Abstract
All thrombolytic agents in current clinical usage are plasminogen activators. Although effective, plasminogen activators uniformly increase the risk of bleeding complications, especially intracranial hemorrhage, and no laboratory test is applicable to avoid such bleeding. We report results of a randomized, blinded, dose-ranging comparison of tissue-type plasminogen activator (TPA) with a direct-acting thrombolytic agent, plasmin, in an animal model of fibrinolytic hemorrhage. This study focuses on the role of plasma coagulation factors in hemostatic competence. Plasmin at 4-fold, 6-fold, and 8-fold the thrombolytic dose (1 mg/kg) induced a dose-dependent effect on coagulation, depleting antiplasmin activity completely, then degrading fibrinogen and factor VIII. However, even with complete consumption of antiplasmin and decreases in fibrinogen and factor VIII to 20% of initial activity, excessive bleeding did not occur. Bleeding occurred only at 8-fold the thrombolytic dose, on complete depletion of fibrinogen and factor VIII, manifest as prolonged primary bleeding, but with minimal effect on stable hemostatic sites. Although TPA had minimal effect on coagulation, hemostasis was disrupted in a dose-dependent manner, even at 25% of the thrombolytic dose (1 mg/kg), manifest as rebleeding from hemostatically stable ear puncture sites. Plasmin degrades plasma fibrinogen and factor VIII in a dose-dependent manner, but it does not disrupt hemostasis until clotting factors are completely depleted, at an 8-fold higher dose than is needed for thrombolysis. Plasmin has a 6-fold margin of safety, in contrast with TPA, which causes hemorrhage at thrombolytic dosages.
Introduction
Thrombolytic therapy with plasminogen activators is a highly effective modality for achieving both vascular reperfusion and clinical benefit in patients with arterial occlusions, for example, acute myocardial infarction, peripheral arterial occlusion, and ischemic stroke.1 However, such therapy is unfortunately accompanied by a significant risk of intracranial hemorrhage (ICH), which occurs in 1% to 2% of patients who receive continuous treatment for 2 to 24 hours.2,3 ICH is especially prevalent in patients who are older and smaller, those who are hypertensive or have a history of transient ischemic attack or stroke, and those treated with tissue plasminogen activator (TPA).4,5 Patients treated for ischemic stroke are especially prone to symptomatic or lethal ICH, with an occurrence of 10% to 16% after receiving TPA or streptokinase (SK), about 10-fold greater than in patients who receive only anticoagulation.6 To date, the use of every effective thrombolytic agent causes this adverse outcome, and even newly approved TPA mutants7,8 or reduced-dosage regimens of TPA9 share this risk for ICH.
We recently reported on an alternative to plasminogen activator-type thrombolytic agents, namely, plasmin, a direct fibrinolytic enzyme that does not require plasminogen to achieve thrombolysis.10First tested for efficacy more than 40 years ago, plasmin was ineffective when administered by the intravenous route11because it was neutralized by plasma antiplasmin.12However, our regional administration of plasmin by catheter directly to the thrombus avoids antiplasmin neutralization, and rabbit arterial and venous thrombolysis models clearly document thrombolytic activity of plasmin.10,13 In addition, our studies show a significant advantage of plasmin over TPA in its safety from fibrinolytic hemorrhage.10 Thrombolytic doses of plasmin (2 or 4 mg/kg) did not cause bleeding, whereas thrombolytic doses of TPA (1 or 2 mg/kg) induced fibrinolytic hemorrhage.10 Our explanation for safety using plasmin is that antiplasmin neutralizes plasmin that appears in the circulation, whereas TPA circulates, binds to hemostatic plugs at sites of vascular injury, degrades fibrin, and induces fresh hemorrhage.10
Although plasmin clearly shows safety from bleeding with therapeutic (thrombolytic) dosages, important questions still remain regarding its pharmacophysiology. For example, does a higher dosage of plasmin consume antiplasmin, thereby allowing free plasmin to circulate, degrade clotting factors, and induce hemorrhage? If so, our hypothesis regarding the mode of action of plasmin would be strengthened. In addition, such results would indicate that laboratory monitoring of antiplasmin could provide a useful predictive marker for bleeding. Because there is no currently applicable laboratory assay available for predicting hemorrhage in a patient receiving a plasminogen activator, such a monitoring tool for thrombolytic therapy with plasmin would represent a unique advantage for its use. Further, does bleeding follow antiplasmin consumption or does bleeding occur only after further consumption of clotting factors such as fibrinogen and factor VIII? Observations regarding these laboratory end points could provide insights for the pathophysiology of plasmin-induced bleeding and for its prevention.
Accordingly, this study uses an animal model of fibrinolytic hemorrhage14 to evaluate escalating dosages of plasmin, above the 2-mg/kg dose that dissolves experimental thrombi,13 to determine if a threshold dose for bleeding exists and if such bleeding can be predicted or at least explained by changes in blood coagulation or fibrinolytic parameters. As control, and to test what dose of TPA can be administered without inducing bleeding, TPA at progressively lower doses, below that which is optimal for thrombolysis (1 mg/kg),13 were compared for bleeding manifestations and for blood coagulation parameters.
Materials and methods
Human plasminogen was purified from plasma by affinity chromatography on lysine–Sepharose 4B (Amersham Pharmacia, Uppsala, Sweden),15,16 eluted with 0.1 M glycine and 0.03 M lysine at pH 3.0, and stabilized by addition of ε-aminocaproic acid (EACA; 0.02 M final concentration). After adjustment to pH 7.5, the plasminogen was activated to plasmin with a 100:1 molar ratio of plasminogen to streptokinase (American Diagnostica, Greenwich, CT) in 0.05 M glycine, 0.015 M l-lysine, 0.01 M EACA, and 10% glycerol. After 16 hours at 4°C, activation was stopped with sodium chloride and EACA, final concentrations 0.5 M and 0.25 M, respectively. The pH was adjusted to 8.5 and SK-activated plasminogen was applied to a benzamidine–Sepharose 6B (Amersham Pharmacia Biotech, Piscataway, NJ) column equilibrated with 0.05 M Tris (tris(hydroxymethyl)aminomethane), 0.5 M sodium chloride, 0.25 M EACA buffer at pH 8.5. Plasmin was eluted with 1.0 M EACA, pH 7.5, and SK was removed by octyl–Sepharose 4 FF (Amersham Pharmacia) chromatography at pH 3.4 after equilibration with 0.1 M glycine, 0.03 M lysine. Polyacrylamide gel electrophoretic analysis with sodium dodecyl sulfate (SDS-PAGE) showed a single nonreduced band and bands corresponding to heavy and light chains under reduced conditions. Final preparations showed N-terminal lysine, contained 18 to 23 caseinolytic U/mg17 and were not contaminated by SK, as measured by enzyme-linked immunosorbent assay (ELISA).
The experimental system for assessing fibrinolytic hemorrhage used the rabbit ear puncture model based on the “rebleed phenomenon.”14 18 The experimental protocol was approved by the Animal Care and Use Committee at Los Angeles Orthopaedic Hospital. Thirty New Zealand white rabbits (Hazelton Research Products, Denver, PA) weighing 3.5 to 4.5 kg were assigned to 6 treatment groups in a randomized, prospective manner. Following these observations, baseline measurements were obtained in an additional group of 5 rabbits, which received saline only, in an open, unblinded manner.
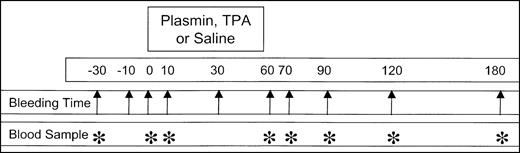
Animals were anesthetized with 40 mg/kg ketamine HCl (Phoenix Pharmaceuticals, St Joseph, MO) and 5 mg/kg xylazine (Phoenix Pharmaceuticals; 2.7-3.25 mL total volume) by intramuscular injection. After anesthesia was achieved, the skin of both ears and the neck was shaved and a depilatory (Nair; Sally Hansen Division, Del Laboratory, Farmingdale, NY) was applied to the ears. The left external jugular vein was exposed surgically, catheterized with a 30-cm 7F 3-lumen catheter (Braun, Bethlehem, PA), and attached to syringes containing saline, anesthesia mixture, and experimental infusion. Anesthesia was maintained by intermittent injections of the ketamine HCI/xylazine mixture every 3 to 10 minutes as required. Primary bleeding times were determined after full-thickness punctures (3.5 mm width) of the ear with a number 11 surgical blade (Feather Safety Razor, Medical Division, Osaka, Japan), at 30 and 10 minutes and immediately prior to initiation of TPA or plasmin infusion. The duration of bleeding was measured by absorption of blood onto 11-cm circular filter paper discs (Whatman International, Maidstone, United Kingdom) at 30-second intervals. Thrombolytic was administered by continuous pump infusion (Sage Instruments, Orion Research, Boston, MA) of a 10-mL sample over 50 to 55 minutes. Each rabbit received 1 of 30 coded samples, prospectively randomized and blinded to the observer, containing TPA (Genentech, South San Francisco, CA; 1, 2, or 4 mg) or human plasmin (16, 24, or 32 mg). Plasmin used in these experiments had a pH of 3.7 prior to infusion. All infusates were prepared at Bayer Corporation, stored at −80°C, then thawed at 32°C just before infusion. After initiation of the infusion, ear bleeding times were performed at 10, 30, 60, 70, 120, and 180 minutes. During the 3-hour observation interval after initiation of infusion, all lesions were monitored for renewed bleeding. No infused animal died prior to being killed at 180 minutes with intravenous sodium pentobarbital (48 mg/2 mL, Kentosol injection; Med-Pharmex, Pomona, CA). Figure1 illustrates the experimental plan, showing the times for ear puncture bleeding times (arrow) and blood samples (*) relative to agent or saline infusion.
Schematic representation of the experimental design.
Diagram shows the bleeding times (arrows) and blood samples (*) relative to the 50- to 60-minute infusion of plasmin or TPA. Infusions into rabbits (4 kg body weight) were performed in a randomized, blinded manner, using coded vials containing plasmin (16, 24, or 32 mg) or TPA (1, 2, or 4 mg) in a total volume of 10 mL. Data were pooled by group for statistical analysis, without knowledge of treatment. Control infusions of 10 mL saline to 5 animals were performed in an open-label manner at the end of the study.
Schematic representation of the experimental design.
Diagram shows the bleeding times (arrows) and blood samples (*) relative to the 50- to 60-minute infusion of plasmin or TPA. Infusions into rabbits (4 kg body weight) were performed in a randomized, blinded manner, using coded vials containing plasmin (16, 24, or 32 mg) or TPA (1, 2, or 4 mg) in a total volume of 10 mL. Data were pooled by group for statistical analysis, without knowledge of treatment. Control infusions of 10 mL saline to 5 animals were performed in an open-label manner at the end of the study.
Blood samples were obtained at 30 minutes and immediately prior to the start of infusion, and at 10, 60, 70, 90, 120, and 180 minutes after the start. Blood (4.5 mL) was collected on citrate anticoagulant (0.5 mL, 0.25 M sodium citrate) through a dedicated lumen of the external jugular vein catheter and kept at 4°C until the end of each experiment. After centrifugation at 3000g at 4°C for 30 minutes, plasma was tested immediately for factor VIII activity, fibrinogen concentration, and functional antiplasmin activity according to established procedures.19-21
Data were grouped according to coded samples and analyzed without knowledge of the specific agent infused by χ2 and byt test or repeated measure analysis of variance (ANOVA) comparison of means.
Results
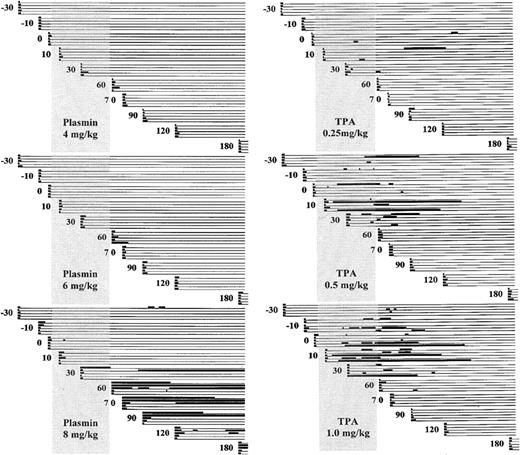
Figure 2 schematically illustrates the ear puncture primary bleeding times (PBTs) and rebleeding episodes for animals that received TPA (0.25, 0.5, or 1.0 mg/kg) or plasmin (4, 6, or 8 mg/kg).
Schematic representation of bleeding.
Bleeding is diagramed before, during, and after infusion of plasmin (left) or TPA (right) to groups of 5 rabbits. PBTs were performed on cohorts of 5 animals at −30 minutes, −10 minutes, and 0 minutes before infusion and at 10, 30, 60, 70, 90, 120, and 180 minutes after the start of infusion. The vertical shaded rectangle represents the infusion period from 0 to 55 to 60 minutes. The heavy lines indicate when bleeding occurred, either the primary bleeding times shown at the extreme left margin of each line, or renewed bleeding at these same sites over the 180-minute observation period. Intervals of stable hemostasis are indicated by the light horizontal lines.
Schematic representation of bleeding.
Bleeding is diagramed before, during, and after infusion of plasmin (left) or TPA (right) to groups of 5 rabbits. PBTs were performed on cohorts of 5 animals at −30 minutes, −10 minutes, and 0 minutes before infusion and at 10, 30, 60, 70, 90, 120, and 180 minutes after the start of infusion. The vertical shaded rectangle represents the infusion period from 0 to 55 to 60 minutes. The heavy lines indicate when bleeding occurred, either the primary bleeding times shown at the extreme left margin of each line, or renewed bleeding at these same sites over the 180-minute observation period. Intervals of stable hemostasis are indicated by the light horizontal lines.
Five animals that were infused with saline control showed no change in PBT, mean of 50 tests 2.25 ± 0.4 minutes (not shown). TPA at 1 mg/kg showed a nonsignificant prolongation of the PBT during infusion (8.2 ± 20.0 versus 2.3 ± 0.9; P = .26), with return to pretreatment values after infusion (Table1). Plasmin at 4 mg/kg had no effect on PBT; at 6 mg/kg, the posttreatment PBT was slightly prolonged. At 8 mg/kg, plasmin strikingly prolonged the PBT, but only after the infusion was completed (25.5 ± 41.0 minutes).
Mean ± SD of PBT relative to start of plasmin or TPA infusion
| . | Plasmin . | TPA . | ||||
|---|---|---|---|---|---|---|
| . | 4 mg/kg . | 6 mg/kg . | 8 mg/kg . | 0.25 mg/kg . | 0.5 mg/kg . | 1 mg/kg . |
| Before treatment | 2.1 ± 0.6 | 2.2 ± 0.4 | 2.6 ± 1.4 | 1.8 ± 0.7 | 2.4 ± 0.9 | 2.3 ± 0.9 |
| Treatment | 2.3 ± 1.9 | 3.0 ± 1.0 | 5.6 ± 8.0 | 2.1 ± 1.3 | 3.2 ± 1.2 | 8.2 ± 20.0 |
| After treatment | 2.6 ± 1.4 | 3.6 ± 2.8* | 25.5 ± 41.0† | 1.9 ± 1.1 | 3.0 ± 1.2 | 2.6 ± 1.2 |
| . | Plasmin . | TPA . | ||||
|---|---|---|---|---|---|---|
| . | 4 mg/kg . | 6 mg/kg . | 8 mg/kg . | 0.25 mg/kg . | 0.5 mg/kg . | 1 mg/kg . |
| Before treatment | 2.1 ± 0.6 | 2.2 ± 0.4 | 2.6 ± 1.4 | 1.8 ± 0.7 | 2.4 ± 0.9 | 2.3 ± 0.9 |
| Treatment | 2.3 ± 1.9 | 3.0 ± 1.0 | 5.6 ± 8.0 | 2.1 ± 1.3 | 3.2 ± 1.2 | 8.2 ± 20.0 |
| After treatment | 2.6 ± 1.4 | 3.6 ± 2.8* | 25.5 ± 41.0† | 1.9 ± 1.1 | 3.0 ± 1.2 | 2.6 ± 1.2 |
Pretreatment times were −30, −10, and 0 minutes; treatment times, 10 and 30 minutes; and posttreatment times, 60, 70, 90, 120, and 180 minutes.
P = .02 versus before treatment.
P = .027 and .04 versus before treatment and after treatment.
Ear puncture sites were continuously scrutinized for renewed bleeding for a maximum of 180 minutes. All 10 rabbits infused with TPA at 1 or 0.5 mg/kg and 4 of the 5 rabbits receiving only 0.25 mg/kg showed renewed bleeding from previously stable ear puncture sites at 36%, 30% and 12%, respectively, of 50 sites. None of the animals exposed to plasmin at 4 mg/kg or 6 mg/kg manifested rebleeding. Rebleeding from stable hemostatic sites occurred in 3 of 5 rabbits that received plasmin at 8 mg/kg, at 16% of the ear puncture sites.
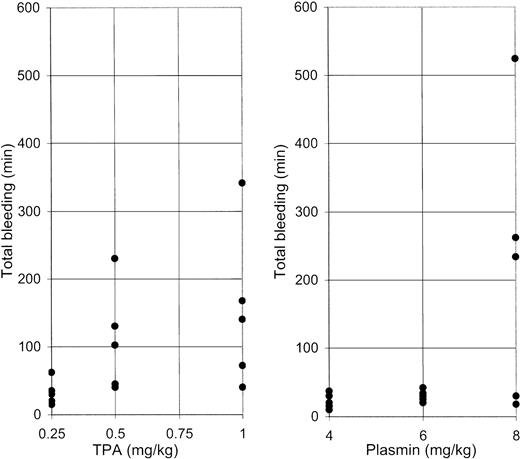
Figure 3 shows the absolute total duration of bleeding for each rabbit. Bleeding for more than 50 minutes occurred more often at higher doses of TPA, in 1 of 5, 3 of 5, and 4 of 5 rabbits receiving TPA at 0.25, 0.5, and 1 mg/kg, respectively, suggesting a dose-dependent pattern of bleeding. Plasmin caused excessive bleeding only at the highest dose (3 of 5 animals), suggesting a distinctive threshold, rather than a dose-dependent, pattern of bleeding.
Total bleeding induced by TPA or plasmin.
Results shown for 5 animals that received 0.25, 0.5, or 1 mg/kg TPA (left panel) or 4, 6, or 8 mg/kg of plasmin (right panel). Total bleeding is calculated for individual animals as the sum of bleeding detected from all 10 puncture sites (Figure 2).
Total bleeding induced by TPA or plasmin.
Results shown for 5 animals that received 0.25, 0.5, or 1 mg/kg TPA (left panel) or 4, 6, or 8 mg/kg of plasmin (right panel). Total bleeding is calculated for individual animals as the sum of bleeding detected from all 10 puncture sites (Figure 2).
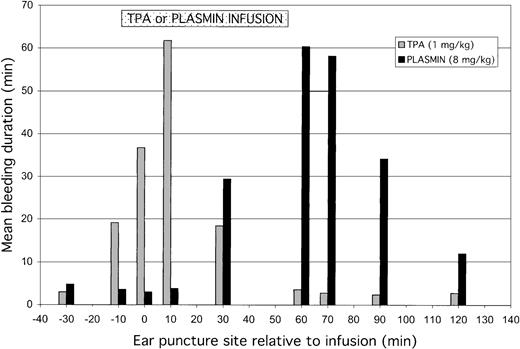
The mean duration of bleeding at each ear puncture site, induced before, during, or after infusion of TPA or plasmin, is shown in Figure4. TPA disrupted hemostasis at sites induced prior to or during infusion, with no effect at sites induced after the infusion (60 minutes and thereafter). By contrast, plasmin had no effect on preinfusion ear puncture sites or on those induced early (10 minutes) into the infusion. Rather, plasmin caused bleeding from puncture sites induced toward the end (30 minutes) and after the infusion.
Total bleeding induced by TPA or plasmin.
Total bleeding induced by TPA (1 mg/kg) or plasmin (8 mg/kg) relative to the time during which agent was infused. Results are expressed as mean of 5 animals for either treatment group. The preinfusion ear puncture bleeding times were performed at −30, −10, and 0 minutes before starting TPA or plasmin. During the 55- to 60-minute infusions, ear punctures were induced at 10 and 30 minutes; postinfusion bleeding times were performed at 60, 70, 90, and 120 minutes. TPA (░) caused increased bleeding from the sites induced before or during infusion, most evident at the 10-minute site, whereas plasmin (▪) caused bleeding from midpoint of infusion and at all subsequent ear puncture sites. Results for bleeding at 180 minutes are not shown because experiments were terminated just after these observations.
Total bleeding induced by TPA or plasmin.
Total bleeding induced by TPA (1 mg/kg) or plasmin (8 mg/kg) relative to the time during which agent was infused. Results are expressed as mean of 5 animals for either treatment group. The preinfusion ear puncture bleeding times were performed at −30, −10, and 0 minutes before starting TPA or plasmin. During the 55- to 60-minute infusions, ear punctures were induced at 10 and 30 minutes; postinfusion bleeding times were performed at 60, 70, 90, and 120 minutes. TPA (░) caused increased bleeding from the sites induced before or during infusion, most evident at the 10-minute site, whereas plasmin (▪) caused bleeding from midpoint of infusion and at all subsequent ear puncture sites. Results for bleeding at 180 minutes are not shown because experiments were terminated just after these observations.
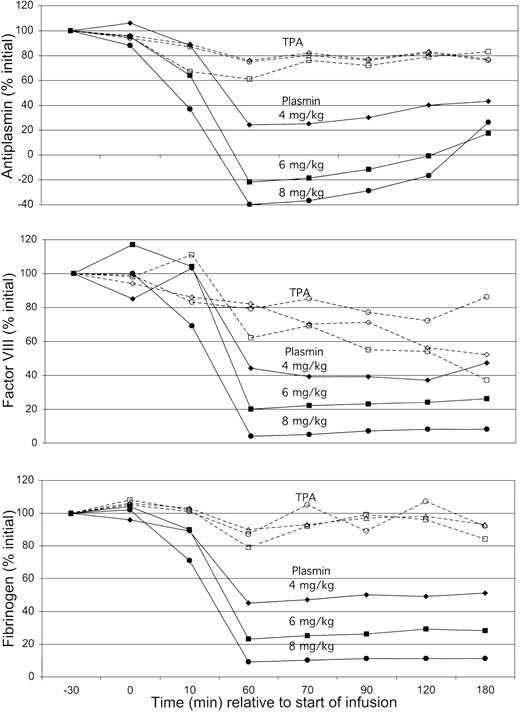
To assess whether blood coagulation contributed to these patterns of bleeding, antiplasmin, factor VIII, and fibrinogen were monitored over the course of each experiment (Figure 5). TPA caused minimal changes in antiplasmin activity or fibrinogen and only a mild decrease in factor VIII to 60% of initial concentration. There was no difference in coagulation factor concentration at TPA doses between 0.25 mg/kg and 1 mg/kg. Results with plasmin were different, showing a dose-dependent decrease in all 3 laboratory parameters, reaching nadir levels at the end of infusion (60 minutes). Antiplasmin activity produced apparent negative nadir values, probably reflecting the presence of catalytically active plasmin entrapped in α2-macroglobulin.22
Concentrations of plasma antiplasmin, factor VIII, and fibrinogen.
Plasma antiplasmin (top), factor VIII (middle), and fibrinogen concentrations (bottom) in animals exposed to TPA (open symbols) or plasmin (solid symbols) over the course of the 180-minute observation. Mean results for each cohort of 5 animals expressed as percent of pretreatment value at −30 minutes, normalized to 100%. Data are corrected for results obtained with saline alone, which was associated with a 5% decrease in all results during the 60-minute infusion and with a 10% decrease in values of samples obtained from 60 to 180 minutes. Results for TPA were not different at dosages of 0.25 mg/kg (○), 0.5 mg/kg (■), or 1.0 mg/kg (⋄). Plasmin showed a dose-dependent decrease in all 3 assays on exposure to increasing dosages of 4 mg/kg (♦), 6 mg/kg (▪), and 8 mg/kg (●). Antiplasmin activity was fully depleted with plasmin at 6 mg/kg, a dose that decreased fibrinogen and factor VIII to 20% to 30% of initial activity. Only at a dose of 8 mg/kg did plasmin cause depletion of fibrinogen and factor VIII to levels at or below the lower limit of quantification.
Concentrations of plasma antiplasmin, factor VIII, and fibrinogen.
Plasma antiplasmin (top), factor VIII (middle), and fibrinogen concentrations (bottom) in animals exposed to TPA (open symbols) or plasmin (solid symbols) over the course of the 180-minute observation. Mean results for each cohort of 5 animals expressed as percent of pretreatment value at −30 minutes, normalized to 100%. Data are corrected for results obtained with saline alone, which was associated with a 5% decrease in all results during the 60-minute infusion and with a 10% decrease in values of samples obtained from 60 to 180 minutes. Results for TPA were not different at dosages of 0.25 mg/kg (○), 0.5 mg/kg (■), or 1.0 mg/kg (⋄). Plasmin showed a dose-dependent decrease in all 3 assays on exposure to increasing dosages of 4 mg/kg (♦), 6 mg/kg (▪), and 8 mg/kg (●). Antiplasmin activity was fully depleted with plasmin at 6 mg/kg, a dose that decreased fibrinogen and factor VIII to 20% to 30% of initial activity. Only at a dose of 8 mg/kg did plasmin cause depletion of fibrinogen and factor VIII to levels at or below the lower limit of quantification.
The disparate dose-dependent effects of TPA and plasmin on coagulation and bleeding are shown in Figure 6. Progressively higher doses of TPA showed no dose-dependent effect on fibrinogen, but did show a stepwise increase in bleeding (P = .051 and P = .055, respectively, for 1 mg/kg and 0.5 mg/kg versus 0.25 mg/kg). On the contrary, plasmin caused a dose-dependent decrease in fibrinogen concentration, but a threshold effect on hemostasis, excessive bleeding occurring only at the highest dose. Excessive bleeding with TPA occurred despite maintenance of fibrinogen concentrations above 150 mg/dL, whereas plasmin at 4 or 6 mg/kg decreased the fibrinogen concentration to 100 mg/dL without causing bleeding. Plasmin caused bleeding only when the fibrinogen concentration was less than the lower limit of quantification (25 mg/dL). The timing of the nadir fibrinogen values caused by plasmin (8 mg/kg; Figure 5) coincided with the time that ear punctures showed prolonged bleeding, near to or after the end of infusion (Figure 2).
Fibrinogen concentrations compared with total bleeding.
Nadir fibrinogen concentrations (top) are compared with total bleeding (bottom) in animals receiving TPA or plasmin. Control values for nadir fibrinogen concentrations, at 0 mg/kg of plasmin or TPA, were determined in plasma samples obtained before infusion (at −30 and 0 minutes). Control values for total bleeding were determined in the 5 rabbits that received saline (mean of 50 determinations, 2.25 ± 0.4 minutes). Decrease in plasma fibrinogen concentration with plasmin was dose-dependent and more profound than with TPA, which did not alter the fibrinogen concentration significantly from the pretreatment level. Nevertheless, TPA induced bleeding in a stepwise manner at increasing dosages, unrelated to the plasma fibrinogen concentration. Plasmin did not induce excessive bleeding at 4 or 6 mg/kg. At a plasmin dose of 8 mg/kg, the nadir fibrinogen concentration dropped below the lower limit of quantification, in association with the appearance of excessive bleeding.
Fibrinogen concentrations compared with total bleeding.
Nadir fibrinogen concentrations (top) are compared with total bleeding (bottom) in animals receiving TPA or plasmin. Control values for nadir fibrinogen concentrations, at 0 mg/kg of plasmin or TPA, were determined in plasma samples obtained before infusion (at −30 and 0 minutes). Control values for total bleeding were determined in the 5 rabbits that received saline (mean of 50 determinations, 2.25 ± 0.4 minutes). Decrease in plasma fibrinogen concentration with plasmin was dose-dependent and more profound than with TPA, which did not alter the fibrinogen concentration significantly from the pretreatment level. Nevertheless, TPA induced bleeding in a stepwise manner at increasing dosages, unrelated to the plasma fibrinogen concentration. Plasmin did not induce excessive bleeding at 4 or 6 mg/kg. At a plasmin dose of 8 mg/kg, the nadir fibrinogen concentration dropped below the lower limit of quantification, in association with the appearance of excessive bleeding.
Discussion
This study clearly distinguishes between TPA and plasmin relative to the relationship of plasma coagulation factors with bleeding. TPA caused bleeding regardless of the plasma concentration of antiplasmin, fibrinogen, or factor VIII (Figure 6), consistent with prior observations that show minimal effect of TPA on coagulation23 and no predictive value of laboratory coagulation assays for a bleeding event caused by TPA.2 24-26 By contrast, plasmin caused no excessive bleeding when fibrinogen and factor VIII were present, but induced excessive bleeding when the factors were absent (below the limit of quantification). Thus, for the first time, a clear profile of plasma coagulation assays is linked to protection from bleeding on administration of a thrombolytic agent, albeit only with plasmin.
In addition to these different effects on coagulation, plasmin and TPA exhibited markedly different patterns of bleeding. First, TPA caused bleeding at therapeutic dosage13 and even at 25% of this amount; plasmin caused no bleeding except at supratherapeutic (8-fold) dosages. Second, TPA caused bleeding in a dose-dependent manner, increasing progressively with incremental doses between 0.25 mg/kg and 1 mg/kg, whereas plasmin showed normal hemostasis at doses of 2 mg/kg,10 4 mg/kg, or 6 mg/kg and excessive bleeding only at the threshold dose of 8 mg/kg (Figure 6). Third, TPA caused bleeding only during administration, whereas plasmin (at 8 mg/kg only) caused bleeding toward the end and after termination of infusion (Figure 4). Fourth, TPA caused rebleeding, that is, renewed oozing from previously stable ear puncture sites, whereas plasmin mostly affected primary hemostasis (Figure 2).
The distinct effects of plasmin and TPA on coagulation and bleeding can be explained by their biochemical properties. TPA avidly binds to fibrin on thrombi and at vascular injury sites, where its enzymatic activity is physiologically focused, but causes only limited degradation of plasma coagulation factors.27-29 The dose-dependent nature of TPA bleeding reflects progressively higher local accumulations on hemostatic plugs, thus inducing bleeding from stable ear puncture sites, even from those incurred before the TPA infusion was started. In patients, this effect is indirectly reflected by the correlation of blood TPA concentration with bleeding risk.30 Once the TPA infusion is terminated, the agent is rapidly cleared31 and at these dosages, newly induced, postinfusion trauma sites do not bleed excessively (Figure 4).
The effects of plasmin can now be explained as follows. Plasmin is rapidly neutralized by antiplasmin32 and is thus prevented from circulating and binding to fibrin of hemostatic plugs. Thus, stable ear puncture sites are immune from disruption by infused plasmin. However, we show that at a high enough dose of plasmin (8 mg/kg), antiplasmin activity was depleted and plasmin-sensitive substrates in blood such as fibrinogen and factor VIII were degraded in a dose-dependent manner (Figure 6). When fibrinogen and factor VIII were completely depleted, a threshold of hemostatic protection was breached, allowing bleeding to occur (Figure 6). Such bleeding was observed only toward the end and after plasmin infusion (Figure 2). So long as fibrinogen and factor VIII concentrations remained below the lower limit of quantification (Figure 5), bleeding continued for the ensuing 120-minute observation period (Figure 2).
There are clinical implications for our observations regarding the safety, efficacy, and monitoring of thrombolytic therapy using plasmin. As to efficacy, plasmin would not be applicable for systemic administration, such as in patients with acute myocardial infarction, because safe dosages of the agent would be neutralized by antiplasmin. However, regional administration of plasmin such as in patients with thrombosed arteriovenous shunts, arterial graft occlusions, deep vein thrombosis, or coronary artery thrombosis after failed percutaneous intervention, should be achievable without the risk of ICH that accompanies regional use of TPA, urokinase, or staphylokinase.33-35 Because the dose of plasmin that causes excessive bleeding was more than 6-fold greater (Figure 6) than the effective thrombolytic dose,13 additional dosages of plasmin could be infused locally to maximize thrombolytic efficacy. This potential of safe administration of supratherapeutic amounts of plasmin contrasts with the situation using plasminogen activators, where even reduced dosages are associated with bleeding36and higher doses of TPA-type agents proportionately increase bleeding risk.2
Our concept of plasmin use for thrombolysis, namely, regional infusion for efficacy and dose selection that preserves coagulation for safety, contrasts with the approach used by Nagai et al37 and by Lapchak et al38 using microplasmin in experimental embolic stroke. These studies used systemic (intravenous) rather than regional (intra-arterial) infusion with the objective of depleting, rather than preserving, antiplasmin activity. The authors conclude that neurologic improvement with microplasmin was achieved without increased risk for parenchymal hemorrhage,38 but because the rate of hemorrhage with placebo was much higher than in historical controls,39 the safety of this approach requires further study.
Our results indicate that the threshold pattern of plasmin-induced bleeding occurred only on complete depletion of clotting factors, thus providing a unique circumstance for laboratory monitoring of treatment. In this scenario, plasmin treatment could be monitored and judged as safe from excessive bleeding so long as significant amounts of clotting factors are present. Such monitoring is not feasible for TPA administration, because changes in clotting factor concentration do not correlate with bleeding complications.26
In conclusion, we have compared plasmin with TPA in a randomized and blinded study in an established rabbit model of fibrinolytic hemorrhage. The data provide insights into rational dose selection of plasmin and the role of plasma coagulation for hemostatic safety during plasmin infusion.
Prepublished online as Blood First Edition Paper, November 21, 2002; DOI 10.1182/blood-2002-08-2546.
Supported by research funding provided by the Los Angeles Orthopaedic Foundation and by a research grant from Bayer Corporation to Los Angeles Orthopaedic Hospital.
V.N. and G.J. are employed by the company whose thrombolytic agent (plasmin) was studied in the present work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Victor J. Marder, Los Angeles Orthopaedic Hospital/UCLA, 2400 South Flower St, Los Angeles, CA 90007; e-mail:vmarder@laoh.ucla.edu.