Abstract
p27 is a cyclin-dependent kinase inhibitor that plays a critical role in regulating G1/S progression, and whose activity is, in part, regulated through interactions with D-type cyclins. Mantle cell lymphoma (MCL) is characterized by the t(11;14) translocation resulting in deregulated cyclin D1. We previously showed that p27 expression in MCL, as assessed by immunohistochemistry (IHC), does not show the usual inverse relationship to proliferate seen in most other lymphomas that do not overexpress cyclin D1. This suggested that the normal expression or control of p27 activity on cell growth might be altered through potential interactions with cyclin D1. Using Western blot and coimmunoprecipitation studies, we assessed the interrelationship between cyclin D1 and p27 in several cyclin D1+ cell lines and primary MCL cases. Similar to our previous results by IHC, typical MCLs showed lower expression of p27 when compared to the more highly proliferative blastic cases or cell lines (mean arbitrary units: 58 versus 236 versus 120). Cyclin D1 was expressed at variable levels in both typical and blastic MCLs. p27 protein could be consistently coimmunoprecipitated with cyclin D1 from both cell lines and cases. Using techniques of exhaustive immunoprecipitation, we could demonstrate that most p27 protein was sequestered into complexes containing cyclin D1. We hypothesize that mantle cell lymphomagenesis results not only from direct consequences of inappropriate cyclin D1 expression, but also from the ability of overexpressed cyclin D1 to buffer physiologic changes in p27 levels, thereby rendering p27 ineffective as an inhibitor of cellular growth.
Introduction
Progression through the various phases of the cell cycle depends on the orderly activation of specific cyclin/cyclin-dependent kinase (CDK) complexes.1 Specific inhibitory proteins as well as positive and negative phosphorylation events play critical roles in regulating the activation of cyclin/CDK complexes during the cell cycle.1-7
p27Kip1 is a CDK inhibitor that plays a critical role in regulating G1/S progression, at a stage called the restriction point, preceding the onset of DNA synthesis.8-11 p27Kip1 binds to both cyclin D/CDK4 complexes and cyclin E/CDK2 complexes with very different effects.5,6 Although p27Kip1 binds more efficiently with cyclin D/CDK4 complexes,3 this interaction does not inhibit the cyclin D/CDK4 kinase activity but rather results in the stabilization of the complex.5 On the contrary, the interaction of p27Kip1 with cyclin E/CDK2 complexes inactivates the kinase activity that is essential for cell cycle progression.12,13 As cells progress through G1, cyclin D levels increase and compete with cyclin E for free p27Kip1 while cyclin-dependent kinase-activating kinase (CAK) begins to activate CDK2.5 These 2 events, respectively, result in decreased levels of free p27Kip1 potentially available to inhibit the cyclin E/CDK2 kinase and in the degradation of p27Kip1 bound to the cyclin E/CDK2 complex. Activated CDK2 phosphorylates p27Kip1 at Thr187,14 triggering its subsequent ubiquitination and degradation by the ubiquitin ligase F-box protein Skp2.15-18 In contrast, activated cyclin D/CDK4 complexes do not seem to be involved in p27Kip1degradation.5 12
We recently reported19 that p27Kip1 expression in normal lymphoid tissue and in lymphoid neoplasms is inversely proportional to the proliferation index as measured by Ki-67, confirming the important role of p27Kip1 as a negative regulator of cell cycle progression. However, in typical mantle cell lymphomas (MCLs), we could detect no or very low levels of p27Kip1 despite their generally low proliferation rate. Paradoxically, the blastic variant of MCL, with a much higher proliferation rate, expressed higher levels of p27Kip1. The lack of immunologically detectable expression of p27Kip1 in typical MCL was not due to p27Kip1 gene deletions because none of the 25 MCL cases examined showed gross rearrangement or deletion of the gene. This finding was consistent with previous studies in a variety of human primary tumors and cancer cell lines that have shown only rare mutations of the p27Kip1gene.20-23
The paradoxical expression profile of p27Kip1 in MCL is particularly interesting because MCLs are characterized by the t(11;14) translocation, which results in activation of theCCDN1 gene and expression of the gene's product, cyclin D1.24 The inappropriate expression of cyclin D1 has been proposed to deregulate the normal control of cell cycle and growth and to contribute to lymphomagenesis in MCL. However, cyclin D1 has little transforming activity and is not sufficient to induce malignant transformation of transfected cells, suggesting that tumorigenesis induced by cyclin D1 requires the cooperation of additional oncogenes or tumor suppressor genes or both.25-28 Given the ability of cyclin D1 to bind p27Kip1 and modulate p27Kip1 levels as part of its normal physiologic function, we wished to explore the relationship of cyclin D1 expression to p27Kip1 expression in MCL and assess the possibility that the inappropriate high levels of cyclin D1 in MCL contribute to lymphomagenesis by sequestering p27Kip1.
Materials and methods
Cell lines and primary tissues
Nine human lymphoid cell lines were selected for study. These included 3 cell lines with high expression of cyclin D1 as a result of the t(11;14) translocation: Granta 51929 and NCEB-130 (MCL cell lines) and KMS1231(myeloma cell line). The 6 remaining cell lines do not express cyclin D1 and included 3 follicular lymphoma cell lines: SUDHL-4, SUDHL-6, SUDHL-10; 1 T-cell lymphoblastic lymphoma cell line: MOLT-4; 1 anaplastic large cell lymphoma cell line: KiJK; and 1 Burkitt lymphoma cell line: Thomas. Thirteen primary lymphoma samples were also selected. Frozen tissue from 7 typical cases of MCL, 1 case of blastic MCL, and 3 cases of B-cell chronic lymphocytic leukemia (CLL) were selected from the files of the Hematopathology Section, Laboratory of Pathology, National Cancer Institute, National Institutes of Health. An additional 2 cases of blastic MCL were obtained from the files of the Pathology Department of the Hospital Clinic of Barcelona, Spain. In all cases the number of reactive CD3+ T cells was estimated. The number of reactive T cells was generally low with a median of 10% (range, 5%-30%). All primary MCLs have been the subject of previous studies.19 32
Preparation of cell line blocks
Tumor cell lines were grown in RPMI 1640 supplemented with 15% fetal calf serum. The cells were harvested during their exponential growth phase, centrifuged at 550g for 5 minutes, and the supernatant removed. The cell pellet was washed and resuspended in 3 drops human plasma to which 3 drops thrombin were added. The resulting clot was fixed in 10% formalin and embedded in paraffin.
Immunoreagents
Rabbit polyclonal antibodies against cyclin D1 (H-295), p27 C-terminal peptide (C-19), and cyclin A (H-432) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The monoclonal antibody against cyclin E (14591A) was purchased from Pharmingen (San Diego, CA). The monoclonal antibodies against p27Kip1, CDK2, and CDK4 were purchased from Transduction Laboratories (Lexington, KY). The protein A/agarose was obtained from Transduction Laboratories.
Protein extraction
Ten sections of 20 μm thickness were cut from frozen tissue blocks embedded in Tissue-Tek OCT compound 4583 (Sakura Finetek, Torrance, CA) and immersed into 5 mL sonication buffer (phosphate-buffered saline [PBS], 1 mM EDTA [ethylenediaminetetraacetic acid], 10 μg/mL leupeptin, 10 μg/mL aprotinin, 10 μg/mL pepstatin, 50 μm AEBSF [4-(2-aminoethyl)-benzenosulfonyl fluoride hydrochloride]). Lysates were centrifuged at 550g for 5 minutes, and the supernatants were discarded. The pellets were resuspended in 1 mL sonication buffer and sonicated 3 times, 30 seconds each on ice with 30-second pauses. The protein concentration was determined by the BCA protein assay reagent kit (Pierce, Rockford, IL).
Western blot analysis
A total of 30 μg protein extracts was separated by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to pure nitrocellulose immobilization membranes (Protran, BA85, Dassel, Germany). Membranes were blocked for 1 hour in 5% nonfat dry milk at 37°C and incubated with the primary antibody for 1 hour. Subsequently, membranes were washed 5 times 5 minutes each in a wash buffer (Tris [tris(hydroxymethyl)aminomethane] 10 mM/L, pH 7.6, NaCl 100 mM/L, and Tween 0.1%) and incubated with an appropriate125I-linked secondary reagent for 1 hour. Radiolabeled protein A and sheep antimouse immunoglobulin were used to detect polyclonal and monoclonal antibodies, respectively. Membranes were washed 5 times in the same wash buffer, and detection was performed by exposing the membranes to Kodak X-OMAT AR film. All assays on each cell line and primary tumors were repeated several times and gave similar results.
The intensity of each of the bands on the Western blots was quantitated in arbitrary units on a Molecular Dynamics (Sunnyvale, CA) computing phosphoimager, using Image Quant software for Macintosh. For each sample, the intensity of the band was expressed as the percentage of the signal obtained to that of Granta 519 cell line (100), which was used as the positive control in all experiments.
Immunoprecipitation studies
For immunoprecipitation studies, we used 300 μg total protein of cell lines and primary tumors in 500 μL of 2 times immunoprecipitation buffer (2% Triton X-100, 300 mM NaCl, 20 mM Tris, pH 7.4, 2 mM EDTA, 2 mM EGTA [ethylene glycol tetraacetic acid], pH 8.0, 0.4 mM sodium orthovanadate, 0.4 mM phenylmethylsulfonyl fluoride [PMSF], 1.0% NP-40), and 400 μL H20. The lysates were incubated overnight with a rabbit polyclonal antibody against either cyclin D1 (H-295) or p27Kip1 (C-19) at a concentration of 2.5 μg/mL or with agarose-conjugated antibodies p27Kip1 (F8), cyclin E (HE 111), and cyclin A (H-432) (Santa Cruz Biotechnology) for 1 hour at a concentration of 5 mg/mL. The immune complexes were collected, when indicated, on protein A conjugated to agarose (Transduction Laboratories) and the pellets were analyzed by SDS-PAGE as described (see “Western blot analysis”).
Immunoprecipitation was also performed following denaturation of protein complexes to evaluate possible masking of immunoreactive epitopes within the complex. To unfold and dissociate protein complexes, 300 mg of cell line extracts were first treated with 8 M urea in wash buffer for 30 minutes at room temperature.33Samples were slowly diluted 20-fold with immunoprecipitation buffer containing p27Kip1 (C-19) antibody. The immune complexes were collected on protein A conjugated to agarose. Control samples were either untreated or brought to a final concentration of 0.4 M (to match conditions of refolded samples) or were untreated but normalized for protein concentration. Bound proteins were separated on SDS-PAGE as described (see “Western blot analysis”).
Immunohistochemistry
All primary cases were previously studied by paraffin section immunohistochemistry (IHC) with a panel of antibodies to assess lymphoid phenotype, as well as to p27, cyclin D1, p53, and MIB-1.19 For the lymphoid cell lines, the expression of p27Kip1 (Transduction Laboratories; dilution 1:1000), cyclin D1 (clone P2D11F11; Novocastra, Newcastle, United Kingdom; dilution 1:10), CD20 (Dako, Carpinteria, CA; dilution 1:200), p53 with the monoclonal antibody DO7 against the wild-type/mutant p53 (Dako; dilution 1:50) and the MIB-1 antibody against the Ki-67 nuclear proliferation antigen (Immunotech, Westbrook, ME; dilution 1:10) was investigated on paraffin-embedded clot sections.
Immunohistochemical staining was performed on an automated immunostainer (Ventana Medical Systems, Tucson, AZ) according to the company's protocol with slight modifications. After deparaffinization and rehydration, the slides were placed in a microwave pressure cooker in boiling 0.01 M citrate buffer (pH 6.0) containing 0.1% Tween 20 (hot start) and heated in a microwave oven at maximum power (800 W) for 8 minutes. Thereafter, sections were washed in Tris-buffered saline (pH 7.6) containing 5% fetal calf serum (Life Technologies, Grand Island, NY) for 30 minutes. p27Kip1, cyclin D1, and CD5 were incubated overnight at room temperature. The rest of the procedure (secondary antibody, avidin-biotin complex [ABC], color development, and counterstain) was performed on the Ventana immunostainer. Positive controls were used to confirm the adequacy of the staining.
Results
Correlation between cyclin D1 expression and p27Kip1 expression in t(11;14) cell lines
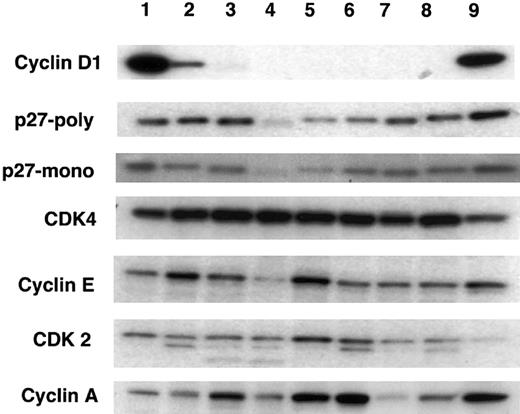
Cyclin D1 expression was detected only in 3 cell lines that had the t(11;14) translocation (Figure1). By measuring the intensity of the signals with a phosphoimager (Table 1), Granta 519 showed the highest levels of cyclin D1 expression (100) followed by the KMS12 (74) and NCEB-1 (10) cell lines, when normalized to Granta 519. The remaining cell lines were negative for cyclin D1 expression, as expected.
Western blot analysis for cyclin D1, p27Kip1, and other cell cycle–related proteins in lymphoid cell lines.
Each lane contains 30 μg protein extract from the following cell lines: lane 1, Granta 519; lane 2, NCEB-1; lane 3, SUDHL-4; lane 4, SUDHL-6; lane 5, Thomas; lane 6, Molt4; lane 7, KiJK; lane 8, SUDHL-10; lane 9, KMS12. Lanes 1 (Granta 519), 2 (NCEB-1), and 9 (KMS12) represent the t(11;14) translocated cell lines. Expression of cyclin D1 is limited to these 3 cell lines. These cell lines also show strong positivity for p27Kip1 although several of the non-t(11;14) translocated cell lines also show relatively high levels of p27Kip1. Note that the cell lines with positivity for cyclin D1 have the strongest positivity for p27Kip1. Cyclin E, cyclin A, CDK4, and CDK2 expression is present in all cell lines with no apparent correlation between any of these proteins and either cyclin D1 or p27Kip1.
Western blot analysis for cyclin D1, p27Kip1, and other cell cycle–related proteins in lymphoid cell lines.
Each lane contains 30 μg protein extract from the following cell lines: lane 1, Granta 519; lane 2, NCEB-1; lane 3, SUDHL-4; lane 4, SUDHL-6; lane 5, Thomas; lane 6, Molt4; lane 7, KiJK; lane 8, SUDHL-10; lane 9, KMS12. Lanes 1 (Granta 519), 2 (NCEB-1), and 9 (KMS12) represent the t(11;14) translocated cell lines. Expression of cyclin D1 is limited to these 3 cell lines. These cell lines also show strong positivity for p27Kip1 although several of the non-t(11;14) translocated cell lines also show relatively high levels of p27Kip1. Note that the cell lines with positivity for cyclin D1 have the strongest positivity for p27Kip1. Cyclin E, cyclin A, CDK4, and CDK2 expression is present in all cell lines with no apparent correlation between any of these proteins and either cyclin D1 or p27Kip1.
Expression of cyclin D1 and p27Kip1 in human lymphoma cell lines by Western blot
| Cell line . | Cyclin D1* . | p27Kip1 . |
|---|---|---|
| Granta 519 | 100 | 100 |
| NCEB-1 | 10 | 68 |
| SUDHL-4 | 0 | 88 |
| SUDHL-6 | 0 | 11 |
| Thomas | 0 | 18 |
| Molt4 | 0 | 48 |
| KiJk | 0 | 78 |
| SUDHL-10 | 0 | 91 |
| KMS12 | 74 | 194 |
| Cell line . | Cyclin D1* . | p27Kip1 . |
|---|---|---|
| Granta 519 | 100 | 100 |
| NCEB-1 | 10 | 68 |
| SUDHL-4 | 0 | 88 |
| SUDHL-6 | 0 | 11 |
| Thomas | 0 | 18 |
| Molt4 | 0 | 48 |
| KiJk | 0 | 78 |
| SUDHL-10 | 0 | 91 |
| KMS12 | 74 | 194 |
Values are given in arbitrary units.
Expression of p27Kip1 was investigated with 2 different antibodies (monoclonal from Transduction Laboratories and a polyclonal [C-19] from Santa Cruz Biotechnology), both of which gave similar but not identical results (Figure 1). High levels of p27Kip1expression were seen in 6 of 9 cell lines examined. These results were somewhat unexpected because the cell lines were harvested during the exponential growth phase, and their proliferation rate was nearly 100% as demonstrated by the proliferation marker Ki-67, indicating that some rapidly proliferating human lymphoma cell lines can tolerate high levels of p27Kip1. Of particular interest, the 3 cell lines expressing cyclin D1 were among the highest p27Kip1-expressing cell lines (Table 1). Levels of expression of cyclin E, cyclin A, CDK4, and CDK2 varied from cell line to cell line with no apparent correlation observed among these proteins and levels of cyclin D1 or p27Kip1.
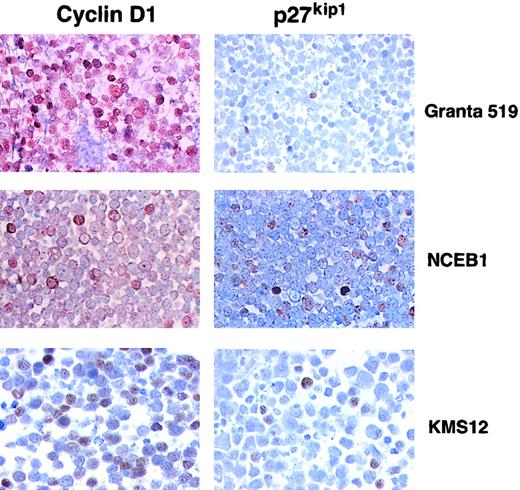
Immunohistochemical analysis of the t(11;14) translocated cell lines (Granta 519, NCEB-1, and KMS12) revealed strong cyclin D1 nuclear staining in the 3 cases (Figure 2). Granta 519 and KMS12 showed cyclin D1 expression in practically 100% of the cell population, whereas NCEB-1 expressed cyclin D1 in a minority of the cells. p27Kip1 was detectable in less than 5%, 10%, and 30% of the cell population in Granta 519, NCEB, and KMS12, respectively. The percentage of p27Kip1+ cells was roughly correlated with the levels of p27Kip1 observed by Western blot.
Immunohistochemical analysis of t(11;14) cell lines for expression of cyclin D1 and p27Kip1.
Cyclin D1 positivity is seen in approximately 100%, 10%, and 60% of the cells in Granta 519, NCEB-1, and KMS12, respectively. In contrast, p27Kip1 is positive in 5%, 20%, and 10% of the cells in Granta 519, NCEB-1, and KMS12, respectively.
Immunohistochemical analysis of t(11;14) cell lines for expression of cyclin D1 and p27Kip1.
Cyclin D1 positivity is seen in approximately 100%, 10%, and 60% of the cells in Granta 519, NCEB-1, and KMS12, respectively. In contrast, p27Kip1 is positive in 5%, 20%, and 10% of the cells in Granta 519, NCEB-1, and KMS12, respectively.
Expression levels of cyclin D1 and p27Kip1 in t(11;14) primary MCL cases
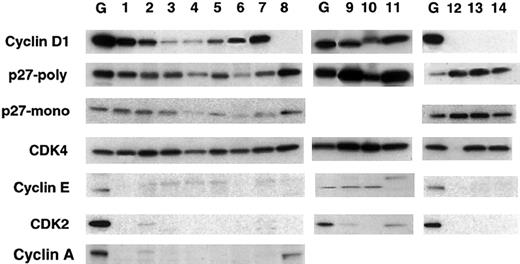
Expression of cyclin D1 was demonstrated in all 10 MCL cases (Figure 3). There were no differences in cyclin D1 expression between the typical and the variant blastic cases. By measuring the intensity of the bands with a phosphoimager, the typical MCL cases showed expression levels between 2 and 26 when compared with the Granta 519 cell line, whereas the blastic cases showed 11 to 41 (Table 2). Expression of p27Kip1 was investigated with 2 different antibodies (monoclonal from Transduction Laboratories and a polyclonal [C-19] from Santa Cruz Biotechnology), both of which gave similar but not identical results. Expression of p27Kip1 was detected in all MCL cases. The typical MCL cases showed expression levels between 42 and 72 (mean, 58) when compared to Granta 519. Cases with higher expression of cyclin D1 tended to express higher levels of p27Kip1. The expression of p27Kip1 in the blastic MCL cases (84-333; mean, 236) was very high, similar or higher than the cyclin D1+ cell lines. The CLL cases used for comparison were negative for cyclin D1 and showed very high expression of p27Kip1 (123-270; mean, 209). The expression of cyclin E, cyclin A, and CDK2 was generally very low in the typical MCL when compared to Granta 519 cell line and did not correlate with the expression of cyclin D1 or p27Kip1. In contrast, cyclin E was higher in the blastic MCL, similar to the cell lines with the t(11;14) translocation.
Western blot analysis for cyclin D1, p27Kip1, and other cell cycle–related proteins in primary MCL.
Each lane contains 30 μg protein extract. Lane G represents the Granta 519 cell line control; lanes 1-7 represent 7 cases of typical MCL. Lanes 8 and 12-14 represent 3 control cases of CLL. Lanes 8 and 14 correspond to the same CLL case. Lanes 9-11 represent 3 cases of blastic MCL. Note that all the MCL cases express cyclin D1 and the CLL cases are negative for cyclin D1. p27Kip1 is expressed in all instances; however, its expression is highest in the blastic variant of MCL (lanes 9-11), followed by the CLL cases (lanes 8 and 12-14). Note that the expression of cyclin A, cyclin E, and CDK2 is very low in the typical MCL cases (lanes 1-7), whereas the expression of these proteins in the blastic variant of MCL is comparable to that of the cell lines. Cyclin A was not performed in the blastic cases because there was not enough tissue.
Western blot analysis for cyclin D1, p27Kip1, and other cell cycle–related proteins in primary MCL.
Each lane contains 30 μg protein extract. Lane G represents the Granta 519 cell line control; lanes 1-7 represent 7 cases of typical MCL. Lanes 8 and 12-14 represent 3 control cases of CLL. Lanes 8 and 14 correspond to the same CLL case. Lanes 9-11 represent 3 cases of blastic MCL. Note that all the MCL cases express cyclin D1 and the CLL cases are negative for cyclin D1. p27Kip1 is expressed in all instances; however, its expression is highest in the blastic variant of MCL (lanes 9-11), followed by the CLL cases (lanes 8 and 12-14). Note that the expression of cyclin A, cyclin E, and CDK2 is very low in the typical MCL cases (lanes 1-7), whereas the expression of these proteins in the blastic variant of MCL is comparable to that of the cell lines. Cyclin A was not performed in the blastic cases because there was not enough tissue.
Expression of cyclin D1 and p27Kip1 in MCLs and CLLs by Western blot
| Cases . | Cyclin D1* . | p27kip1 . |
|---|---|---|
| Granta 519 | 100 | 100 |
| 1 MCL | 26 | 67 |
| 2 MCL | 8 | 55 |
| 3 MCL | 2 | 72 |
| 4 MCL | 2 | 42 |
| 5 MCL | 4 | 52 |
| 6 MCL | 8 | 52 |
| 7 MCL | 18 | 63 |
| 8 CLL | 0 | 179 |
| 9 B-MCL | 29 | 333 |
| 10 B-MCL | 11 | 84 |
| 11 B-MCL | 41 | 291 |
| 12 CLL | 0 | 263 |
| 13 CLL | 0 | 270 |
| 14 CLL | 0 | 123 |
| Cases . | Cyclin D1* . | p27kip1 . |
|---|---|---|
| Granta 519 | 100 | 100 |
| 1 MCL | 26 | 67 |
| 2 MCL | 8 | 55 |
| 3 MCL | 2 | 72 |
| 4 MCL | 2 | 42 |
| 5 MCL | 4 | 52 |
| 6 MCL | 8 | 52 |
| 7 MCL | 18 | 63 |
| 8 CLL | 0 | 179 |
| 9 B-MCL | 29 | 333 |
| 10 B-MCL | 11 | 84 |
| 11 B-MCL | 41 | 291 |
| 12 CLL | 0 | 263 |
| 13 CLL | 0 | 270 |
| 14 CLL | 0 | 123 |
Cases 8 and 14 are different blocks from the same case.
MCL indicates mantle cell lymphoma; B-MCL, blastic variant of MCL; CLL, chronic lymphocytic leukemia.
Values are given in arbitrary units
Cyclin D1 sequesters the majority of p27Kip1 in MCLs
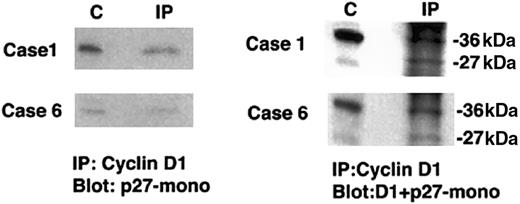
To assess potential physical interactions between cyclin D1 and p27Kip1, we next immunoprecipitated protein extracts from t(11;14) translocated cell lines and from whole tissue from 2 cases (nos. 1 and 6) of typical MCL with anticyclin D1 and probed Western blots for coimmunoprecipitated p27Kip1 (Figure4). In the 2 primary cases, we recovered p27Kip1 protein from cyclin D1 immunoprecipitates, demonstrating the physical association of cyclin D1 and p27Kip1 in typical MCL cases. Similar results were found in all 3 t(11;14) cell lines tested. These results from both primary cases and cell lines indicate that the p27Kip1 protein detected by Western blotting is derived from the tumor cells expressing cyclin D1 and not from other cells such as infiltrating T cells.
Coimmunoprecipitation of p27Kip1 with cyclin D1 in primary typical MCL.
Protein extracts were immunoprecipitated with anticyclin D1. Western blots were probed with either p27Kip1 or cyclin D1 and p27Kip1. Lane C contains positive control and lane IP contains the case immunoprecipitated. In both cases p27Kip1protein is recovered from cyclin D1 immunoprecipitates.
Coimmunoprecipitation of p27Kip1 with cyclin D1 in primary typical MCL.
Protein extracts were immunoprecipitated with anticyclin D1. Western blots were probed with either p27Kip1 or cyclin D1 and p27Kip1. Lane C contains positive control and lane IP contains the case immunoprecipitated. In both cases p27Kip1protein is recovered from cyclin D1 immunoprecipitates.
We next wished to determine what proportion of total cellular p27Kip1 was bound to cyclin D1. For these experiments we used only the t(11;14) translocated cell lines due to the requirement for substantial amount of protein extracts. We first performed exhaustive immunoprecipitations with either anticyclin D1 or anti-p27Kip1. Granta 519, NCEB-1, and KMS12 were each sequentially immunoprecipitated with anticyclin D1, until cyclin D1 was depleted (Figure 5A). This was achieved by the third immunoprecipitation in all cell lines. Thereafter, the supernatants were immunoprecipitated and blotted with anti-p27Kip1 (Figure 5B). Once cyclin D1 was depleted, p27Kip1 could no longer be detected. This indicates that most of the p27Kip1 coprecipitated with cyclin D1. In the inverse assay, the 3 cell lines were sequentially immunoprecipitated with anti-p27Kip1 until p27Kip1 was depleted. This was achieved by the second immunoprecipitation (Figure 5C). Thereafter, the supernatants were immunoprecipitated with anticyclin D1 (Figure 5D). The recovery of cyclin D1 in the third immunoprecipitation, in the absence of p27Kip1, reveals an excess of cyclin D1. These experiments indicate that a likely result of the high cyclin D1 levels present in t(11;14) translocated MCL cell lines is the sequestration of p27Kip1 protein into complexes containing cyclin D1.
Immunodepletion studies of cyclin D1 and p27Kip1.
(A) The cell lines Granta 519 (G), NCEB-1 (N), and KMS12 (K) are sequentially immunoprecipitated with anticyclin D1. By the third immunoprecipitation (3rd IP) all 3 cell lines were depleted of cyclin D1. (B) Supernatants were immunoprecipitated with p27Kip1; however, once cyclin D1 was depleted (4th IP), p27Kip1 was not detectable. (C) In the inverse assay, the 3 cell lines were sequentially immunoprecipitated with anti-p27Kip1. p27Kip1 was depleted by the second immunoprecipitation. (The bands observed in the blot are nonspecific bands seen with the polyclonal p27Kip1 antibody.) (D) The remaining supernatants were immunoprecipitated with anticyclin D1 (3rd IP). The recovery of cyclin D1 in the absence of p27Kip1 reveals excess of cyclin D1 relative to p27Kip1. C indicates positive control; IP, immunoprecipitation; Sup, supernatant.
Immunodepletion studies of cyclin D1 and p27Kip1.
(A) The cell lines Granta 519 (G), NCEB-1 (N), and KMS12 (K) are sequentially immunoprecipitated with anticyclin D1. By the third immunoprecipitation (3rd IP) all 3 cell lines were depleted of cyclin D1. (B) Supernatants were immunoprecipitated with p27Kip1; however, once cyclin D1 was depleted (4th IP), p27Kip1 was not detectable. (C) In the inverse assay, the 3 cell lines were sequentially immunoprecipitated with anti-p27Kip1. p27Kip1 was depleted by the second immunoprecipitation. (The bands observed in the blot are nonspecific bands seen with the polyclonal p27Kip1 antibody.) (D) The remaining supernatants were immunoprecipitated with anticyclin D1 (3rd IP). The recovery of cyclin D1 in the absence of p27Kip1 reveals excess of cyclin D1 relative to p27Kip1. C indicates positive control; IP, immunoprecipitation; Sup, supernatant.
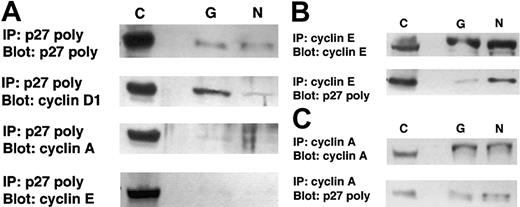
Additional coimmunoprecipitation experiments in the 2 MCL cell lines (Granta 519 and NCEB-1) using anti-p27Kip1 and either anticyclin A or anticyclin E as precipitating antibodies revealed that low but detectable amounts of p27Kip1 could also be identified in these cyclin-containing complexes (Figure6). It is of interest that when p27Kip1 was used as the precipitating antibody, the amount of cyclin E and cyclin A recovered was negligible, despite an extended exposure time, as evidenced by the strong bands in the controls (Figure6A). However, when cyclin E or cyclin A was used as the precipitating antibody, p27Kip1 could be detected, particularly in the NCEB-1 cyclin E coimmunoprecipitates (Figure 6B). This is consistent with a relative inefficiency of the p27Kip1 antibody in immunoprecipitating complexed p27Kip1. It should also be noted that the NCEB-1 cell line has intrinsically high levels of cyclin E and low levels of cyclin D1, which may account for some redistribution of p27Kip1 into cyclin E–containing complexes. In general, the cell lines have a much greater content of cyclin A and cyclin E, in contrast to the primary mantle cell cases, which have extremely low levels of these potential alternative binding partners (Figure 3), and therefore, analysis of cell lines may not accurately reflect the relative distribution of p27Kip1 in typical MCL cases. Nonetheless, these data do suggest that although the major binding partner in MCL is cyclin D1, binding to other cyclins may occur.
Coimmunoprecipitation studies of p27Kip1with cyclin E, cyclin A, and cyclin D1 in t(11;14) cell lines.
(A) The cell lines Granta 519 (G) and NCEB-1 (N) were immunoprecipitated with agarose-conjugated anti-p27Kip1. Western blots were probed with either p27Kip1, cyclin E, cyclin A, or cyclin D1. In both cell lines, the predominant cyclin recovered from p27Kip1 immunoprecipitates is cyclin D1. (B) In the inverse assay, the cell lines were immunoprecipitated with agarose-conjugated anticyclin E and blotted with polyclonal p27Kip1 antibody. p27Kip1 was recovered in cyclin E immunoprecipitates from NCEB-1. (C) The cell lines were immunoprecipitated with agarose-conjugated cyclin A and blotted with p27Kip1. A faint band is observed in Granta 519 and in NCEB-1. C indicates positive control, 60 μg total protein; IP, immunoprecipitation, 300 μg total protein.
Coimmunoprecipitation studies of p27Kip1with cyclin E, cyclin A, and cyclin D1 in t(11;14) cell lines.
(A) The cell lines Granta 519 (G) and NCEB-1 (N) were immunoprecipitated with agarose-conjugated anti-p27Kip1. Western blots were probed with either p27Kip1, cyclin E, cyclin A, or cyclin D1. In both cell lines, the predominant cyclin recovered from p27Kip1 immunoprecipitates is cyclin D1. (B) In the inverse assay, the cell lines were immunoprecipitated with agarose-conjugated anticyclin E and blotted with polyclonal p27Kip1 antibody. p27Kip1 was recovered in cyclin E immunoprecipitates from NCEB-1. (C) The cell lines were immunoprecipitated with agarose-conjugated cyclin A and blotted with p27Kip1. A faint band is observed in Granta 519 and in NCEB-1. C indicates positive control, 60 μg total protein; IP, immunoprecipitation, 300 μg total protein.
Complexed p27Kip1 reacts less efficiently than free p27Kip1 to the C-19 p27Kip1antibody
We previously reported no to weak expression of p27Kip1 using IHC in the typical MCL. In the present study, p27Kip1 was identified in all cases by Western blot analysis, albeit at lower levels when compared to variant blastic MCL or t(11;14) translocated cell lines. Therefore, we wished to determine whether our inability to detect p27Kip1 in typical MCL by IHC could be explained in part by epitope masking, because most cellular p27Kip1 was likely to be present in complexes containing cyclin D1. To assess this possibility, we examined the ability of the p27Kip1 (C-19) antibody to recognize native versus denatured complexes containing p27Kip1 in extracts of Granta 519 cell line. The p27Kip1 (C-19) antibody immunoprecipitated significantly more p27Kip1 protein after denaturation of cell lysates with 8 M urea (Figure7). The amount of p27Kip1immunoprecipitated with native conditions and in lysates treated with 0.4 mM urea was very similar (100 versus 122, respectively). In contrast, the amount of p27Kip1 recovered after denaturing the complexes with 8 M urea was more than double (227) compared with native conditions. This experiment indicates that the C-19 antibody used in this study detects unbound p27Kip1 protein better than when p27Kip1 is complexed with cyclin D1 and provides a possible explanation why immunohistochemical detection of p27Kip1 is generally negative or weak in these lymphomas.
Immunoprecipitation of p27Kip1 following denaturation with 8 M urea.
Prior to immunoprecipitation with the polyclonal anti-p27Kip1 (C-19), the protein extracts were either treated with 8 M urea and subsequently diluted 1:20 as described in “Materials and methods” (lane 8M) or treated by dilution with buffer not containing urea (N), or diluted with buffer in a final concentration of 0.4 M urea (lane 0.4M). The p27Kip1 antibody immunoprecipitates significantly more p27Kip1 protein following denaturation of cell lysates with 8 M urea. Note that there is practically no difference in the expression of p27Kip1 between the extracts with native conditions and the one treated with 0.4 M urea.
Immunoprecipitation of p27Kip1 following denaturation with 8 M urea.
Prior to immunoprecipitation with the polyclonal anti-p27Kip1 (C-19), the protein extracts were either treated with 8 M urea and subsequently diluted 1:20 as described in “Materials and methods” (lane 8M) or treated by dilution with buffer not containing urea (N), or diluted with buffer in a final concentration of 0.4 M urea (lane 0.4M). The p27Kip1 antibody immunoprecipitates significantly more p27Kip1 protein following denaturation of cell lysates with 8 M urea. Note that there is practically no difference in the expression of p27Kip1 between the extracts with native conditions and the one treated with 0.4 M urea.
Discussion
In this study we analyzed the relationship of p27Kip1protein and cyclin D1 expression in t(11;14) translocated primary MCL cases and cell lines. We found that the cyclin D1+ cell lines and the blastic variant of MCL have the highest levels of p27Kip1 expression, whereas the less proliferative typical MCLs generally show more modest levels of p27Kip1 protein. The levels of p27Kip1 detected correlate roughly with the levels of cyclin D1 in typical MCLs because cases with higher cyclin D1 tended to have higher expression of p27Kip1. Immunodepletion studies showed that most of the p27Kip1protein was bound to cyclin D1 and that there was excess of cyclin D1 in relation to p27Kip1. This was true for the typical MCL cases that express moderate levels of p27Kip1 protein, as well as for the cell lines and the variant blastic MCL cases, which express the highest levels of p27Kip1 protein. We suggest that the high levels of cyclin D1 in MCLs and t(11;14) translocated cell lines alter the balance of free and bound p27Kip1, favoring the inactive bound form, and thereby contributing to lymphomagenensis.
p27Kip1 is a CDK inhibitor that plays a critical role in the physiologic process of cell cycle commitment.13 In early G1 the level of free p27Kip1 in the cells is high, but as G1 progresses the accumulating cyclin D1/CDK4 complexes bind to and sequester p27Kip1, effectively lowering the levels of free p27Kip1.1 Of critical importance is the recent information from several laboratories indicating that p27Kip1 does not inhibit the kinase activity of the cyclin D1/CDK4/p27Kip1 complex itself.5,34,35On the contrary, sequestration of p27Kip1 has 2 important positive consequences in stimulating cell cycle progression: (1) the release of its inhibitory effect on cyclin E/CDK2 complexes, which allows the phosphorylation of retinoblastoma (RB) protein to be completed so that progression into S phase may occur, and (2) the stabilization of cyclin D1/CDK4 complexes.5,34,36Recently, it has been shown that the Cip/Kip proteins not only are potent inhibitors of cyclin E– and cyclin A–dependent CDK2, but they also act as positive regulators of cyclin D–dependent kinases.5 In particular, p27Kip1 appears to be necessary for assembly of cyclin D1/CDK4 complexes, and its presence in the complex increases the half-life of cyclin D1 from 10 to 25 minutes.5 Due to the close relationship between p27Kip1 and cyclin D1 in controlling cell cycle commitment, it is perhaps not unexpected that MCL, a neoplasia with constitutive overexpression of cyclin D1, displays perturbations in p27Kip1 homeostasis.
In typical MCL, we found low expression of p27Kip1 protein relative to the more aggressive blastic variant. This is surprising because the vast majority of other lymphoid tumors show an inverse correlation between p27Kip1 expression levels and proliferation rates, and typical MCLs have low to intermediate rates of proliferation, whereas the blastic variants have high proliferative rates. The explanation for this finding may be related to overexpression of cyclin D1 in these lymphomas. In vitro studies have shown that NIH 3T3 fibroblasts that overexpress cyclin D1 stoichiometrically titrate more p27Kip1 into cyclinD1/CDK4 complexes, thereby facilitating the initial activation of cyclin E/CDK2 as cells approach the restriction point. Active cyclin E/CDK2 triggers p27Kip1 ubiquitination and degradation, ensuring the irreversibility of the transition.34,37,38 In further support of this finding, a recent study showed that typical MCLs have increased ubiquitination activity capable of degrading p27Kip1.39 Of note is that despite the increased p27Kip1 degradation in MCL shown by Chiarle et al,39 recent studies have failed to demonstrate any abnormal expression of the F-box protein Skp2 in MCL,40,41which is the only known protein, so far, capable of degrading p27Kip1.16,17 These findings suggest that the down-regulation of p27Kip1 in MCL does not depend only on its degradation by Skp2 and that other posttranscriptional mechanisms influence the regulation of p27Kip1 protein levels.42 Our results, together with these previous studies, are consistent with a 2-step process in which low expression of p27Kip1 in typical MCL results from sequestration of p27Kip1 by overexpressed cyclin D1, followed by increased degradation of p27Kip1 by activated cyclin E/CDK2 complexes.
In the current study we report low but detectable levels of p27Kip1 as assessed by Western blot analysis in all cases of typical MCL studied. The nearly complete lack of p27Kip1protein by IHC in typical MCL cases, as reported in our previous study,19 as well as by others,39 might be due to one of 2 possibilities. A simple explanation would be that the low levels of p27Kip1 expression found in these tumors are below the sensitivity of IHC. A second more interesting possibility is that the antibodies used in this study are unable to recognize p27Kip1 efficiently when complexed with cyclinD1/CDK4. This latter possibility is supported by immunoprecipitation experiments in which we showed that more p27Kip1 could be detected in the cyclin D1+ Granta 519 cell line when the nuclear extracts were denatured with 8 M urea prior to being immunoprecipitated with the C-19 antibody. A similar phenomenon was described recently with 2 antibodies against certain epitopes of p21Cip1 due to conformational changes induced by complex formation with cyclin A/CDK2.33 Clearly, further assessment of this issue is warranted.
In contrast to typical MCL, the blastic variant and the cyclin D1+ cell lines have high expression of p27Kip1protein, despite their high proliferative activity. The vast majority of the p27Kip1 is bound to cyclin D1, as shown in the immunoprecipitation and immunodepletion studies. Our findings are similar to those of Fredersdorf et al,43 who found high levels of p27Kip1 expression in 12 highly proliferative human breast cancer cell lines. Moreover, most of the cell lines that expressed high levels of p27Kip1 also showed the highest levels of cyclin D1. One can speculate that the increased expression of p27Kip1 might reflect a homeostatic feedback mechanism linked to the level of cyclin D1. In addition, the frequent loss of other negative cell cycle regulatory genes (p53, p16INK4a, and p21Cip) in the blastic variant of MCL, shown by our group32 44 might be responsible for further disruption of the normal control of the cell cycle and lead to compensatory increases in p27Kip1 levels.
Inactivation of p27Kip1 through sequestration into cyclin D complexes has been suggested, in at least another lymphoid neoplasia. Sanchez-Beato et al45 reported high levels of p27Kip1 and cyclin D3 expression in a group of high-grade diffuse large B-cell non-Hodgkin lymphomas (DLBLs). Similar to our results, the authors demonstrated by immunoprecipitation studies that all p27Kip1 coprecipitated with cyclin D3, and they were also unable to recover p27Kip1 in CDK2 complexes. However, in contrast to our study, they were able to demonstrate p27Kip1 by IHC, probably reflecting differences in the interaction between cyclin D3 and p27Kip1 from that of cyclin D1 and p27Kip1. Furthermore, it has also been suggested that the functional consequences of these interactions might be different.35
Interestingly, we found that typical MCL cases express very low levels of cyclin A, cyclin E, and CDK2. Because the ubiquitin-dependent degradation of cyclin E is also induced by active CDK2,46 47 it is possible that the same mechanism explains the low expression of both cyclin E/CDK2 complexes and p27Kip1. Alternatively, the high levels of cyclin D1 may result in the down-regulation of cyclin E and cyclin A as a negative, compensatory feedback mechanism. The low levels of cyclin A and cyclin E in typical MCL cases are in contrast with our findings in blastic cases and cell lines, where these 2 cyclins can be highly expressed (eg, cyclin E in NCEB-1 and cyclin A in KMS12). This difference may be a result of further deregulation of cell cycle pathways in the more proliferative MCL variants and cell lines. Due to these important differences, the results of the immunoprecipitation studies in the cell lines must be interpreted with caution and may not accurately reflect the relative distribution of p27Kip1 in primary typical MCL cases.
The immunoprecipitation studies that we performed in both typical MCL cases and in MCL cell lines demonstrate that the majority of cellular p27Kip1 appears to be complexed with cyclin D1. We should note that detectable levels of p27Kip1 were recovered from cyclin A and cyclin E complexes in MCL cell lines that have elevated levels of these cyclins. This suggests that there is a competition for p27Kip1 between various cyclins, but is still consistent with the notion that the abnormally high levels of cyclin D1 in MCLs result in a redistribution of p27Kip1 among different cyclin/CDK complexes. The immunodepletion studies showing an excess of cyclin D1 over p27Kip1 further suggest that high cyclin D1 expression may also act to buffer physiologic increases in cellular p27Kip1, thereby interfering with p27Kip1function in its role as a negative regulator of cell cycle progression. This effect may be particularly important in the early pathogenesis of MCL enabling the t(11;14) translocated cell to escape from physiologic growth controls mediated through p27Kip1.
In summary, our study suggests that one consequence of overexpressed cyclin D1 in MCL is the sequestration of free p27Kip1 into cyclin D1/CDK4 complexes. We hypothesize that this would effectively decrease the active form of p27Kip1 in the cell, reducing the inhibition on cyclin E/CDK2 complexes, facilitating entry into S phase, and potentially contributing to lymphomagenesis.
The authors are grateful to Alan Epstein for providing SUDHL-4, -6, and -10 cell lines; Dr D. Saltman for the NCEB-1 cell line; Takemi Otsuki for the KMS12 cell line; and Martin Dyer for the Granta 519 cell line. Cell lines not mentioned above were obtained from the American Type Cell Culture (ATCC; Rockville, MD). We also thank Ralph L. Isenberg for his photographic assistance.
Prepublished online as Blood First Edition Paper, December 19, 2002; DOI 10.1182/blood-2002-01-0263.
Supported in part by the German Science Fund Wilhelm Sander Sstiftung (L.Q.-M. and F.F.) and Comision Interministerial de Ciencia y Tecnologia (CICYT) SAF 99/20 (E.C.), and by the European Union QLRT-1999-30687.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Mark Raffeld, Hematopathology Section, Laboratory of Pathology, National Cancer Institute, National Institutes of Health, 9000 Rockville Pike, Bethesda, MD 20892; e-mail:mraff@box-m.nih.gov.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal