Abstract
Little is known about the requirements for human T-cell leukemia virus type I (HTLV-I) entry, including the identity of the cellular receptor(s). Recently, we have generated an HTLV-I surface glycoprotein (SU) immunoadhesin, HTSU-IgG, which binds specifically to cell-surface protein(s) critical for HTLV-I–mediated entry in cell lines. Here, expression of the HTLV-I SU binding protein on primary cells of the immune system was examined. The immunoadhesin specifically bound to adult T cells, B cells, NK cells, and macrophages. Cell stimulation dramatically increased the amount of binding, with the highest levels of binding on CD4+ and CD8+ T cells. Naive (CD45RAhigh, CD62Lhigh) CD4+ T cells derived from cord blood cells, in contrast to other primary cells and all cell lines examined, bound no detectable HTLV-I SU. However, following stimulation, the level of HTSU-IgG binding was rapidly induced (fewer than 6 hours), reaching the level of binding seen on adult CD4+ T cells by 72 hours. In contrast to HTLV-I virions, the soluble HTSU-IgG did not effect T-cell activation or proliferation. When incubated with human peripheral blood mononuclear cells in a mixed leukocyte reaction, HTSU-IgG inhibited proliferation at less than 1 ng/mL. These results indicate that cell-surface expression of the HTLV SU binding protein is up-regulated during in vitro activation and suggest a role for the HTLV-I SU binding proteins in the immunobiology of CD4+ T cells.
Introduction
The human T-cell leukemia virus type I (HTLV-I) retrovirus, which belongs to the deltaretrovirus subfamily of retroviruses, is endemic in certain geographic areas, including southwestern Japan, the Caribbean basin, and parts of West Africa, South America, and Melanesia.1 Although HTLV-I causes no disease in the majority of 10 million infected individuals, HTLV-I is the etiologic agent in a severe lymphocyte neoplasia called adult T-cell leukemia/lymphoma (ATL)2,3 and an inflammatory neurological disease known as HTLV-I–associated myelopathy/tropical spastic paraparesis (HAM/TSP).4,5 HTLV-I infection is also the causal agent of infectious dermatitis of children6 and uveitis7 and has been associated with several other inflammatory and immune-mediated conditions including polymyositis, arthropathy, and Sjogren syndrome.
HTLV-I can be transmitted perinatally, sexually, through mother's milk, by blood transfusion, or by sharing needles during intravenous drug use. The major route of mother-to-child transmission is by breast milk, with a seroconversion rate of 20%-40% reported for breast-fed children and 0%-13% for bottle-fed children born to HTLV-I–seropositive mothers.8 Transfusion is believed to be the most efficient mode of virus transmission; the probability of seroconversion can be 40%-60%9,10 with a rate of 74% from blood stored for fewer than 5 days.11 ATL develops after a long incubation period (reviewed by Green and Chen12), and individuals infected early in life are at the highest risk for ATL.13
ATL is a malignancy of CD4+ T cells, and the majority of the cells infected by HTLV-I in vivo are CD4+ T cells.14,15 However, recent studies indicate that both CD4+ and CD8+ T cells serve as viral reservoirs in HAM/TSP patients.16 The closely related human T-cell leukemia virus type II (HTLV-II), which shares a common receptor with HTLV-I,17,18 infects both CD4+ and CD8+ cells in vivo, with a greater proviral burden in CD8+ T cells.19,20 Both HTLV-I and HTLV-II can infect all human lymphocytic subsets, causing polyclonal and monoclonal T-cell lymphocytoses.12
Entry of retroviruses into target cells involves the interaction of 2 envelope (Env) glycoproteins, a surface glycoprotein (SU) and a transmembrane glycoprotein (TM), with at least one specific host-cell receptor.21 Since HTLV-I is poorly infectious, assays other than susceptibility to infection have been used to examine the distribution of the HTLV-I receptor(s). In earlier studies, the level and distribution of the HTLV-I receptor(s) on different cell types had been inferred from their ability to form syncytia with cells expressing HTLV-I Env or from the relative titer of viruses pseudotyped with HTLV-I Env on these cells. These studies suggested that the HTLV receptor is widely expressed on established cell lines of both human and nonhuman origin.22-24 Recent work in our laboratory and others, using a soluble form of HTLV-I SU, indicates that all established cell lines of vertebrate-origin tested express cell-surface molecules capable of specifically binding HTLV-I SU.25 26
As part of that work, we observed that the level of expression of the HTLV SU binding molecule is greater on activated primary CD4+ T cells than on established cell lines. Here, the regulation of expression of the HTLV-I SU binding protein(s) on primary cells of the immune system was examined. These data indicate that HTLV-I SU binding proteins are regulated on several cell types during an immune response, absent on resting naive cord blood CD4+T cells, and possibly play a role in the immunobiology of activated T cells.
Materials and methods
Expression of the immunoadhesin proteins
To generate the immunoadhesins, 293 cells were plated on a 100-mm plate and transfected 24 hours later according to the manufacturer's protocol using FuGene 6 (Roche Molecular Biochemicals, Indianapolis, IN) with 8 μg of plasmid vector encoding HTSU-IgG (HTSU-IgG/pSK100)26 or a plasmid encoding a similar fusion protein (SUA-rIgG/pSK100) containing the SU protein from the avian retrovirus ALSV-A (SUA-rIgG).27 The latter was used as a negative control in all studies. The transfected cells were re-fed with OptiMEM (Invitrogen, Carlsbad, CA) containing 1% fetal calf serum (FCS) 20 hours prior to harvesting. Following harvesting, the cell culture supernatant was frozen at −80°C, and the concentration of immunoadhesins determined by enzyme-linked immunosorbent assay (ELISA), as previously described.26
Flow cytometric analysis of immunoadhesin binding to human leukocytes
Specific binding of HTSU-IgG to target cells was examined essentially as previously described.26 Briefly, 1 × 106 target cells were incubated with cellular supernatant containing immunoadhesin (either HTSU-IgG or, as a negative control, SUA-IgG used in excess) to the final volume of 0.5 mL for 30 minutes at 20°C. The cells were washed, incubated for 30 minutes on ice with fluorescein isothiocyanate (FITC)–conjugated antibody specific for rabbit immunoglobulins (Sigma, St Louis, MO), washed again, and fixed in 400 μL ice-cold phosphate-buffered saline (PBS)/4% paraformaldehyde. For experiments involving large numbers of samples, cells were fixed in 4% paraformaldehyde for 30 minutes on ice prior to exposure to the immunoadhesins, and the binding to the immunoadhesins was carried out on ice rather than at room temperature. Ten-to-twenty thousand live cell events were measured either on an EPICS XL-MCL (Coulter, Hialeah, FL) and analyzed using Coulter System II software version 3.0 or on a FACScan (BD PharMingen; San Diego, CA) and analyzed using FlowJo software (Treestar; Aurora, CA).
Binding studies for analysis by 2- and 3-color flow cytometry were performed as follows. After binding with the immunoadhesin as described above, cells were blocked with 10% human AB serum for 25 minutes, washed once with PBS/2% FCS/0.02% sodium azide, and the FITC-conjugated antibody specific for rabbit IgG was added. After 45 minutes of incubation on ice, cells were incubated with a second (phycoerythrin [PE]–labeled) and, as noted, a third (PerCP-labeled) antibody at 1:20 dilution to the final volume of 200 μL. After incubating on ice for 45 minutes, cells were washed and fixed with 4% paraformaldehyde. For the experiments involving 2-color flow cytometry, PE-labeled anti-CD45RA or anti-CD45RO antibodies (Coulter) were used as the second antibody. For the 3-color flow cytometric analysis, sorted resting CD4+ T cells were incubated with the immunoadhesin and the FITC-labeled anti–rabbit IgG, followed by either PE-labeled anti-CD45RA and PerCP-labeled anti-CD62L antibodies, or followed by PE-labeled anti-CD4 and either PerCP-labeled anti-CD45RA or anti-CD45RO antibodies as indicated in “Results.” The human monoclonal antibodies directed against epitopes of HTLV-I SU (PRH-1, PRH-4, PRH-7A, and PRH-11A) and the isotype control R0-4 (generous gifts of Ken Hadlock and Steve Foung, Stanford University, Palo Alto, CA) have been previously described.28
Isolation, activation, and cell culture of human leukocytes
Leukopaks of peripheral blood were collected from adult donors by the National Institutes of Health (NIH) transfusion branch (Bethesda, MD), and cord blood samples were collected from Frederick Memorial Hospital (Frederick, MD), according to the approved NIH IRB protocols. Cord blood lymphocytes were isolated by Ficoll-Hypaque gradient centrifugation. The light density fraction was collected, washed twice with PBS, then CD4+ and CD8+ T cells were isolated by negative selection using magnetic-activated cell-sorting (MACs) methods according to the manufacturer's instructions (Miltenyi Biotec, Sunnyvale, CA). For the peripheral blood, after Ficoll density separation, lymphocytes and monocytes were separated by counter-current elutriation. CD4+ and CD8+ T cells, B cells, and NK cells were then isolated from peripheral blood by positive selection using MACs. Samples are passed over the magnetic extraction column several times to complete the isolation. In some experiments CD4+ cells were further isolated by CD45RA or CD45RO positivity by passage over the magnetic separation columns. These cell populations were consistently more than 95% pure.
After isolation, the cells were either immediately analyzed or cultured and activated in RPMI 1640 medium supplemented with 10% FCS, 2 mM/L glutamine, sodium pyruvate (1 mM), and antibiotics for 1-7 days as follows. Monocytes were activated using 10 μg/mL lipopolysaccharide (Difco, Detroit, MI) and cultured in nonadherent plates. B cells were activated with staphylococcal enterotoxin A (SEA) and pokeweed mitogen at 10 μg/mL. NK cells were activated in 500 U/mL interleukin-2 (IL-2). T cells were cultured in 20 U/mL of IL-2 and activated with either phytohemagglutinin (PHA) (1 μg/mL) and IL-2, anti-CD3, and anti-CD28 antibody beads at a ratio of 3 beads per cell (a gift of Carl June, National Cancer Institute, Bethesda, MD) or phorbol myristate acetate (PMA) (10 ng/mL) and ionomycin (1 ng/mL). All cells were cultured in a humidified atmosphere at 37°C in an incubator containing 5% CO2.
Mixed leukocyte reaction
Peripheral blood samples were taken from 2 healthy donors, and the PBMCs were isolated by Ficoll-Hypaque gradient centrifugation. PBMCs from one donor, to be used as stimulator cells, were irradiated with 3000 rad, then suspended at 5 × 106/mL in RPMI containing 10% human AB and 2 mM/L glutamine. 100 μL containing 5 × 105 cells of the irradiated cells were dispensed into each well of 96-well round-bottom plate and incubated with 50 μL supernatant containing various dilutions of HTSU-IgG, SUA-IgG (negative control), anti-CD2 antibody (10 μg/mL; positive control), or Dulbecco modified Eagle medium (DMEM) alone. After 30 minutes, 50 μL responder cells were added (105 cells into each well). The cells were incubated for 6 days in a humidified 37°C incubator with 5% CO2. The plates were pulsed with 3H-thymidine (0.5 μCi [0.0185 MBq]/well; NEN, Boston, MA). The cells were harvested 20 hours after the pulse, and the amount of radioactivity incorporated was determined in a scintillation counter (Wallac 1410; Wallac, Gaithersburg, MD). The results are presented as percentage of the counts per minute (cpm) of the control well (stimulator and responder cells incubated with DMEM) as means of triplicate cultures.
Production of HTLV virus
MT2, an HTLV-I producer cell line, and 729pH6neo, an HTLV-II producer cell line (a gift of Pat Green, Ohio State University Medical Center, Columbus, OH) were maintained in RPMI 1640 medium and Iscove modified Dulbecco medium, respectively, with 10% FCS. Cell lines were re-fed 18 hours prior to cell-free harvest, and the viral concentration was determined using a p19 ELISA kit (Zeptometrix, Buffalo, NY).
Results
HTLV-I SU immunoadhesin binds specifically to CD4+ T cells
Recently, we have generated a soluble form of the HTLV-I SU glycoprotein by generating an HTLV-I SU immunoadhesin, HTSU-IgG.26 Using established cell lines, we and others have observed that HTLV-I SU immunoadhesins bind specifically to cell-surface protein(s) critical for HTLV-I Env-mediated binding and entry.25 26 In this study, a similar approach was used to examine the regulation of the HTLV SU binding protein on primary cells of the immune system.
Initially, the binding of HTSU-IgG to proliferating adult CD4+ T cells was examined. The level of HTSU-IgG bound to the CD4+ T cells was determined as mean fluorescence intensity (MFI) by fluorescence-activated cell-sorter (FACS) analysis. As a control for nonspecific binding, the amount of binding to a similar immunoadhesin SUA-rIgG, which contains the SU protein from an unrelated retrovirus, was determined in parallel. A dramatic increase in binding was observed when CD4+ T cells were incubated with HTSU-IgG as compared to cells incubated with SUA-IgG (Figure 1A). A dose-response curve showed the binding of HTSU-IgG to CD4+ T cells was saturable at 400-700 ng/mL (data not shown).
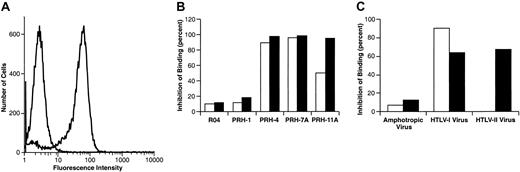
HTLV-IgG specifically binds to activated primary CD4+ T cells.
The HTLV-I SU immunoadhesin was generated and the binding assays were performed as described in “Materials and methods.” (A) PHA-activated IL-2–dependent primary CD4+ T cells (106) isolated from adult PBMCs were incubated with HTSU-IgG (80 ng) or, as a negative control, a similar immunoadhesin containing the SU protein of an unrelated retrovirus (SUA-IgG; 400 ng). The cells were then incubated with an FITC-conjugated antibody specific for rabbit IgG, and the amount of binding was determined by FACS as described in “Materials and methods.” The left peak represents the amount of binding to SUA-IgG; the right peak represents binding to HTSU-IgG. (B) HTSU-IgG was preincubated with anti–HTLV-I SU human monoclonal antibodies and binding to activated CD4+ T cells determined. Supernatant containing 50 ng/mL HTSU-IgG was incubated with either 2 μg/mL (■) or 10 μg/mL (▪) human mAb directed against HTLV-I SU (PRH-1, PRH-4A, PRH-7A, PRH-11A) or an isotype control directed against a 64-kDa protein of cytomegalovirus (R04)28 for 1 hour on ice before performing the binding assay. The MFI was determined for each of the samples, the value of the MFI of the control (SUA-IgG) subtracted from the MFI of HTSU-IgG samples, and the percent inhibition determined using the following formula: 100-(MFI of experimental/MFI of media-only control × 100). (C) Cell-free HTLV-I and HTLV-II were harvested from the supernatant of producer cell lines (MT-2 and 729pH6neo, respectively), and the relative amount of viral particles determined using a p19 ELISA kit as previously described.26 Stimulated adult CD4+ T cells were preincubated for 30 minutes on ice with a high concentration (50 ng/mL of p19/mL) of HTLV-I or a low concentration (0.2 ng of p19/mL) of HTLV-I or HTLV-II. As controls, the cells were incubated with media without viral particles or with high and low infectious doses of an unrelated mouse retrovirus (amphotropic murine leukemia virus) (gift of Sandra Ruscetti, National Cancer Institute-Frederick). HTSU-IgG (400 ng/mL) was then added to each sample, and binding assays were performed. ■ indicates low concentration of virus; and □, high concentration.
HTLV-IgG specifically binds to activated primary CD4+ T cells.
The HTLV-I SU immunoadhesin was generated and the binding assays were performed as described in “Materials and methods.” (A) PHA-activated IL-2–dependent primary CD4+ T cells (106) isolated from adult PBMCs were incubated with HTSU-IgG (80 ng) or, as a negative control, a similar immunoadhesin containing the SU protein of an unrelated retrovirus (SUA-IgG; 400 ng). The cells were then incubated with an FITC-conjugated antibody specific for rabbit IgG, and the amount of binding was determined by FACS as described in “Materials and methods.” The left peak represents the amount of binding to SUA-IgG; the right peak represents binding to HTSU-IgG. (B) HTSU-IgG was preincubated with anti–HTLV-I SU human monoclonal antibodies and binding to activated CD4+ T cells determined. Supernatant containing 50 ng/mL HTSU-IgG was incubated with either 2 μg/mL (■) or 10 μg/mL (▪) human mAb directed against HTLV-I SU (PRH-1, PRH-4A, PRH-7A, PRH-11A) or an isotype control directed against a 64-kDa protein of cytomegalovirus (R04)28 for 1 hour on ice before performing the binding assay. The MFI was determined for each of the samples, the value of the MFI of the control (SUA-IgG) subtracted from the MFI of HTSU-IgG samples, and the percent inhibition determined using the following formula: 100-(MFI of experimental/MFI of media-only control × 100). (C) Cell-free HTLV-I and HTLV-II were harvested from the supernatant of producer cell lines (MT-2 and 729pH6neo, respectively), and the relative amount of viral particles determined using a p19 ELISA kit as previously described.26 Stimulated adult CD4+ T cells were preincubated for 30 minutes on ice with a high concentration (50 ng/mL of p19/mL) of HTLV-I or a low concentration (0.2 ng of p19/mL) of HTLV-I or HTLV-II. As controls, the cells were incubated with media without viral particles or with high and low infectious doses of an unrelated mouse retrovirus (amphotropic murine leukemia virus) (gift of Sandra Ruscetti, National Cancer Institute-Frederick). HTSU-IgG (400 ng/mL) was then added to each sample, and binding assays were performed. ■ indicates low concentration of virus; and □, high concentration.
Several studies verified that the HTSU-IgG specifically bound to primary CD4+ T cells. When HTSU-IgG was preincubated with any of the 3 antibodies directed against conformational epitopes on the HTLV-I SU and previously shown to block HTLV-I–induced syncytia formation at a concentration of 10 μg/mL,28 the mean fluorescence was inhibited by more than 95% (Figure 1B). In contrast, preincubation of HTSU-IgG with either a human monoclonal antibody of the same isotype previously shown not to inhibit syncytia formation (PRH-1)28 or a human monoclonal antibody directed against a cytomegalovirus viral antigen (R04) had only a minor effect on binding (Figure 1B). Furthermore, incubation of HTSU-IgG with a lower concentration of these antibodies (2 μg/mL) revealed that the relative ability of these antibodies to block binding paralleled their ability to block syncytia formation28; PRH-11A was less efficient at blocking binding at lower concentrations than PRH-4 and PRH-7A.
To demonstrate further that binding of the HTLV-I SU immunoadhesin was receptor-mediated, the ability of HTLV virions to compete with HTSU-IgG for binding to target cells was examined. Preincubation of CD4+ T cells with 50 ng/mL HTLV-I virions, but not a murine retrovirus (amphotropic murine leukemia virus; A-MLV), blocked binding of HTSU-IgG (Figure 1C). When target cells were preincubated with equal, nonsaturating concentrations of either HTLV-I or HTLV-II virions, binding of the immunoadhesin was similarly inhibited (Figure1C). These results indicate that the SU portion of the immunoadhesin is conformationally intact and that binding of HTSU-IgG to target cells involves specific interactions between SU and cell-surface molecules critical for HTLV-I Env-mediated binding and fusion.
Expression of HTLV SU binding protein on cells of the immune system
We next used the HTSU-IgG binding assay to compare the level of the cell-surface protein(s) that specifically binds the HTLV-I SU on different cells of the adult immune system. CD4+ and CD8+ T cells, B cells, monocytes/macrophages, and NK cells isolated from adult peripheral blood were tested either immediately after isolation (Figure 2; left panels) or 7 days after stimulation (Figure 2; right panels). All of the cell types bound HTSU-IgG at significant levels immediately after isolation. The CD4+ and CD8+ T cells bound higher levels of HTSU-IgG than the other cell types, as determined by MFI (Figure 2; compare panels A and C to E, G, and I). For all the cell types, stimulation of the cells dramatically increased the amount of HTSU-IgG binding from the level observed in resting cells (Figure 2; right panels). Binding of the negative control (SUA-IgG) was less than 1.5% of the population under these conditions. The observation that primary cells of the immune system expressed the HTLV-I SU binding protein is consistent with the belief that the receptor(s) for HTLV is ubiquitously expressed22-24 and recent observations by our laboratory and others that soluble HTLV-I SU binds to all established cell lines of vertebrate origin tested to date.25 26
HTSU-IgG binds to resting and activated primary cells of the immune system.
CD4+ (A-B) and CD8+ T cells (C-D), B cells (E-F), monocytes (G), monocyte-derived macrophages (H), and NK cells (I-J) were isolated from adult peripheral blood leukopaks as described in “Materials and methods.” The cells were either tested immediately for HTSU-IgG binding (80 ng/mL) (left panels) or activated for 7 days and reassayed for HTSU-IgG binding (right panels). Binding to the SUA-IgG (200 ng/mL) was no more than 0.8% of the HTSU-IgG binding for these samples. Data are from a representative experiment out of 3 performed. HTSU-IgG binding is on the y-axis and FL-2 is on the x-axis.
HTSU-IgG binds to resting and activated primary cells of the immune system.
CD4+ (A-B) and CD8+ T cells (C-D), B cells (E-F), monocytes (G), monocyte-derived macrophages (H), and NK cells (I-J) were isolated from adult peripheral blood leukopaks as described in “Materials and methods.” The cells were either tested immediately for HTSU-IgG binding (80 ng/mL) (left panels) or activated for 7 days and reassayed for HTSU-IgG binding (right panels). Binding to the SUA-IgG (200 ng/mL) was no more than 0.8% of the HTSU-IgG binding for these samples. Data are from a representative experiment out of 3 performed. HTSU-IgG binding is on the y-axis and FL-2 is on the x-axis.
HTLV-I SU binding protein is expressed at low levels on resting cord blood T cells
We next examined the level of expression of the HTLV-I SU binding protein on cells that previously had not been activated. The level of binding of HTSU-IgG to T-cell subsets derived from freshly isolated, unstimulated cord blood was determined. The HTLV-I SU immunoadhesin bound at low levels to both CD4+ (Figure 3Aii) and CD8+ (panel v) T cells. Donor-dependent variation was observed in the percentage of cells expressing the HTLV-I SU binding protein; the percentage of positive cells was as high as 14% for CD4+ and 11% for CD8+ T cells (data not shown). Following 5 days of in vitro stimulation, both CD4+ and CD8+ T cells bound HTSU-IgG at high levels (Figure 3Aiii,vi), similar to that observed for stimulated CD4+ and CD8+ from adult blood (Figure 2).
Next, freshly isolated cord blood T cells, enriched for the CD4+ population, were sorted into naive (CD45RAhigh) and memory (CD45RO+) populations. To avoid possible stimulation of the cells during magnetic sorting, cells were first depleted of CD8+ T cells and monocytes. A low percentage of memory cells in the CD4+ subset of T cells derived from cord blood was observed in some of the samples, presumably due to maternal contamination. HTSU-IgG binding on cells from 2 representative donors are shown in Figure 3B. In unstimulated cord blood T cells from one donor, memory as well as naive cells could be isolated. In those samples, significant binding to the CD45RO+ population (Figure 3Bii) but not to the CD45RAhigh population (Figure 3Bi) was observed. In the CD4+ T cells from a second donor, where the percentage of memory cells was not detectable, CD45RAhigh T cells also did not bind HTSU-IgG (Figure 3Biii). To date, we have examined HTSU-IgG binding to naive CD4+ T cells from cord blood cells from 23 individual donors. No detectable binding to CD45RAhigh CD4+ T cells was seen in any of these samples.
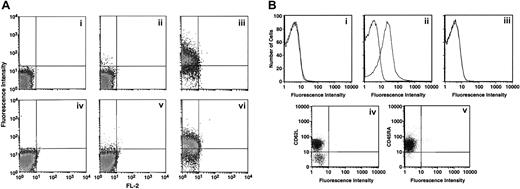
HTSU-IgG binding to unstimulated CD4+ T cells isolated from cord blood is restricted to the CD45RO+(memory) subset.
(A) Cord blood lymphocytes were enriched for CD4+ and CD8+ T cells as described in “Materials and methods,” and the binding assays performed. Panels i-iii, CD4+ T cells; panels iv-vi, CD8+ T cells. The level of HTSU-IgG binding was determined for unactivated cells (panels ii,v) and for cells activated for 5 days (panels iii,vi). Panels i and iv show the level of binding to negative control SUA-IgG. (B) Panels i-iii show freshly isolated cord blood lymphocytes enriched for CD4+ T cells that were sorted for CD45RO and CD45RA, and binding of HTSU-IgG to these cell populations from freshly isolated, unstimulated cord CD4+ T lymphocytes was determined. Panel i shows binding to CD45RA+ T cells from one donor, panel ii shows binding to CD45RO+ cells from the same donor as panel i. Panel iii shows binding to CD45RA+ T cells from a different donor. Panels iv and v show freshly isolated cord blood lymphocytes enriched for CD4+ T cells. Three-color flow cytometry was performed, using PE-labeled anti-CD62L antibody, PerCP-labeled anti-CD45RA antibody, and HTSU-IgG indirectly labeled with FITC as described in “Materials and methods.” Panel iv shows analysis performed by gating on the population positive for binding to the anti-CD45RA antibody. X-axis, binding of HTSU-IgG; y-axis, binding to anti-CD62L antibody. Panel v shows analysis performed by gating on the population positive for binding to the anti-CD62L antibody. X-axis, binding of HTSU-IgG; y-axis, binding to anti-CD45RA antibody.
HTSU-IgG binding to unstimulated CD4+ T cells isolated from cord blood is restricted to the CD45RO+(memory) subset.
(A) Cord blood lymphocytes were enriched for CD4+ and CD8+ T cells as described in “Materials and methods,” and the binding assays performed. Panels i-iii, CD4+ T cells; panels iv-vi, CD8+ T cells. The level of HTSU-IgG binding was determined for unactivated cells (panels ii,v) and for cells activated for 5 days (panels iii,vi). Panels i and iv show the level of binding to negative control SUA-IgG. (B) Panels i-iii show freshly isolated cord blood lymphocytes enriched for CD4+ T cells that were sorted for CD45RO and CD45RA, and binding of HTSU-IgG to these cell populations from freshly isolated, unstimulated cord CD4+ T lymphocytes was determined. Panel i shows binding to CD45RA+ T cells from one donor, panel ii shows binding to CD45RO+ cells from the same donor as panel i. Panel iii shows binding to CD45RA+ T cells from a different donor. Panels iv and v show freshly isolated cord blood lymphocytes enriched for CD4+ T cells. Three-color flow cytometry was performed, using PE-labeled anti-CD62L antibody, PerCP-labeled anti-CD45RA antibody, and HTSU-IgG indirectly labeled with FITC as described in “Materials and methods.” Panel iv shows analysis performed by gating on the population positive for binding to the anti-CD45RA antibody. X-axis, binding of HTSU-IgG; y-axis, binding to anti-CD62L antibody. Panel v shows analysis performed by gating on the population positive for binding to the anti-CD62L antibody. X-axis, binding of HTSU-IgG; y-axis, binding to anti-CD45RA antibody.
Studies have shown that naive CD4+ T cells can be further divided by double expression of the CD45RA isoform and L-selectin (CD62L).29 30 Three-color flow cytometry was performed to measure the expression of CD45RA, CD62L, and the HTLV-I SU binding protein(s) on freshly isolated CD4+ cord blood T cells. CD62Lhigh CD45RAhigh T cells did not bind HTSU-IgG at significant levels (Figure 3Biv,v). Thus, naive nonactivated CD4+ T cells derived from cord blood lymphocytes, in contrast to other primary leukocytes, express little or no HTLV-I SU binding protein(s).
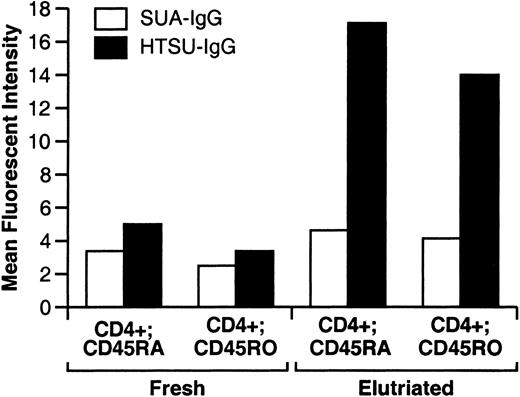
The results obtained with unactivated T cells derived from cord blood contrasted with what we had observed for unactivated T cells isolated from adult donors (compare Figure 3Aii and iii with Figure 2i and iii). The expression level of the HTLV-I SU binding protein(s) to T-cell subsets derived from unactivated CD4+ T cells isolated from adult donors was further examined. In these initial studies, in contrast to T cells derived from cord blood, both naive (CD45RAhigh) and memory (CD45RO+) CD4+ T cells bound significant levels of the HTSU-IgG (data not shown). Since one difference between adult and cord blood was method of isolation (adult peripheral blood leukocytes were concentrated and elutriated), it was not clear whether there was a true difference between unstimulated, phenotypically naive CD4+ T cells derived from adult and cord blood or whether the result was a reflection of this difference in isolation. To address this, we isolated adult PBLs using the same method used for the isolation of cord blood lymphocytes (Ficoll-Hypaque gradient centrifugation). A portion of the cells were then immediately examined for cell-surface expression of the HTLV SU binding protein(s), CD4, and CD45RA or CD45RO. Following this method of separation, approximately 10%-20% of naive and memory CD4+ T cells bound HTSU-IgG with an MFI only slightly above that of the negative control immunoadhesin (Figure 4). The remainder of the lymphocytes were separated by counter-current elutriation and examined for expression of the same markers. Elutriation dramatically increased the amount of HTSU-IgG binding to both CD45RAhighand CD45RO+ CD4+ T cells (Figure 4). The number of cells positive for HTSU-IgG binding increased to between 70% and 80% for both the CD45RAhigh or CD45RO+subsets, with an MFI significantly above that of the negative control. Thus, resting adult CD4+ T cells express low levels of HTLV SU binding protein on the cell surface, and methods of cell isolation can stimulate increased cell-surface expression.
Freshly isolated unstimulated CD4+ T cells isolated from adult blood bind low levels of HTSU-IgG.
Lymphocytes were isolated by Ficoll-Hypaque gradient centrifugation from leukopaks of peripheral blood from adult donors. A portion of the cells were then analyzed by flow cytometry using the immunoadhesins (HTSU-IgG, 100 ng/mL, or SUA-IgG, 400 ng/mL) and anti-CD4 and either anti-CD45RA or anti-CD45RO antibodies as described in “Materials and methods.” Analysis was performed by gating on the population positive for binding to the CD4+ population, and binding of the immunoadhesins and the anti-CD45 antibodies was determined. The MFI of the immunoadhesin on each double-labeled population was then determined. The remainder of the cells was then separated by counter-current elutriation, and similar flow cytometric analyses were performed.
Freshly isolated unstimulated CD4+ T cells isolated from adult blood bind low levels of HTSU-IgG.
Lymphocytes were isolated by Ficoll-Hypaque gradient centrifugation from leukopaks of peripheral blood from adult donors. A portion of the cells were then analyzed by flow cytometry using the immunoadhesins (HTSU-IgG, 100 ng/mL, or SUA-IgG, 400 ng/mL) and anti-CD4 and either anti-CD45RA or anti-CD45RO antibodies as described in “Materials and methods.” Analysis was performed by gating on the population positive for binding to the CD4+ population, and binding of the immunoadhesins and the anti-CD45 antibodies was determined. The MFI of the immunoadhesin on each double-labeled population was then determined. The remainder of the cells was then separated by counter-current elutriation, and similar flow cytometric analyses were performed.
HTLV-I SU binding protein is expressed rapidly during activation of CD4+ T cells
The increase in HTLV-I SU immunoadhesin binding on cells following elutriation suggested that the HTLV-I SU binding protein was rapidly activated. We next examined the kinetics of expression of the SU binding protein(s) following the in vitro stimulation of cord blood lymphocytes. Freshly isolated lymphocytes from cord blood, enriched for CD4+ T cells, were stimulated by exposure to bead-immobilized anti-CD3 and anti-CD28 antibodies.31 At 0, 6, 24, and 48 hours after stimulation, the expression of the HTLV-I SU binding protein(s) and the activation status of the T cells (as determined by CD45RA expression) was measured by 2-color flow cytometry (Figure 5A). Consistent with the results from Figure 3, the freshly isolated, unstimulated cord blood CD4+ cells did not bind significant levels of HTSU-IgG (Figure 5A). In contrast, significant levels of binding to HTSU-IgG were observed as early as 6 hours after stimulation (Figure 5A). It should be noted that the cells that bound HTSU-IgG at 6 hours after activation were mainly phenotypically naive, as judged by their CD45RA phenotype. The level of HTSU-IgG binding was higher at 24 hours, and by 48 hours nearly all of the T cells were expressing significant levels of the HTLV-I SU binding protein(s) and were CD45RAlow(Figure 5A). Similar experiments, in which the activation status of the T cells was determined from binding of antibodies directed against the RO isoforms of CD45, gave similar results (data not shown). The ability of HTSU-IgG to bind to T cells that are still naive, as judged by their CD45RAhigh, CD45RO− phenotype, suggests that the HTLV-I SU binding protein is an early marker for T-cell activation and that activated naive T cells can be targets for HTLV-I infection.
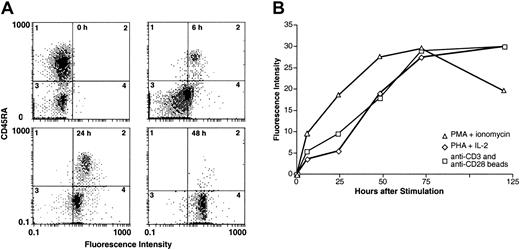
Rapid cell surface expression of HTSU-IgG binding proteins is observed following different methods of immune activation of cord blood T lymphocytes.
(A) Freshly isolated CD4+ cord blood lymphocytes were activated by exposure to bead-immobilized anti-CD3 and anti-CD28 antibodies. Two-color flow cytometry was performed with FITC-labeled anti–rabbit IgG (Sigma) and PE-labeled anti-CD45RA antibodies (Coulter). HTSU-IgG binding assays were performed at 0, 6, 24, and 48 hours after stimulation. X-axis, binding of HTSU-IgG; y-axis, binding of anti-CD45RA antibody. (B) Kinetics of expression of HTLV-I SU binding protein following different methods of T-cell activation was determined. CD4+ cord blood T lymphocytes were activated by treatment with PMA and ionomycin (▵), exposure to bead-immobilized anti-CD3 and anti-CD28 antibodies (■), or by PHA and IL-2 (⋄) as described in “Materials and methods.” The amount of binding was determined prior to activation and at 6, 24, 48, 72, and 120 hours after activation. The MFI of HTSU-IgG binding - MFI of SUA-IgG binding is plotted on the y-axis. Data are a representative experiment of 3 performed.
Rapid cell surface expression of HTSU-IgG binding proteins is observed following different methods of immune activation of cord blood T lymphocytes.
(A) Freshly isolated CD4+ cord blood lymphocytes were activated by exposure to bead-immobilized anti-CD3 and anti-CD28 antibodies. Two-color flow cytometry was performed with FITC-labeled anti–rabbit IgG (Sigma) and PE-labeled anti-CD45RA antibodies (Coulter). HTSU-IgG binding assays were performed at 0, 6, 24, and 48 hours after stimulation. X-axis, binding of HTSU-IgG; y-axis, binding of anti-CD45RA antibody. (B) Kinetics of expression of HTLV-I SU binding protein following different methods of T-cell activation was determined. CD4+ cord blood T lymphocytes were activated by treatment with PMA and ionomycin (▵), exposure to bead-immobilized anti-CD3 and anti-CD28 antibodies (■), or by PHA and IL-2 (⋄) as described in “Materials and methods.” The amount of binding was determined prior to activation and at 6, 24, 48, 72, and 120 hours after activation. The MFI of HTSU-IgG binding - MFI of SUA-IgG binding is plotted on the y-axis. Data are a representative experiment of 3 performed.
We next compared the expression of the HTLV-I SU binding protein(s) using different modes of T-cell activation (Figure 5B). Freshly isolated cord blood lymphocytes were activated by PMA and ionomycin, PHA and IL-2, or exposure to bead-immobilized anti-CD3 and anti-CD28 antibodies, as described in the legend to Figure 5. All 3 methods of stimulation resulted in the expression of detectable levels of the HTLV-I SU binding protein by 6 hours after activation. Cell-surface levels of the HTLV SU binding proteins on 3 different donors (as determined by MFI) reached maximal levels by 72 hours after the stimulation of the cord blood CD4+ T cells. Dose-response experiments showed that HTSU-IgG binding was saturable between 400 and 800 ng/mL on activated CD4+ cord blood T cells and, at the highest concentrations tested, no binding was seen on resting naive cord blood cells (Figure 6, and data not shown).
Specific binding of HTSU-IgG to activated primary CD4+ T cells shows saturable dose-dependent kinetics.
PHA-activated IL-2 dependent (♦) or unactivated (●) primary CD4+ cord blood T cells (106) isolated from cord blood were incubated with the indicated amount of HTSU-IgG or, as a negative control, SUA-IgG (125 ng/mL). Since this experiment involved a large number of samples being analyzed in parallel, the cells were fixed in 4% paraformaldehyde for 30 minutes on ice prior to exposure to the immunoadhesin. The cells were then incubated with an FITC-conjugated antibody specific for rabbit IgG and the amount of binding determined as described in “Materials and methods.” MFI of HTSU-IgG binding minus MFI of SUA-IgG binding is plotted on the y-axis and the concentration of HTSU-IgG on the x-axis. Data are a representative experiment of 3 performed.
Specific binding of HTSU-IgG to activated primary CD4+ T cells shows saturable dose-dependent kinetics.
PHA-activated IL-2 dependent (♦) or unactivated (●) primary CD4+ cord blood T cells (106) isolated from cord blood were incubated with the indicated amount of HTSU-IgG or, as a negative control, SUA-IgG (125 ng/mL). Since this experiment involved a large number of samples being analyzed in parallel, the cells were fixed in 4% paraformaldehyde for 30 minutes on ice prior to exposure to the immunoadhesin. The cells were then incubated with an FITC-conjugated antibody specific for rabbit IgG and the amount of binding determined as described in “Materials and methods.” MFI of HTSU-IgG binding minus MFI of SUA-IgG binding is plotted on the y-axis and the concentration of HTSU-IgG on the x-axis. Data are a representative experiment of 3 performed.
HTLV-I SU immunoadhesin was not mitogenic for T cells but blocked a mixed lymphocyte reaction
The observation that the HTLV-I SU binding protein was expressed rapidly after stimulation raised the possibility that this cell-surface protein may play a role in immune regulation. To investigate this, the effect of HTSU-IgG on mitogenic responses and in vitro alloresponses was examined. HTLV virions have been shown to be mitogenic for quiescent CD4+ and CD8+ T cells.32 33 Since heat-inactivated virions retain this mitogenic activity, it is believed to involve interactions between a virion surface molecule and a specific receptor on the T cells. To determine whether the HTLV-I SU immunoadhesin possesses this activity, we examined the effect of HTSU-IgG on T-cell activation and proliferation. Addition of HTSU-IgG at 400 ng/mL to cultures of unstimulated adult CD4+ T cells neither induced T-cell activation, as measured by cell-surface expression of CD69, nor stimulated proliferation, as measured by thymidine incorporation in DNA (data not shown). Under these same conditions, HTLV-I virions induced T-cell activation in 70% of the cells and stimulated proliferation.
We next examined whether HTSU-IgG could block in vitro alloresponses requiring cell-cell interactions. Stimulator and responder cells in a mixed lymphocyte reaction (MLR) were incubated with different concentrations of HTSU-IgG. Incubation of the MLR with as little as 0.15 ng/mL HTSU-IgG significantly inhibited proliferation compared to SUA-IgG, which contains the same rabbit IgG portion as HTSU-IgG fused to the SU protein from an avian retrovirus (Figure7). At higher concentrations, HTSU-IgG inhibited proliferation in a dose-dependent manner. However, although the effect was less than the inhibition by HTSU-IgG, the SUA-IgG also had an inhibitory effect of the MLR, indicating that some nonspecific binding was occurring, probably through Fc receptor.34 35
HTSU-IgG blocks proliferation in a mixed lymphocyte reaction.
PBMCs from 2 healthy adult donors were isolated. PBMCs from one donor served as responder, and the PBMCs from another donor were irradiated and used as stimulator cells as described in “Materials and methods.” The stimulator cells were incubated with media containing various dilutions of HTSU-IgG or with SUA-IgG, media alone, or with anti-CD2 antibody (10 μg/mL; positive control) for 30 minutes. Responder cells were then added, the cultures incubated for 6 days, and the amount of proliferation determined from the amount of3H thymidine incorporated during a 20-hour pulse, as described in “Materials and methods.” The percent proliferation was determined using the following formula: (cpm of experimental/cpm of media-only control ×100). Each bar represents the average of triplicates. Data are a representative experiment of 3 performed.
HTSU-IgG blocks proliferation in a mixed lymphocyte reaction.
PBMCs from 2 healthy adult donors were isolated. PBMCs from one donor served as responder, and the PBMCs from another donor were irradiated and used as stimulator cells as described in “Materials and methods.” The stimulator cells were incubated with media containing various dilutions of HTSU-IgG or with SUA-IgG, media alone, or with anti-CD2 antibody (10 μg/mL; positive control) for 30 minutes. Responder cells were then added, the cultures incubated for 6 days, and the amount of proliferation determined from the amount of3H thymidine incorporated during a 20-hour pulse, as described in “Materials and methods.” The percent proliferation was determined using the following formula: (cpm of experimental/cpm of media-only control ×100). Each bar represents the average of triplicates. Data are a representative experiment of 3 performed.
Discussion
The expression of HTLV-I SU binding protein(s) on primary cells of the immune system was examined. An HTLV-I SU immunoadhesin specifically bound to adult T-cell subsets, B cells, NK cells, and macrophages. This is consistent with recent work using HTLV-I SU immunoadhesins to directly examine the expression of the SU binding proteins on established cell lines. These studies revealed that all cell lines of vertebrate origin tested express cell-surface molecules capable of specifically binding HTLV-I SU.25 26 Cell stimulation dramatically increased the amount of binding, with the highest levels observed on CD4+ and CD8+ T cells, the main targets of HTLV-I and HTLV-II. Naive (CD45RAhigh, CD62Lhigh) resting CD4+ T cells derived from cord blood cells do not bind detectable levels of HTLV-I SU, while naive and memory resting adult CD4+ T cells bind low levels. Following stimulation, regardless of the mode of activation, the HTLV SU binding protein is rapidly expressed on the cell surface of naive T cells. Within 72 hours of activation, the level of binding of the HTLV-I SU immunoadhesin to cord blood CD4+ T cells is similar to that seen for adult CD4+ T cells. Thus, an HTLV SU binding protein is an early activation marker for T cells.
The rapid increase in the level of HTSU-IgG binding following T-cell activation suggests that HTLV SU binding proteins could play a role in immune regulation. As one approach to investigate this, the effect of the immunoadhesins on in vitro alloresponses was examined. When incubated with human PBMCs in a mixed leukocyte reaction, HTSU-IgG inhibited proliferation at 0.15 ng/mL, while the nonspecific SUA-IgG had no effect. These results suggest a role for the HTLV-I SU binding proteins in the immunobiology of CD4+ T cells. Recent studies showing that dendritic cells from HTLV-I–infected monocytes and from ATL patients cannot stimulate autologous and heterologous CD4+ T cells36 suggest a clinical relevance to the inhibition by HTSU-IgG. At higher concentrations, HTSU-IgG inhibited proliferation in a dose-dependent manner. However, the SUA-IgG also had an inhibitory effect of the MLR, although this effect was less than the inhibition by HTSU-IgG. It is possible that the IgG portion of the immunoadhesins is binding to the Fc receptor on monocytic antigen-presenting cells (APCs)34,35 and thus indirectly blocking APC–T-cell interactions in an MLR. Since dendritic cells do not possess any Fc receptors except CD32, a low-affinity Fc receptor for aggregated IgG,37 studies are currently under way to determine the effect of HTLV-I SU on pure DC–T-cell interactions.
The concept that HTLV-I SU binding proteins may be important for T-cell immunobiology must be reconciled with previous observations that cell-surface molecules capable of specifically binding HTLV-I SU are ubiquitously expressed on vertebrate cells.22-26 One possible explanation is that the cell-surface protein(s) that specifically binds the HTLV-I SU varies genetically among different cell types and/or species.
An intriguing alternative possibility is suggested by recent reports that both HTLV-I Env-mediated syncytia formation and T-cell antigen-receptor signaling require the presence of lipid rafts.38,39 These studies, along with our observations that the HTSU-IgG blocks proliferation in an MLR, raise the possibility that the requirements for these 2 processes, while distinct, could be overlapping. In addition, a number of cell-surface molecules appear to play a critical role in both these functions. Several earlier studies have shown that molecules important for the T-cell immune response play a role in regulating HTLV-I Env-mediated syncytia formation, including VCAM-140 and major histocompatibility complex class II molecules.41 The ability of antibodies against these molecules to block Env-mediated fusion was shown to reflect protein crowding and/or steric effects of the immunoglobulin molecules bound to the cell surface, rather than by blocking SU-receptor interactions.40 41 It is possible that the effect of HTSU-IgG on the MLR was similarly indirect.
Previously, it has been reported that HTLV-I virions stimulate T-cell activation.32,33 In contrast, HTSU-IgG did not stimulate T-cell activation, even at concentrations where a significant amount of cell-surface binding was observed. Up-regulation of LFA-1 or LFA-3 on T cells is sufficient to cause activation of resting T cells.42 Similarly, it has previously been reported that HTLV-I–infected T cells can activate resting T cells through this pathway; this activation is inhibited by antibodies directed against LFA-1, LFA-3, CD2, IL-2, and IL-2Rα. However, in this context, antibodies to HTLV-I-Env do not block T-cell activation.33,43 T-cell activation by virions involves the CD2 receptor activation pathway43-45 and most likely reflects CD2-LFA-3 interactions.45 These previous reports are consistent with the inability of soluble HTSU-IgG to activate T cells. However, it is still possible that HTLV SU could have a role in the activation of T cells in the context of the SU/TM trimer and the viral membrane.
Although HTLV-I SU binding proteins are ubiquitously expressed, infectivity in vivo is essentially limited to T lymphocytes. It has been assumed that this reflects blocks in the viral life cycle in non-T cells that occur after virus-cell fusion. However, the observations that the HTLV-I SU binding protein is expressed in primary T lymphocytes at higher levels than that observed for established T and other cell lines (Figures 1-2)26 raises the possibility that the in vivo tropism reflects in part the higher density and/or affinity of HTLV SU binding proteins on T cells. For HIV, receptor density has been shown to play a critical role in the susceptibility of different cell types to infection.46 47
The observation that naive cord blood T lymphocytes lack the protein critical for binding and entry of the HTLV-I virus may explain some clinical aspects of HTLV-I infection. Postnatally infected individuals are at the highest risk for ATL.48 Studies of the route of postnatal infection have revealed that intrauterine infection is extremely rare and that that mother-to-child transmission is primarily by breast feeding.49 Our results suggest that the poor transmission during these periods could reflect the absence of a significant level of HTLV-I receptor(s) on appropriate target cells in cord blood.
The failure to identify an individual receptor for HTLV has raised the possibility that more than one cell-surface molecule critical for HTLV-Env–mediated fusion and entry exists. This molecule, perhaps restricted to CD4+ T cells, would confer efficient susceptibility to HTLV-I infection. The ability of HTSU-IgG to distinguish between efficient binding and little or no binding may prove to be a useful tool in addressing this possibility.
The content of this publication does not reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government.
The authors thank John Young, Ken Bradley, Sara Klucking, and Jessica Bernestrom (University of Wisconsin, Madison, WI) for reagents and useful discussions; Ken Hadlock and Steve Foung for reagents; and Andrea Baines for many useful comments. The publisher or recipient acknowledges the right of the US government to retain a nonexclusive, royalty-free license in and to any copyright covering this article.
Prepublished online as Blood First Edition Paper, December 27, 2002; DOI 10.1182/blood-2002-07-2277.
Supported in whole or in part by federal funds from the National Cancer Institute under contract no. NO1-CO-12400.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Francis W. Ruscetti, Basic Research Laboratory, Bldg 567, Rm 253, National Cancer Institute at Frederick, Frederick, MD 21702-1201; e-mail:ruscettif@mail.ncifcrf.gov.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal