Abstract
We report the detection of an interleukin-4 (IL-4) variant whose expression is tightly associated with deprivation apoptosis. It is detected with the 8D4 anti–IL-4 monoclonal antibody (mAb) not only in T helper-2 (Th2) but also in Th1 clones, and primary T cells, and it is a nonsecreted molecule. It is not expressed during primary necrosis. Our data suggest that de novo IL-4 transcription of an alternative IL-4 mRNA (IL-4δ13) is induced during deprivation apoptosis. In HIV-infected patients, increased expression of IL-4 in T cells is highly correlated to increased apoptosis, restricted to 8D4 reactivity (r2 = 0.84 between % 8D4-8+ and % 7- amino-actinomycin D–positive [7-AAD+] peripheral T cells, P < .0001), and associated with disease progression. The particular reactivity of apoptotic T cells to 8D4 mAb may explain some discordances among studies analyzing the Th1/Th2 balance in HIV infection and questions the function of this intracellular type 2 signal.
Introduction
The cytokine network and the apoptotic pathway exhibit multiple regulatory interactions. Ligands from the tumor necrosis factor receptor (TNF-R) family induce death receptor–dependent apoptosis that down-regulates clonal expansion following antigen-dependent T-cell receptor activation,1and, within T cells, T helper–1 (Th1) and Th2 cell subsets are differentially susceptible to activation-induced cell death (AICD).2-4 Growth factor deprivation apoptosis in previously activated cells is related to the defect of signaling via the interleukin-2 receptor γ chain by cytokines such as IL-2, IL-4, IL-7, IL-9, or IL-15, and it is associated with the down-regulation of antiapoptotic homologues of Bcl-2.5 In HIV-infected persons, both death receptor–induced apoptosis and deprivation apoptosis are increased, and it has been suggested that the apoptosis contributes to T-cell deletion associated with this disease.6-8 Alteration in Th1/Th2 balance has been suggested to contribute to progression to AIDS.9,10 In particular, increase in IL-4 expression in patients' peripheral blood mononuclear cells (PBMCs) was reported by several groups,11,12 but this observation is still controversial.4,13 14 In this study, we have identified a nonsecreted variant of IL-4 whose expression is tightly associated with deprivation apoptosis, including in patients' PBMCs, and our data suggest that this variant is the major form detected in HIV-infected patients.
Study design
Cells and antibodies
PBMCs were obtained from either healthy donors or chronically HIV-infected patients who were untreated or receiving bitherapy (ie, 2 reverse-transcriptase inhibitors). CD4+ Th1 and Th2 cell clones were derived from HIV+ patients and characterized for polarized interferon γ (IFNγ) or IL-4 production in enzyme-linked immunosorbent assays (ELISAs) (M.F., S. Le Borgne, C. Marty, A. Tallarmin, Y. Riviere, submitted manuscript). Lymphoblastic T-cell lines MT4 and CEM13 and monocytic U937 cell line were also used. Cell surface and cytokine-specific monoclonal antibodies (mAbs) included the following: anti-CD3 (clone SK7, Becton Dickinson, Pont de Claix, France); anti-IFNγ (clone B27); anti–IL-4 mAbs (clones MP4-25D2 and 8D4-8; Pharmingen, La Jolla, CA). Recombinant IL-4 was from R&D Systems (Abingdon, United Kingdom).
Apoptosis induction and detection
Deprivation apoptosis was induced in patients' PBMCs by 24-hour incubation in culture medium,7 and in cell lines by 5-day culture without renewal of culture medium. AICD was induced in PBMCs by 16-hour polyclonal stimulation with 50 ng/mL phorbol myristate acetate (PMA) and 300 ng/mL ionomycin in the presence of 10 μg/mL brefeldin A (BFA; Sigma-Aldrich, France) added during the last 12 hours of the culture to inhibit protein secretion.3 Primary necrosis was induced by 15-minute hyperthermia at 56°C.15 To simultaneously detect apoptosis and cytokine synthesis, cultured cells were first costained with fluorescein isothiocyanate (FITC)–conjugated anti-CD3 mAb and 20 μg/mL nuclear dye 7-amino-actinomycin D (7-AAD; St. Quentin-Fallavier, Sigma-Aldrich) for 30 minutes at 4°C, as described.3 Then, intracellular cytokine staining was performed after cell fixation with 1% paraformaldehyde for 15 minutes at 4°C and permeabilization in saponin buffer. Flow cytometry analysis was performed on a FacsCalibur flow cytometer (Becton Dickinson, Oxford, United Kingdom) with Cell Quest software (Becton Dickinson).3
IL-4 detection
Several methods were used to detect IL-4 expression, including intracellular cytokine staining by fluorescence-activated cell-sorter (FACS) analysis (above) or confocal microscopy, ELISA, and reverse transcriptase–polymerase chain reaction (RT-PCR). IL-4 detection by ELISA was performed using the EASIA kit (Medgenix, Brussels, Belgium) on culture supernatants harvested after 16-hour culture of 106 PBMCs/mL either in medium or in the presence of PMA+ionomycin. For IL-4 detection in lysates, whole-cell lysates were obtained from cell pellets treated with 60 mM phenylmethylsulfonyl fluoride (PMSF) protease inhibitor (Roche Molecular Biochemicals, Indianapolis, IN) after one cycle of freezing at −80°C and 4 pulses of sonification followed by 15-minute 50 000-rpm centrifugation. Samples were stored at −20°C before analysis. For confocal microscopy analysis, intracellular IL-4 staining was performed with unlabeled mAb (either 8D4 or MP4-25D2) followed by 1:200 diluted cyanine 3 (Cy3)–conjugated antimouse mAb or rhodamine (RD)–conjugated antirat mAb (Amersham, Orsay, France), respectively. Stained cells were washed, and mounted in 133 mg/mL Mowiol (Aventis, Strasbourg, France), 33% glycerol, and 133 mM Tris (tris(hydroxymethyl)aminomethane) HCl (pH 8.5). Series of optical sections at 0.7-μm intervals were recorded using a Leica TCS4D microscope (Leica, Bensheim, Germany) and analyzed with the Adobe Photoshop software (San Jose, CA). For analysis of the size and relative expression of IL-4 mRNA, total RNA was extracted from 106 to 107 PBMCs with TRIZOL (Gibco-BRL, Cergy-Pontoise, France). Single-strand cDNA was synthesized and amplified, using the RNA PCR Core Kit (Perkin Elmer, Boston, MA), by 35 cycles of PCR (annealing temperature, 64°C) with a couple of IL-4–specific primers targeted on exon 1 in 5′ (ATGGGTCTCACCTCCCAACTGCT) and on exon 4 in 3′ (CGAACACTTTGAATATTTCTCTCTCAT). Then, 4 μL of RT-PCR products were submitted to one cycle of elongation in the presence of a fluorescent FAM-labeled 5′ primer. After migration, analysis of the run-off products was performed using Applied Biosystem 373A DNA sequencer (Foster City, CA) and the Immunoscope software (Institut Pasteur, Paris, France).16
Statistical analyses
Nonparametric tests (Mann-Whitney U test and Spearman regression analysis) were used. A P value less than .05 was considered significant.
Results and discussion
Following polyclonal stimulation in the presence of BFA, Th1 and Th2 clones were intracellularly costained with IFNγ mAb and either MP4 or 8D4 IL-4-specific mAbs. Figure 1A shows that the Th1 clone expressed IFNγ but not IL-4, detected by MP4 mAb, whereas the Th2 clone was highly stained by IL-4 MP4 mAb and not stained by IFNγ mAb (Figure 1B). Surprisingly different patterns were obtained when IL-4-specific 8D4 mAb was used. Indeed, this mAb stained the Th2 clone (Figure 1B), but also recognized the Th1 clone (Figure 1A). The specificity of this staining was shown following preincubation of cells with recombinant IL-4 (Figure 1A-B). Confocal microscopy analysis of the Th2 clone intracellularly stained with either MP4 or 8D4 anti–IL-4 mAbs revealed different staining patterns: MP4 mAb induced a cytoplasmic staining, and in some sections a typical secretion cone was observed. In contrast, 8D4 mAb induced a more diffuse intracellular staining that persisted in the absence of BFA (Figure 1B). This was confirmed by flow cytometry: in the presence of BFA, 2 populations were detected, a double-positive MP4+8D4+ population and cells stained with only 8D4 mAb, the latter persisting in the absence of BFA while the former disappeared (Figure 1B, bottom panel). In the absence of activation, no MP4 staining was observed on this clone (data not shown). Altogether, these observations suggest that the 8D4 mAb recognizes a nonsecreted form of IL-4.
Detection of nonsecreted IL-4 in Th1 and Th2 clones using 8D4-8 mAb.
T-cell clones were stimulated for 5 hours by 50 ng/mL PMA and 300 ng/mL ionomycin. (A) Dual intracellular stainings of a Th1 clone using anti-IFNγ mAb and MP4 or 8D4 anti–IL-4 mAb. The right panel shows specific inhibition of 8D4 IL-4 staining by preincubation of 8D4-8 anti–IL-4 mAb with 0.1 μg/mL recombinant IL-4. (B) In the top panels, the same experiments were performed on the Th2 clone. Bottom panels, left: T-cell clones were stimulated in the presence or absence of BFA and further stained with MP4 and 8D4 anti–IL-4 mAb. Bottom panel, right: confocal analysis of MP4 or 8D4 anti–IL-4 mAb staining on the Th2 clone, after stimulation in the presence or absence of BFA. The arrow indicates a cytoplasmic cone of secretion. (C) Determination on the Th2 clone of the susceptibility to apoptosis of MP4+ and 8D4+ cells using the nuclear dye 7-AAD.
Detection of nonsecreted IL-4 in Th1 and Th2 clones using 8D4-8 mAb.
T-cell clones were stimulated for 5 hours by 50 ng/mL PMA and 300 ng/mL ionomycin. (A) Dual intracellular stainings of a Th1 clone using anti-IFNγ mAb and MP4 or 8D4 anti–IL-4 mAb. The right panel shows specific inhibition of 8D4 IL-4 staining by preincubation of 8D4-8 anti–IL-4 mAb with 0.1 μg/mL recombinant IL-4. (B) In the top panels, the same experiments were performed on the Th2 clone. Bottom panels, left: T-cell clones were stimulated in the presence or absence of BFA and further stained with MP4 and 8D4 anti–IL-4 mAb. Bottom panel, right: confocal analysis of MP4 or 8D4 anti–IL-4 mAb staining on the Th2 clone, after stimulation in the presence or absence of BFA. The arrow indicates a cytoplasmic cone of secretion. (C) Determination on the Th2 clone of the susceptibility to apoptosis of MP4+ and 8D4+ cells using the nuclear dye 7-AAD.
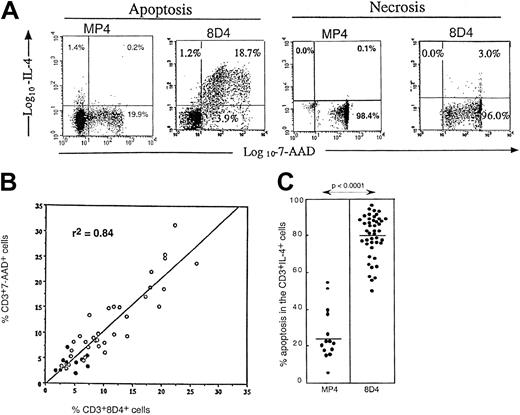
While studying the relationship between intracellular cytokine expression and deprivation apoptosis in the Th clones, we found that MP4-reactive cells were mostly living cells, whereas 8D4-reactive cells were apoptotic (7-AAD+) in the Th2 clone (Figure 1C). The association between 8D4 reactivity and apoptosis was found in all cell types analyzed including Th1 clones, T cells, and monocytic cell lines. For example, we tested MT4, CEM13, and U937 cell lines and observed that none was stained with MP4 mAb, though all were stained with 8D4 mAb (60.3%, 69.3%, and 98.0%, respectively) when apoptosis was induced by medium impoverishment and measured by 7-AAD staining (74.0%, 46.8%, and 100.0%, respectively). As recently reported by Stein et al, various tumor cell lines also express intracellular 8D4 mAb signal when dying from apoptosis.17 We also observed this association between 8D4 reactivity and deprivation apoptosis on peripheral blood CD3 T cells, and a strong correlation (P < .0001) was found between the percentage of 8D4-8+ and 7-AAD+ peripheral T cells following overnight incubation in medium (Figure2B). PBMCs reacted only weakly with MP4 mAb both in HIV+ patients (18.8 ± 8.3% 8D4 reactive cells vs < 1% MP4 reactive cells, n = 35) and in healthy donors (5.6 ± 1.9% 8D4 reactive cells vs < 1% MP4 reactive cells, n = 10), but a significant difference was observed in patients versus controls for 8D4 reactivity (P < .0005) (Figure 2B). 8D4 reactivity was not associated with primary necrosis (Figure 2A). Following PMA+ionomycin stimulation, expression of IL-4 detected with 8D4 mAb was still strongly associated with apoptosis, whereas that detected with MP4 mAb was mainly associated with living cells (Figure 2C). 8D4 expression in T cells was inversely correlated with ex vivo CD4 T-cell counts (r = −0.56,P < .0002, n = 40) and positively correlated with the viral load (r = 0.39, P < .03, n = 34). The particular reactivity of patients' apoptotic T cells to 8D4 mAb may explain some discordances reported in studies analyzing the Th1/Th2 balance in HIV infection. Indeed, studies reporting increased IL-4 expression in patients' T cells all used 8D4 mAb,11,12though this increase was not detected when other mAbs were used.13 14 Our work confirms that, in HIV disease, increased expression of IL-4 is restricted to 8D4 reactivity and suggests that this type 2 signal, associated with disease progression, is tightly related to increased T-cell apoptosis.
Relationship between 8D4+ IL-4 production and apoptosis.
(A) Dual anti–IL-4 (MP4 and 8D4 mAb) and 7-AAD staining on CD3+ T cells from an HIV-infected patient, after either 24-hour culture in medium to induce deprivation apoptosis, or after induction of primary necrosis by 15-minute hyperthermia at 56°C. (B) Correlation curve (Spearman regression test) between the percentage of 8D4 anti–IL-4 mAb positive cells among CD3+ T cells and the percentage of 7-AAD+ cells among CD3+ T cells in PBMCs from controls (●) or HIV-infected patients (○) after 24-hour culture in medium. (C) Susceptibility to apoptosis (evaluated by 7-AAD staining) of MP4+CD3+ or 8D4+CD3+T cells from HIV-infected patients after 16-hour PMA+ionomycin stimulation (Mann-Whitney test).
Relationship between 8D4+ IL-4 production and apoptosis.
(A) Dual anti–IL-4 (MP4 and 8D4 mAb) and 7-AAD staining on CD3+ T cells from an HIV-infected patient, after either 24-hour culture in medium to induce deprivation apoptosis, or after induction of primary necrosis by 15-minute hyperthermia at 56°C. (B) Correlation curve (Spearman regression test) between the percentage of 8D4 anti–IL-4 mAb positive cells among CD3+ T cells and the percentage of 7-AAD+ cells among CD3+ T cells in PBMCs from controls (●) or HIV-infected patients (○) after 24-hour culture in medium. (C) Susceptibility to apoptosis (evaluated by 7-AAD staining) of MP4+CD3+ or 8D4+CD3+T cells from HIV-infected patients after 16-hour PMA+ionomycin stimulation (Mann-Whitney test).
Since an inhibitory variant of IL-4 (IL-4δ2) resulting from an alternative mRNA splicing has been described not only in human placenta but also in PBMCs and mastocytes,18 we asked whether a molecular variant of IL-4 was expressed in HIV-infected patients, which could account for this reactivity to 8D4 mAb. The relative frequency of IL-4 mRNA variants was determined in PBMCs from 2 controls and 5 HIV-infected persons either ex vivo or following 3-day culture in medium. IL-4δ2 mRNA (47-bp deletion in exon 2, IL-4δ47) was not predominant ex vivo or after 72-hour culture in PBMCs from control donors or HIV+ patients (Figure 3). Interestingly, we also detected a distinct IL-4 mRNA variant, deleted of 13 bp (IL-4δ13) compared with the complete PCR product (Figure3). IL-4δ13 was rarely expressed ex vivo in PBMCs from control donors or HIV+ patients (Figure 3). Though, following 72-hour incubation in medium (conditions in which spontaneous apoptosis is maximal) the expression of IL-4δ13 was increased, and it represented up to 9% of IL-4 mRNA species in PBMCs from control donor; in some HIV-infected patients, it was the only molecular form of IL-4 mRNA detected (Figure3). Our data therefore suggest that de novo IL-4 transcription is induced during deprivation apoptosis, which is compatible with the recent report of an early (< 5 hours) de novo gene expression in the intrinsic apoptotic pathway.19
Expression of IL-4 mRNA variant transcripts in PBMCs.
(A) Structure of the IL-4 mRNA (E indicates exon; SP, signal peptide) and positions of primers used for the generation of cDNA (arrows). (B) Identification of major IL-4 mRNA, and 42-bp and 13-bp deleted variants. IL-4 mRNA species were detected after hybridization of RT-PCR products with a fluorescent 5′ primer (Immunoscope assay). Profiles from a control donor and 2 HIV-infected patients, ex vivo (J0) or after 72-hour culture in medium (J3), are shown. (C) Relative representation of IL-4 mRNA variants in PBMCs from 2 healthy controls and 5 HIV+ patients.
Expression of IL-4 mRNA variant transcripts in PBMCs.
(A) Structure of the IL-4 mRNA (E indicates exon; SP, signal peptide) and positions of primers used for the generation of cDNA (arrows). (B) Identification of major IL-4 mRNA, and 42-bp and 13-bp deleted variants. IL-4 mRNA species were detected after hybridization of RT-PCR products with a fluorescent 5′ primer (Immunoscope assay). Profiles from a control donor and 2 HIV-infected patients, ex vivo (J0) or after 72-hour culture in medium (J3), are shown. (C) Relative representation of IL-4 mRNA variants in PBMCs from 2 healthy controls and 5 HIV+ patients.
The intracellular IL-4 variant that we detect with 8D4 mAb is a nonsecreted molecule and this was confirmed by the selective detection of IL-4 by ELISA in whole-cell lysates but not in the supernatant from 8D4+MP4− peripheral cells (data not shown). This lack of secretion is likely related to a defect in the glycosylation of the protein, since 8D4 mAb was initially derived against a modified IL-4, which included amino acid substitutions in positions 39 and 105 in order to prevent the glycosylation process.20 The relations between IL-4 and apoptosis are complex. Stein et al have shown that the intracellular expression of IL-4 in apoptotic cells can be inhibited by the use of the pancaspase inhibitor z-VAD-fmk.17 IL-4 is known to induce apoptosis in several cell types, and in mastocytes it directly induces mitochondrial damage, a primary event in deprivation apoptosis.21 Although exogenous IL-4 can protect T cells from deprivation apoptosis,5 the balance between apoptotic/proliferative effects of IL-4 could be biased toward apoptosis in a fraction of T cells, due to their high expression of the IL-4 receptor interacting protein (FRIP).22 Finally, the intracellular accumulation of the anti-inflammatory mediator IL-4 in apoptotic cells but not in primary necrotic cells, could represent a key event in the balance between the anti-inflammatory or proinflammatory status of macrophages after engulfment of dying cells.23
We thank Emmanuelle Perret (Institut Pasteur) for her expertise in confocal analysis.
The authors would like to dedicate this paper to the memory of Professor René Roué, head of the Infectious Diseases Department in Bégin Hospital.
Prepublished online as Blood First Edition Paper, December 19, 2002; DOI 10.1182/blood-2002-08-2499.
Supported by grants from the Agence Nationale de Recherche sur le SIDA (ANRS); Sidaction; and the European Union, contract nos. BMH4-CT 97-2055 and ERB-IC15-CT97-0901.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
M.-L. Gougeon, Département de Médecine Moléculaire, Institut Pasteur, 28 rue du Dr Roux, 75724 Paris cedex 15, France; e-mail:mlgougeo@pasteur.fr.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal