrates for Atm1p and ABC7 have yet to be clearly identified, defects caused by mutations in yeast Atm1p reduce the amount of cytosolic iron-sulfur cluster proteins such as Leu1p, but not mitochondrial iron-sulfur cluster proteins,13 suggesting that Atm1p exports the iron-sulfur cluster generated in mitochondria for the assembly of the cytosolic proteins. On the other hand, the present study demonstrated that overexpression of ABC7 in nonerythroid and erythroid cells resulted in an increase in not only a cytosolic iron-sulfur cluster protein thioredoxin but also ferrochelatase. At present, therefore, it is not clear which proteins are controlled by the function of ABC7 in mammalian cells. It is possible that ABC7 is involved in the expression of ferrochelatase. We speculate that iron positively regulates not only the generation of cytosolic proteins containing iron-sulfur clusters but also the function of mitochondria via the formation of iron-sulfur clusters and heme in mammalian cells.
We previously reported that the cellular content of heme decreased slightly when erythroleukemia K562 cells were treated with desferrioxamine, indicating that the decrease in ferrochelatase causes the decrease in the synthesis of heme.6 However, protoporphyrin did not accumulate in desferrioxamine-treated K562 cells where the formation of heme was suppressed. Moreover, no accumulation of any porphyrin was observed in MEL cells treated with antisense oligonucleotide to ABC7 mRNA (K.K., T.F., and S.T., unpublished observations, 2002). In contrast, defects in ferrochelatase cause the accumulation of protoporphyrin in patients with erythropoietic protoporphyria,33 a phenomenon inconsistent with the findings of this study. These results suggest that iron regulates the de novo synthesis of porphyrin, which is consistent with the observation that in erythroid cells protoporphyrin IX levels are coupled to the availability of iron as a result of a translational induction of erythroid-specific δ-aminolevulinic acid synthase (ALA-S2) by iron.34 Because ALA-S2 mRNA contains an iron-responsive element in its 5′-untranslated region, the translation of ALA-S2 mRNA depends on the availability of iron.35 A molecular defect in ALA-S2 is responsible for X-linked sideroblastic anemia, which is characterized by hypochromic and microcytic anemia with the appearance of ring sideroblasts and large mitochondrial iron deposits.36 Thus, a deficiency of ALA-S2 causes a phenotype very similar to that caused by ABC7, suggesting that the synthesis of heme is tightly coupled with iron homeostasis in erythroblasts. In addition, Kim et al reported a nitric oxide (NO)-induced loss of heme in primary cultures of rat hepatocytes, where ALA-S as well as ferrochelatase activity was markedly reduced.37 Intracellular iron is a major target of NO,38 which impairs the metabolism of mitochondrial iron,39 indicating that hepatic porphyrin production is regulated by iron. Based on these observations, ABC7 would dominate the availability of iron in the mitochondria of ABC7-expressing tissues, which might regulate the expression of the 2 enzymes, ferrochelatase and ALA-S, resulting in the control of heme production in erythroid and nonerythroid cells.
Shirihai et al reported that ABC-me is expressed at high levels in the erythroid tissues of embryos and adults.10 Overexpression of ABC-me in MEL cells enhanced the increase in heme caused by Me2SO, whereas heme suppressed the expression of ABC-me, suggesting that ABC-me plays a role in the trafficking of heme intermediates. The present study demonstrates that ABC7 contributes to the metabolism of heme in mitochondria in a fashion similar to ABC-me. This raises the question of whether the functions of ABC7 and ABC-me differ in the biosynthesis of heme. Considering that mutants deficient in ABC7 exhibit X-linked sideroblastic anemia and mitochondrial ABC transporters function in iron homeostasis,9 ABC-me may be responsible for the unidentified sideroblastic anemia in the autosomal tract. However, it is not clear if a molecular defect of ABC-me is related to this inherited disease. Further study is required to clarify the molecular mechanisms underlying the shared and unique functions of ABC7, MTABC3, and ABC-me during the differentiation of erythroid cells.
We thank Dr Takashi Osumi, Himeji Institute of Technology, for the antibody for rat catalase, and Dr Y. Sokawa for valuable suggestions.
Prepublished online as Blood First Edition Paper, December 12, 2002; DOI 10.1182/blood-2002-04-1212.
Supported in part by grants from the Ministry of Education, Science, Sports and Culture of Japan and the Yamanouchi Foundation for Research on Metabolic Disorders.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Shigeru Taketani, Department of Biotechnology, Kyoto Institute of Technology, Kyoto 606-8585, Japan; e-mail: taketani@ipc.kit.ac.jp.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal