Abstract
Development of an epitope-based vaccination strategy designed to enhance Epstein-Barr virus (EBV)–specific CD8+cytotoxic T lymphocytes (CTLs) is increasingly being considered as a preferred approach for the treatment of EBV-associated relapsed Hodgkin disease (HD) and nasopharyngeal carcinoma (NPC). EBV-encoded latent membrane proteins, LMP1 and LMP2, are the only target antigens available for therapeutic augmentation of CTL responses in patients with HD and NPC. Here, we describe preclinical studies using a recombinant poxvirus vaccine that encodes a polyepitope protein comprising 6 HLA A2–restricted epitopes derived from LMP1. Human cells infected with this recombinant polyepitope construct were efficiently recognized by LMP1-specific CTL lines from HLA A2 healthy individuals. Furthermore, immunization of HLA A2/Kb mice with this polyepitope vaccine consistently generated strong LMP1-specific CTL responses to 5 of the 6 epitopes, which were readily detected by both ex vivo and in vitro assays. More important, this polyepitope vaccine successfully reversed the outgrowth of LMP1-expressing tumors in HLA A2/Kb mice. These studies provide an important platform for the development of an LMP-based polyepitope vaccine as an immunotherapeutic tool for the treatment of EBV-associated HD and NPC.
Introduction
The concept of a role for the immune system in the control and elimination of virus-infected malignant cells has existed for many years, giving rise to the theory of immunologic surveillance against tumors. More recently, it has been established that tumor rejection is mediated by lymphocytes and, most notably, by cytotoxic T lymphocytes (CTLs). This concept is based mainly on the assumption that, like normal virus-infected cells, tumor cells can present virus-specific epitopes on their surface in conjunction with major histocompatibility complex (MHC) molecules, which can be recognized by CTLs. Indeed, Epstein-Barr virus (EBV)–associated Hodgkin disease (HD) and nasopharyngeal carcinoma (NPC) represent 2 of the classic examples of virus-infected malignancies that are characterized by the expression of EBV nuclear antigen 1 (EBNA1) and latent membrane proteins (LMP) 1 and 2.1,2 Molecular analysis of both NPC and HD have shown that unlike other EBV-associated malignancies, such as Burkitt lymphoma, in vitro–established tumor cell lines from these malignancies are highly susceptible to lysis by EBV-specific CTLs and express normal levels of antigen-processing genes (TAP-1 andTAP-2).3 4
In spite of the highly immunogenic phenotypes associated with these malignancies, tumor cells in NPC and HD can escape CTL-mediated immune control in vivo. A number of possible mechanisms have been proposed to explain this immune evasion strategy. Immunologic and biochemical analysis of LMP1 sequences associated with NPC and HD have shown that these sequences are not only highly oncogenic but also seem to be poorly immunogenic in murine models when compared with the LMP1 sequences derived from normal EBV-infected B cells.5,6Moreover, independent studies by different groups also have shown that Reed-Sternberg cells in HD secrete anti-inflammatory cytokines (such as interleukin 10 [IL10] and tumor growth factor β [TGFβ]), which may interfere with the activation of LMP-specific CTLs.7
It has been proposed that strategies designed to augment LMP1- and/or LMP2-specific CTL responses in HD and NPC patients may provide a therapeutic benefit for these malignancies. Indeed, adoptive transfer of polyclonal EBV-specific autologous CTLs into patients with advanced HD recently has been reported.8 In this study, all patients who received this adoptive therapy had incidental improvement, including reduction of high virus load, increase in virus-specific CTL precursor frequency, resolution of some symptoms, and stabilization of the disease. Unfortunately, all of these patients failed to recover from the advanced stages of HD. One of the major limitations of this approach was that the CTLs transferred in this study were expanded by stimulating the peripheral blood lymphocytes with autologous EBV-transformed lymphoblastoid cell lines (LCLs), which are known to preferentially stimulate T cells specific for EBNA antigens rather than LMP1 and LMP2.9 In spite of these limitations, it was encouraging to see a short-term therapeutic effect, and further improvement of the CTL activation strategy may allow selective expansion of T cells that are specific for a limited range of viral antigens expressed in HD and NPC. In the present study, we have developed a novel strategy based on an LMP1 polyepitope vaccine, which allows an efficient activation of LMP1-specific CTL responses in vivo, and these CTLs in turn provide a strong therapeutic benefit against LMP1-expressing tumors.
Materials and methods
Construction of a recombinant LMP polyepitope expression vector
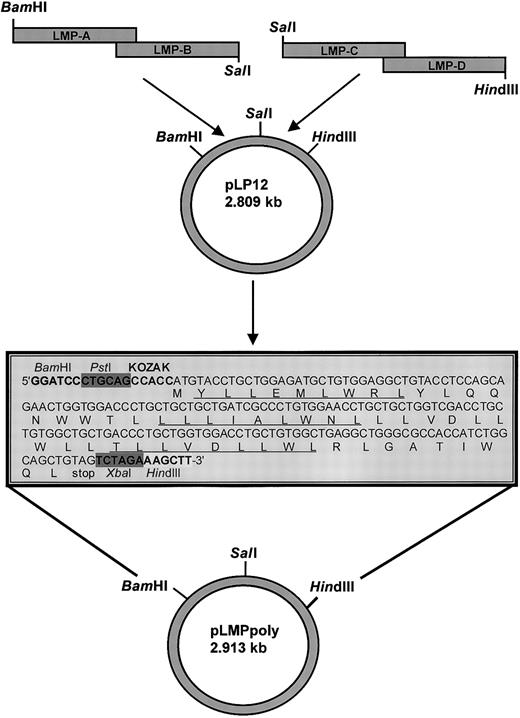
The amino acid sequences of 6 contiguous minimal LMP1 CTL epitopes (Figure 1) were translated to DNA using mammalian frequency codon usage. The resulting LMP polyepitope insert was constructed using 4 overlapping synthetic oligonucleotides: LMP-A 5′-CGCGGATCCCTGCAGGCCACCATGTACCTGCTGGAGATGCTGTGGAGGCTGTACCTC- CAGCAGAACTGGT-3′, LMP-B 5′-CCGGTCGACCAGCAGCAGGTTCCACAGGGCGATGACCAGCAGCA GGGTCCACCAGTTCTGCTGGAGGTA-3′, LMP-C 5′ CCGGTCGACCTGCTGTGGCTGCTGACCCTGCTGGTGGACCTGCTGTGGCTGAGGCTGG-3′, LMP-D 5′-CCCAAGCTTTCTAGACTACAGCTGCCAGATGGTGGCGCCCAGC- CTCAGCCACAGCAGG-3′ (Sigma Genosys, Sydney, Australia). The synthetic fragment included BamHI and PstI restriction sites at the 5′ end, a Kozak consensus sequence, ATG start codon, 6 contiguous minimal LMP1 CTL epitopes, a stop codon, and XbaI andHindIII restriction sites. These oligonucleotides were designed such that both LMP-A and LMP-B, and LMP-C and LMP-D included a complimentary 20–base pair overlap as indicated in Figure 1. The oligonucleotide pairs were annealed and extended using Pfupolymerase (Stratagene, La Jolla, CA). The resulting fragments were cloned sequentially into the BamHI/SalI andSalI/HindIII sites of pUC19, respectively. The resulting full-length LMP polyepitope was subcloned into theBamHI and HindIII sites of the 7.37-kb vaccina virus shuttle vector pTK-7.5A and used to create recombinant vaccinia virus designated as Vacc.polyLMP. We constructed a thymidine kinase–negative (TK−) recombinant virus (Vacc.TK−) using marker rescue recombination as described previously.10
Schematic description of the construction of a recombinant vaccinia virus that expresses a synthetic DNA encoding for a polyepitope protein that contains 6 HLA A2–restricted LMP1 epitopes.
See the box in this figure and Table 1 for details about the LMP1 epitopes. Each of the alternate epitope sequences is underlined. The DNA sequence encoding this polyepitope protein was constructed using epitope sequence–specific primers (referred to as LMP-A, LMP-B, LMP-C, and LMP-D) and a technique based on mutual priming and overlap extension as described in “Materials and methods.” The nucleic acid sequence of the fragment coded (from the 5′ end)BamHI and PstI restriction sites, a Kozak sequence, a methionine start codon, 6 contiguous minimal LMP1 CTL epitopes, a stop codon, and XbaI and HindIII restriction sites at the 3′ end. This DNA insert was subcloned behind the vaccinia p7.5 promoter in the plasmid shuttle vector pTK-7.5A using specific restriction enzymes for the construction of recombinant vaccinia virus.
Schematic description of the construction of a recombinant vaccinia virus that expresses a synthetic DNA encoding for a polyepitope protein that contains 6 HLA A2–restricted LMP1 epitopes.
See the box in this figure and Table 1 for details about the LMP1 epitopes. Each of the alternate epitope sequences is underlined. The DNA sequence encoding this polyepitope protein was constructed using epitope sequence–specific primers (referred to as LMP-A, LMP-B, LMP-C, and LMP-D) and a technique based on mutual priming and overlap extension as described in “Materials and methods.” The nucleic acid sequence of the fragment coded (from the 5′ end)BamHI and PstI restriction sites, a Kozak sequence, a methionine start codon, 6 contiguous minimal LMP1 CTL epitopes, a stop codon, and XbaI and HindIII restriction sites at the 3′ end. This DNA insert was subcloned behind the vaccinia p7.5 promoter in the plasmid shuttle vector pTK-7.5A using specific restriction enzymes for the construction of recombinant vaccinia virus.
Establishment and maintenance of human and mouse cell lines
EBV-transformed LCLs were established from seropositive donors by exogenous virus transformation of peripheral B cells using the B95.8 virus isolate. These cell lines were routinely maintained in RPMI 1640 (Gibco Invitrogen, Carlsbad, CA) supplemented with 2 mM L-glutamine, 100 IU/mL penicillin, and 100 μg/mL streptomycin plus 10% fetal calf serum (FCS) (growth medium). In addition, a mouse thymoma tumor cell line EL4 expressing an HLA A2/Kb chimeric molecule (referred to as EL4-A2/Kb) also was used in this study. These cells were maintained in growth medium supplemented with G418 (500 μg/mL). EL4-A2/ Kb cells were transfected with an expression vector encoding full-length genomic B95.8 LMP1 (pSG5-LMP1; referred to as EL4-A2/Kb-LMP1) and cultured in growth medium supplemented with G418 (500 μg/mL) for 4 to 6 weeks. Expression of LMP1 protein in EL4-A2/ Kb-LMP1 cells was confirmed by immunofluorescence and Western blotting using an LMP1-specific CS1-4 antibody (Dako, Botany, Australia).
Immunization of HLA A2/Kb transgenic mice with an LMP polyepitope vaccine
HLA A2/Kb transgenic mice used in this study have been described elsewhere11 (a kind gift from Dr L. Sherman, Scripps Research Institute). These mice express a chimeric class I molecule composed of the alpha 1 and 2 domains of the human A*0201 allele and the alpha 3 domains of the mouse H-2Kbclass I molecules. These animals were vaccinated intraperitoneally with 5 × 107 plaque-forming units (PFUs) of recombinant vaccinia encoding the LMP polyepitope. After 3 weeks, splenocytes were harvested, and 4 × 106 splenocytes from each mouse were restimulated with syngeneic irradiated (2000 rad) 1 × 106 lipopolysaccharide (LPS) blasts.12The LPS blasts were sensitized with peptide (10 μg/mL for 1 hour at 37°C) and washed twice prior to use. Cells were cultured in growth medium supplemented with 5 × 105 M mercaptoethanol (Sigma, Sydney, Australia). On day 6 the cultures were used as effectors in standard 5-hour 51Cr release assays against EL4-A2/Kb target cells,32 which were sensitized with the indicated peptide (10 μg/mL) at the same time as being radiolabeled and washed twice prior to use. The remaining CTL cultures were restimulated as described above on day 6 and tested for CTL activity again on day 10.
Synthesis of peptides
Peptides, synthesized by the Merrifield solid phase method, were purchased from Chiron Mimotopes (Melbourne, Australia), dissolved in dimethyl sulphoxide, and diluted in serum-free RPMI 1640 medium for use in standard CTL assays. Purity of these peptides were tested by mass spectrometry and showed more than 90% purity.
Assessment of T-cell responses by IFN-γ ELISPOT assay
This assay is based on a modification of the interferon γ (IFN-γ) ELISPOT method described previously.13,14 Ninety-six well-mixed cellulose ester membrane plates (Millipore, Bedford, MA) were coated overnight at 4°C with 75 μL/well of 8 μg/mL anti–IFN-γ, capture monoclonal antibodies (mAbs) (BD PharMingen, Los Angeles, CA; clone R4-6A2) in freshly prepared filter-sterilized 0.1M NaHCO3, pH 8.4. The plates were washed 6 times with phosphate-buffered saline (PBS) prior to blocking for 1 hour at 37°C with PBS containing 5% FCS. Splenocytes were harvested into growth medium supplemented with 5 × 105 M 2-mercaptoethanol. These cells were plated in ELISPOT plates (106/well), and then synthetic peptide epitopes were added into the wells at a final 1 μ/g concentration. For negative controls, splenocytes were incubated without peptide. The plates were incubated at 37°C with 5% CO2 overnight (about 16-20 hours), then washed 3 times with PBST (0.05% Tween-20 in PBS) and 3 times with PBS before addition of 75 μL/well of 1 μg/mL anti–IFN-γ-biotin (PharMingen, La Jolla, CA; clone XMG1.2) and incubated at room temperature for a further 4 hours. After incubation, plates were washed again 3 times each with PBS-Tween (PBST) and PBS, and 100 μL/well of 1 μg/mL streptavidin-alkaline phosphatase conjugate was added and incubated at room temperature for 2 hours. After a final PBS wash, BCIP/NBT (5-bromo, 4-chloro, 3-indoylphosphate/nitroblue tetrazolium) developing/substrate solution (Sigma) was added at 100 μL/well and kept at room temperature until individual IFN-γ–producing cells were detected as dark spots (3-5 minutes). Color development was stopped by thoroughly washing the plates in tap water prior to drying. Spots were counted automatically using an image analysis software (ImagePro)15 and were expressed as spot-forming cells (SFCs) per 106 PBMCs. The number of IFN-γ–secreting T cells was calculated by first correcting for the background by the subtraction of the negative control SFC.
Establishment of human polyclonal CTL lines and LMP1-specific CTL clones
Polyclonal CTL lines and LMP1-specific CTL clones were established according to previously published methods.3Briefly, 2 × 106 PBMCs from 2 HLA A2–positive healthy virus carriers (referred to as donor no. 1 and donor no. 2) were stimulated with 1 × 106 autologous lymphocytes (responder-to-stimulator ratio of 2:1) pulsed with 10 μM peptide for 1 hour. After 3 days, growth medium with IL-2 (10 U/mL) was added, and the cells were further expanded. These lymphocytes were restimulated on day 7 with γ-irradiated (8000 rad) autologous LCLs. After 10 days in culture medium, the cells were used as polyclonal effectors in a standard 51Cr-release assay against peptide-sensitized autologous phytohemagglutinin (PHA) blasts.
To generate peripheral blood CTL clones specific for the LMP1-derived peptides, PBMCs (2 × 106) of healthy donors were reactivated with peptide-sensitized (10 μg of peptide/mL) autologous lymphocytes (1 × 106) in 2-mL wells of a 24-well plate in growth medium. After 3 days, the cells were seeded onto 0.35% low melting gel agarose and maintained in T-cell growth medium containing recombinant IL2 (rIL2) (50 IU/mL). After another 3 days, growing clones were transferred to 96-well round-bottom tissue culture plates (Life Technologies, Sydney, Australia) and cultured in T-cell growth medium containing rIL2 (50-100 IU/mL).
In vitro cytotoxicity assays
Target cells presensitized with synthetic peptide epitopes or infected with recombinant vaccinia encoding the LMP polyepitope were incubated with 51Cr for 90 minutes. Following incubation, these cells were washed in growth medium and used as targets in standard 5-hour 51Cr-release assays.
In vivo cytotoxicity assays
LMP1 epitope-specific in vivo CTL activity was assessed by using carboxyfluorescein diacetate succinimidyl ester (CFSE) dye-labeled (Molecular Probes, Eugene, OR) target cells.16Briefly, HLA A2/Kb splenocyte cell suspensions were divided into 2 populations following red cell lysis. One population was pulsed with an LMP1 epitope (1 μg/mL) for 90 minutes at 37°C, washed in PBS, and labeled with a high concentration (5 μM) of CFSE. Control, uncoated target cells were labeled with a low concentration of CFSE (0.5 μM). Cells (107) of each population were mixed in 200 μL of PBS and injected intravenously into Vacc.polyLMP- or Vacc.TK−-immunized mice. Specific in vivo cytotoxicity was determined by collecting the cells from spleen from recipient mice 18 hours after injection, and the number of cells in each target cell population was determined by flow cytometry. The ratio between the percentages of uncoated versus LMP1 peptide-coated (CFSElow/CFSEhigh) cells was calculated to obtain a numeric value of cytotoxicity.
Tumor challenge and polyepitope immunization
To assess the efficacy of the LMP polyepitope vaccine, 2 different vaccination strategies were used. In the first set of experiments, HLA A2/Kb mice were immunized intraperitoneally with either Vacc.polyLMP or Vacc.TK−(107 PFU/mouse). These mice were challenged subcutaneously with live 107 EL4-A2/Kb-LMP1 cells 3 weeks after the immunization. Following challenge, these animals were regularly monitored for 21 days, and the tumor size measured by a caliper. In the second set of experiments, HLA A2/Kb mice were first challenged with EL4-A2/Kb-LMP1 (107cells/mouse) tumor cells. These mice were immunized with either Vacc.polyLMP or Vacc.TK− 12 days after the challenge, when the tumor size was approximately 0.4 cm in diameter. The therapeutic efficacy of the LMP polyepitope vaccine was assessed by regular monitoring of tumor regression. Any mice showing a tumor size larger than 1.0 cm in diameter were killed according to the guidelines of the institute animal ethics committee.
Results
LMP1-specific CTL lines efficiently recognize target cells infected with a recombinant LMP polyepitope
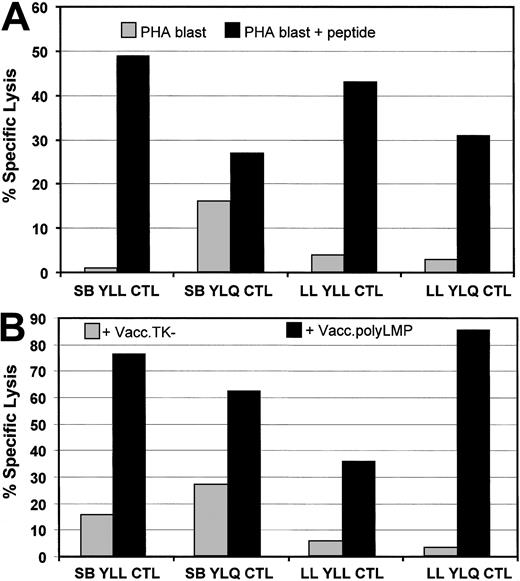
A recombinant LMP polyepitope vaccinia virus (Vacc.polyLMP) encoding 6 different HLA A2–restricted epitopes (Table1) was derived using homologous recombination. To test whether the LMP1 epitopes encoded by this polyepitope were endogenously processed, target cells infected with Vacc.polyLMP were exposed to LMP1-specific CTL polyclonal/clonal lines specific for YLLEMLWRL (referred to as YLL) and YLQQNWWTL (referred to as YLQ) epitopes. These CTL lines were generated from healthy virus carriers, and their specificity was confirmed by their ability to lyse HLA A2–positive target cells coated with respective peptide epitopes (Figure 2A). HLA A2–positive fibroblasts infected with Vacc.polyLMP also were efficiently recognized by YLL- and YLQ-specific CTL lines (Figure 2B). These results clearly show that HLA class I–restricted CTL epitopes included in the LMP polyepitope are efficiently processed and presented to the target cells.
List of HLA A2-restricted LMP1 epitopes included in the polyepitope vaccine
| Epitope sequence . | Epitope code . | EBV antigen . | LMP1 localization . | HLA restriction . | Reference . |
|---|---|---|---|---|---|
| YLLEMLWRL | YLL | LMP1 | aa125-133 | HLA A2 | 3 |
| YLQQNWWTL | YLQ | LMP1 | aa159-167 | HLA A2 | 3 |
| TLLVDLLWL | TLL | LMP1 | aa166-174 | HLA A2 | 3 |
| LLVDLLWLL | LLV | LMP1 | aa167-175 | HLA A2 | 3 |
| LLLIALWNL | LLL | LMP1 | aa92-100 | HLA A2 | 3 |
| RLGATIWQL | RLG | LMP1 | aa132-140 | HLA A2 | 3 |
| Epitope sequence . | Epitope code . | EBV antigen . | LMP1 localization . | HLA restriction . | Reference . |
|---|---|---|---|---|---|
| YLLEMLWRL | YLL | LMP1 | aa125-133 | HLA A2 | 3 |
| YLQQNWWTL | YLQ | LMP1 | aa159-167 | HLA A2 | 3 |
| TLLVDLLWL | TLL | LMP1 | aa166-174 | HLA A2 | 3 |
| LLVDLLWLL | LLV | LMP1 | aa167-175 | HLA A2 | 3 |
| LLLIALWNL | LLL | LMP1 | aa92-100 | HLA A2 | 3 |
| RLGATIWQL | RLG | LMP1 | aa132-140 | HLA A2 | 3 |
Endogenous processing of LMP1 CTL epitopes encoded by Vacc.polyLMP.
(A) LMP1 epitope-specific lysis by YLL- and YLQ-specific CTL lines derived from 2 healthy virus carriers (donor no. 1 and donor no. 2). Peptide-sensitized and uncoated HLA A2–positive PHA blasts were used as target cells in the CTL assay. (B) HLA A2–positive fibroblasts were infected with either Vacc.polyLMP or Vacc.TK− for 18 hours and then exposed to YLL- and YLQ-specific CTL lines from donors donor no. 1 and donor no. 2. An effector-target ratio of 10:1 was used for both assays.
Endogenous processing of LMP1 CTL epitopes encoded by Vacc.polyLMP.
(A) LMP1 epitope-specific lysis by YLL- and YLQ-specific CTL lines derived from 2 healthy virus carriers (donor no. 1 and donor no. 2). Peptide-sensitized and uncoated HLA A2–positive PHA blasts were used as target cells in the CTL assay. (B) HLA A2–positive fibroblasts were infected with either Vacc.polyLMP or Vacc.TK− for 18 hours and then exposed to YLL- and YLQ-specific CTL lines from donors donor no. 1 and donor no. 2. An effector-target ratio of 10:1 was used for both assays.
Generation of LMP1-specific CTL responses in HLA A2/Kb mice vaccinated with a LMP polyepitope
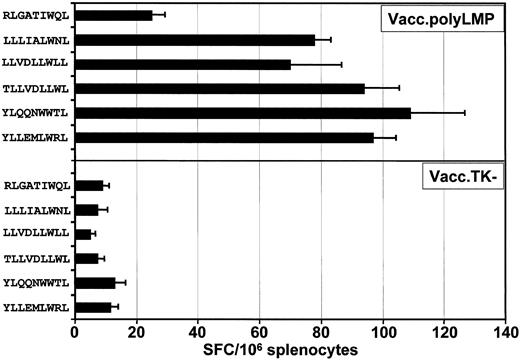
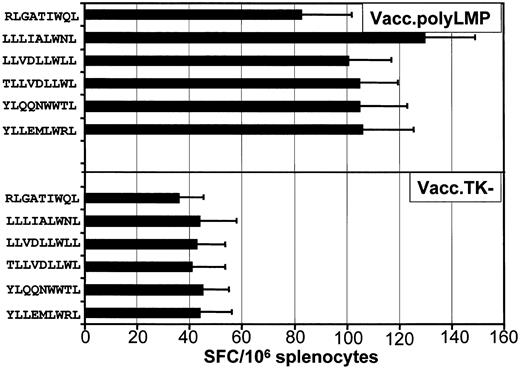
To determine whether the LMP polyepitope construct was capable of raising CTL responses in vivo, HLA A2/Kb transgenic mice were vaccinated with either the Vacc.polyLMP or Vacc.TK−, and 21 days following immunization, CTL responses to each of the 6 epitopes were assessed. Three different methods were used to assess T-cell responses. In the first set of experiments, ex vivo T-cell reactivity to each of the peptide epitopes was assessed by ELISPOT technology. A minimum of 6 animals was assessed in each group. Splenocytes from these mice were used as responder cells for the detection of epitope-responsive T cells. Data presented in Figure3 clearly show that all mice consistently responded to 5 (YLL, YLQ, TLL, LLV, and LLL) of the 6 LMP1 CTL epitopes included in the polyepitope vaccine. On the other hand, low levels of T-cell response to RLG epitope were detected (Figure 3).
Ex vivo functional analysis of LMP1 epitope-specific T cells following immunization with Vacc.polyLMP.
HLA A2/Kb mice were immunized intraperitoneally with 107PFU/mouse (Vacc.polyLMP or Vacc.TK−), and 21 days after immunization, LMP1 epitope-specific reactivity was assessed in the splenocytes by ELISPOT assays as described in “Materials and methods.” A minimum of 6 mice from each group were assessed for LMP1-specific T-cell reactivity. The results are expressed as mean ± SE of spot-forming cells (SFCs) per 106splenocytes.
Ex vivo functional analysis of LMP1 epitope-specific T cells following immunization with Vacc.polyLMP.
HLA A2/Kb mice were immunized intraperitoneally with 107PFU/mouse (Vacc.polyLMP or Vacc.TK−), and 21 days after immunization, LMP1 epitope-specific reactivity was assessed in the splenocytes by ELISPOT assays as described in “Materials and methods.” A minimum of 6 mice from each group were assessed for LMP1-specific T-cell reactivity. The results are expressed as mean ± SE of spot-forming cells (SFCs) per 106splenocytes.
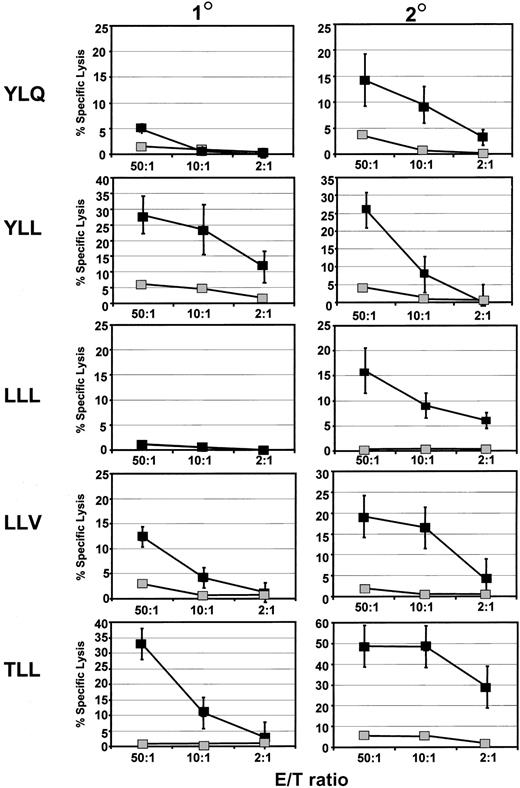
In the next set of experiments, these precursor T cells were stimulated in vitro to determine whether epitope-specific CTL effectors could be expanded following immunization with the LMP polyepitope. Representative data from a group of 6 animals are shown in Figure4. Following a single stimulation with peptide-sensitized LPS blasts, strong CTL responses to 2 epitopes (YLL and TLL) were detected, while a low-to-moderate response to the LLV epitope was detected. No epitope-specific lysis was detected for LLL and YLQ epitopes. However, a significant increase in the levels of CTL lysis was observed following secondary stimulation with peptide-sensitized LPS blasts. It is important to mention here that no CTL effectors were expanded following stimulation with the RLG peptide. These observations are consistent with our ELISPOT data presented in Figure 3.
Activation of LMP1 epitope-specific CTL lines from HLA A2/Kb mice immunized with Vacc.ployLMP.
CTL reactivity specific for LMP epitopes (YLQ, YLL, LLL, LLV, and TLL) was assessed in splenocytes of Vacc.polyLMP immunized mice following primary (1°) and secondary (2°) stimulation with indicated peptide epitope sensitized LPS blasts. These T-cell lines were used as effectors against EL4 A2/Kb cells sensitized with the same peptide (▪) or no peptide (░). Mean lysis values (±SE) for each group of mice are presented. A minimum of 6 animals was assessed for each epitope.
Activation of LMP1 epitope-specific CTL lines from HLA A2/Kb mice immunized with Vacc.ployLMP.
CTL reactivity specific for LMP epitopes (YLQ, YLL, LLL, LLV, and TLL) was assessed in splenocytes of Vacc.polyLMP immunized mice following primary (1°) and secondary (2°) stimulation with indicated peptide epitope sensitized LPS blasts. These T-cell lines were used as effectors against EL4 A2/Kb cells sensitized with the same peptide (▪) or no peptide (░). Mean lysis values (±SE) for each group of mice are presented. A minimum of 6 animals was assessed for each epitope.
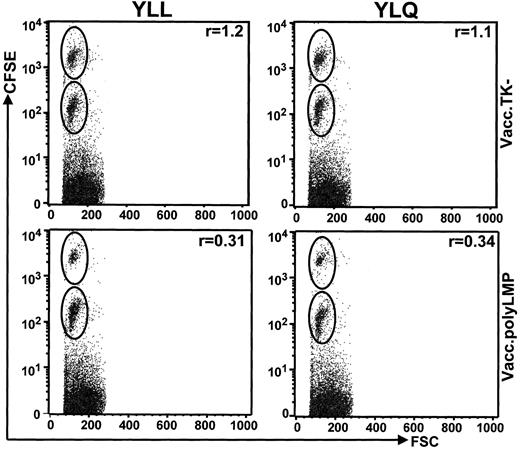
Finally, LMP1 epitope-specific CTL activity was observed in Vacc.polyLMP-immunized mice using an in vivo cytotoxicity assay to monitor depletion of target cells labeled with immunogenic peptide and CFSE dye. Vacc.polyLMP and Vacc.TK− mice were inoculated with CFSE-labeled target cells coated with LMP epitopes (1 μg/mL), and clearance of these cells was compared with that of targets labeled with CFSE but no peptide. The ratio (r) of the percentage of unlabeled cells and the percentage of epitope-coated cells represents the relative cytotoxic CD8+ CTL activities. Data presented in Figure 5 clearly demonstrate that Vacc.polyLMP-immunized mice cleared the great majority of peptide-coated target cells, while uncoated cells were not lysed. On the other hand, Vacc.TK−-immunized mice showed minimal difference in the clearance of peptide-coated and uncoated target cells. Taken together these analyses clearly demonstrate that immunization with the Vacc.polyLMP vaccine can efficiently generate CTL responses to LMP1 epitopes and that these T cells display strong lytic activity against peptide-sensitized HLA A2–positive target cells.
Vacc.polyLMP immunization generates strong CTL activity in vivo.
Vacc.polyLMP or Vacc.TK−-immunized mice were inoculated with CFSE-labeled uncoated and peptide-sensitized (YLL or YLQ) splenocytes at 21 days after immunization. The clearance of these cells was monitored 18 hours after inoculation by FACScalibur. Ratios in the top right corner of each plot represent the percentage of peptide-pulsed, high CFSE-labeled cells to the percentage of low CFSE-labeled uncoated splenocytes retrieved from inoculated mice. A ratio of 1 indicates little or no clearance of peptide-labeled cells. Figures are representative values from individual mouse from a group of 4 mice. Data are representative of 2 independent experiments.
Vacc.polyLMP immunization generates strong CTL activity in vivo.
Vacc.polyLMP or Vacc.TK−-immunized mice were inoculated with CFSE-labeled uncoated and peptide-sensitized (YLL or YLQ) splenocytes at 21 days after immunization. The clearance of these cells was monitored 18 hours after inoculation by FACScalibur. Ratios in the top right corner of each plot represent the percentage of peptide-pulsed, high CFSE-labeled cells to the percentage of low CFSE-labeled uncoated splenocytes retrieved from inoculated mice. A ratio of 1 indicates little or no clearance of peptide-labeled cells. Figures are representative values from individual mouse from a group of 4 mice. Data are representative of 2 independent experiments.
Immunization with Vacc.polyLMP affords protection against LMP1-expressing tumors
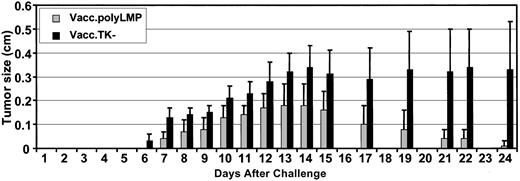
To test whether the Vacc.polyLMP vaccine-induced T-cell responses can afford protection against LMP1-expressing tumor cells, 2 groups of HLA A2/Kb mice (10 mice in each group) were first immunized with Vacc.polyLMP or Vacc.TK− and then challenged with EL4-A2/Kb-LMP1 cells. These mice were regularly monitored for tumor outgrowth. Although both groups of animals developed tumors, the tumor outgrowth in Vacc.TK− was highly aggressive and showed no evidence of protection from tumor challenge (Figure6). On the other hand, these tumors grew much less aggressively in animals immunized with Vacc.polyLMP, and this outgrowth was completely resolved in 90% of the animals by the end of the observation period. By day 24, the average tumor load in Vacc.polyLMP-immunized mice was 30- to 33-fold lower when compared with Vacc.TK−-immunized mice (Figure 6). It is important to mention here that animals immunized with Vacc.TK− or Vacc.polyLMP showed no protection against challenge with EL4/A2Kb cells, indicating that the epitope-specific immune response was critical for this protection (data not shown).
Immunization with Vacc.polyLMP affords protection against LMP1-expressing EL4-A2/kb tumor cells.
Two groups of 10 mice were immunized with Vacc.polyLMP or Vacc.TK− (107 PFU/mouse), respectively. Twenty-one days after immunization, mice were challenged subcutaneously with EL4-A2/Kb-LMP1 cells (107 cell/mouse) and monitored for tumor size for 24 days after challenge. Data are presented as mean ± SE of tumor size.
Immunization with Vacc.polyLMP affords protection against LMP1-expressing EL4-A2/kb tumor cells.
Two groups of 10 mice were immunized with Vacc.polyLMP or Vacc.TK− (107 PFU/mouse), respectively. Twenty-one days after immunization, mice were challenged subcutaneously with EL4-A2/Kb-LMP1 cells (107 cell/mouse) and monitored for tumor size for 24 days after challenge. Data are presented as mean ± SE of tumor size.
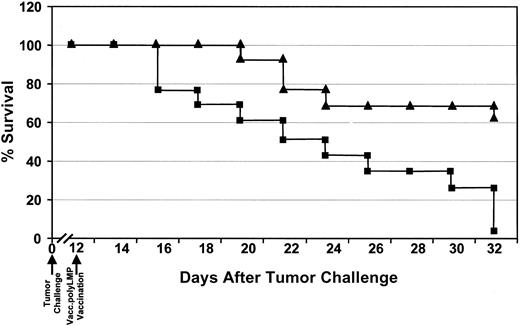
The use of the LMP1 polyepitope vaccine as a therapeutic tool for the treatment of an actively proliferating tumor is one of the major challenges for its translation for human use. To explore the therapeutic efficacy of the polyepitope vaccine, a group of 40 HLA A2/Kb mice were challenged subcutaneously with 1 × 107EL4-A2/Kb-LMP1 cells and monitored for tumor load. On day 12, when the tumor size was approximately 0.4 cm in diameter, mice were divided into 2 groups and immunized with Vacc.TK− (20 mice) or Vacc.polyLMP (20 mice). Following immunization, the tumor size was regularly measured, and any mice showing a tumor size larger than 1.0 cm in diameter were killed. Representative data from one such therapeutic vaccination is shown in Figure7. The tumor size progressively increased in almost all animals immunized with Vacc.TK−, and by day 20 after immunization (day 32 after tumor challenge), almost 100% were dead. In contrast, a dramatic reduction in the tumor load was observed in mice immunized with Vacc.polyLMP, and more than 60% of the mice were completely tumor free by day 20 after immunization and showed long-term protection. Moreover, the average tumor load in Vacc.polyLMP-treated mice was significantly lower when compared with the Vacc.TK−-vaccinated mice (data not shown).
Regression of LMP1-expressing tumors in HLA A2/Kb mice following therapeutic immunization with Vacc.polyLMP.
HLA A2/Kb mice were challenged with 107EL4-A2/Kb-LMP1 tumor cells. Twelve days after the challenge, when the tumor size was approximately 0.4 cm in diameter, these mice were immunized with either Vacc.polyLMP (▴) or Vacc.TK− (▪). The therapeutic efficacy of the LMP polyepitope vaccine was assessed by regular monitoring of tumor regression. Any mice showing tumor size larger than 1.0 cm in diameter were killed according to the guidelines of the ethics committee. Data are presented as percentage of mice surviving after immunization with Vacc.polyLMP or Vacc.TK−.
Regression of LMP1-expressing tumors in HLA A2/Kb mice following therapeutic immunization with Vacc.polyLMP.
HLA A2/Kb mice were challenged with 107EL4-A2/Kb-LMP1 tumor cells. Twelve days after the challenge, when the tumor size was approximately 0.4 cm in diameter, these mice were immunized with either Vacc.polyLMP (▴) or Vacc.TK− (▪). The therapeutic efficacy of the LMP polyepitope vaccine was assessed by regular monitoring of tumor regression. Any mice showing tumor size larger than 1.0 cm in diameter were killed according to the guidelines of the ethics committee. Data are presented as percentage of mice surviving after immunization with Vacc.polyLMP or Vacc.TK−.
Comparative analysis of T-cell responses to LMP1 epitopes in HLA A2/Kb mice following tumor challenge
To determine whether the CTL responses in vivo correlated with the tumor protection, LMP1-specific T-cell responses were assessed following tumor challenge. Data from one such analysis is presented in Figure 8. All animals immunized with Vacc.polyLMP vaccine and showing complete protection from tumor challenge demonstrated strong ex vivo LMP1 epitope–specific CTL responses. One of the interesting aspects of this result was that a significant increase in the T-cell response to the RLGATIWQL epitope was observed following tumor challenge. Our initial studies (Figure 3) had indicated that very low levels of CTL responses are generated to RLGATIWQL epitope following immunization with Vacc.polyLMP vaccine. Furthermore, the levels of T-cell responses to the LMP1 epitopes in individual mice showed strong correlation with the tumor load on day 21. Those mice with higher precursor frequency for LMP1 epitopes showed complete resolution of tumor, whereas small tumors were evident in those mice that had lower precursor frequency. These observations clearly indicate that a strong T-cell response to LMP1 is crucial for a successful rejection of the LMP-expressing tumors. This contention is further supported by lack of tumor protection in Vacc.TK−-immunized mice, which showed 2- to 2.5-fold lower LMP1 epitope–specific T-cell responses following tumor challenge.
Comparative analysis of T-cell responses to LMP1 epitopes in HLA A2/Kb mice following tumor challenge.
LMP1 epitope–specific reactivity was assessed in the splenocytes by ELISPOT assays as described in “Materials and methods.” A minimum of 6 mice from each group were assessed for LMP1-specific T-cell reactivity. The results are expressed as mean ± SE of spot-forming cells (SFC) per 106splenocytes.
Comparative analysis of T-cell responses to LMP1 epitopes in HLA A2/Kb mice following tumor challenge.
LMP1 epitope–specific reactivity was assessed in the splenocytes by ELISPOT assays as described in “Materials and methods.” A minimum of 6 mice from each group were assessed for LMP1-specific T-cell reactivity. The results are expressed as mean ± SE of spot-forming cells (SFC) per 106splenocytes.
Discussion
Over the past several decades, the goal of curing the majority of patients with primary HD and NPC has been reached. In the past 3 decades, 2 powerful tools, irradiation and multiagent chemotherapy, have emerged as the mainstays of modern treatment.17 Although both these therapeutic strategies are most efficient in eradicating a proportion of tumors, the nonspecific nature of these treatments often results in significant side effects, including long-term toxicities, development of secondary cancers, and infectious complications.18 Moreover, a small but significant proportion of HD and NPC patients relapse following chemotherapy and radiotherapy and also fail to respond to conventional therapeutic salvage strategies. More recently, there has been an increasing emphasis on the development of novel therapeutic strategies, which are specifically designed to prime the patient's own immune system to recognize EBV antigens expressed in malignant cells of HD and NPC and to specifically destroy these cells with minimal or no associated toxicities.19 20
The present study illustrates the possibility of using multiple HLA class I–restricted LMP1 CTL epitopes as a polyepitope vaccine for the treatment of EBV-associated HD and NPC. The LMP polyepitope vaccine-induced LMP1 epitope-specific CTLs of multiple specificities in HLA-A2/Kb transgenic mice and the epitopes encoded by the recombinant virus were recognized by CTL lines from HLA A2–positive healthy seropositive donors. These observations indicate that 5 of the 6 epitopes encoded by the polyepitope construct are efficiently processed and presented by antigen-presenting cells. It is important to stress here that the polyepitope vaccine technology also overcomes the potential limitation of the use of LMP1 protein as a vaccine for the induction of antigen-specific T cells. It is now firmly established that expression of LMP1 protein alone can transform normal cells and initiate the oncogenic process. Moreover, the transmembrane localization of LMP1 restricts its accessibility to the cytosolic degradation pathways and thus limits its presentation through the classic class I pathway. Previous studies have shown that a polyepitope protein is highly unstable and is rapidly degraded through the proteasome pathway, thus allowing more efficient presentation of all the epitopes encoded within this recombinant protein.
The therapeutic efficacy of the polyepitope vaccine was assessed using a quasi-HD/NPC tumor model in HLA A2/Kb transgenic mice. This tumor model was based on the EL4-A2/Kb cells, which express the LMP1 oncogene as a transgene. Subcutaneous injection of these tumor cells (1 × 107 cells/mice) consistently resulted in tumor outgrowth in more than 95% of the HLA A2/Kb mice. Prior immunization of HLA A2/Kb mice with the LMP polyepitope vaccine provided a high degree of protection, and the tumor outgrowth was significantly reduced when compared with the TK− mice. Ex vivo CTL analysis indicated that this protection was coincident with the generation of strong LMP1 epitope–specific responses. More importantly, the LMP1 polyepitope vaccine was not only efficient as a prophylactic therapy but also was used successfully to reverse the outgrowth of pre-existing tumors. Immunization of tumor-bearing HLA A2/Kb mice dramatically arrested the tumor outgrowth, and by day 32 after tumor challenge, more than 60% of the mice showed complete resolution of tumor outgrowth. On the other hand, immunization with Vacc.TK− failed to arrest the tumor outgrowth, and 100% mice were dead by day 32 after tumor challenge. These results further confirmed the efficacy of the LMP polyepitope vaccine for the treatment of LMP1-expressing tumors. It is important to stress here that although the tumor model used in this study displays immunologic phenotype similar to that seen in HD and NPC, a proper validation of polyepitope vaccine approach will require a formal clinical trial in human patients.
The data presented here provide an important platform for the future development of immunotherapeutic strategies for the treatment of EBV-associated relapsed HD and NPC. Although HLA A2 is one of most common HLA class I alleles, the wider application of LMP-based polyepitope technology will require the inclusion of CTL epitopes restricted through other HLA class I alleles prevalent in NPC endemic regions of the world (HLA A11, A24, B27, and B57). Moreover, inclusion of additional epitopes from LMP1 and LMP2 also will allow the targeting of both antigens. We anticipate that the translation of polyepitope vaccine technology for human application will require other delivery modalities that are more likely to be approved by safety and human ethics committees. These include replication deficient adenovirus, poxvirus (Modified Vaccinia Ankara) vectors, naked DNA, or transduced autologous dendritic cells.21-23 Considering the highly immunosuppressive nature of malignant cells of HD, it is possible that LMP1 and LMP2 CTL induction might need to be facilitated by codelivery of cytokines and/or prime boost strategies.24
Prepublished online as Blood First Edition Paper, December 5, 2002; DOI 10.1182/blood-2002-10-3092.
J.D. and M.S. contributed equally to this work, and their order should be considered arbitrary.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Rajiv Khanna, Queensland Institute of Medical Research, Bancroft Centre, 300 Herston Rd, Brisbane, Australia 4029; e-mail: rajivk@qimr.edu.au.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal