Abstract
Myelofibrosis and osteosclerosis are prominent features arising in mice overexpressing thrombopoietin (TPO). The pivotal role of transforming growth factor β1 (TGF-β1) in the pathogenesis of myelofibrosis has been documented, but the mechanisms mediating osteosclerosis remain unclear. Here, we used mice deficient in osteoprotegerin (OPG), a secreted inhibitor of bone resorption, to determine whether osteosclerosis occurs through a deregulation of osteoclastogenesis. Marrow cells from opg-deficient mice (opg−/−) or wild-type (WT) littermates were infected with a retrovirus encoding TPO and engrafted into anopg−/− or WT background for long-term reconstitution. The 4 combinations of graft/host (WT/WT,opg−/−/opg−/−,opg−/−/WT, and WT/opg−/−) were studied. Elevation of TPO and TGF-β1 levels in plasma was similar in the 4 experimental groups and all the mice developed a similar myeloproliferative syndrome associated with severe myelofibrosis. Osteosclerosis developed in WT hosts engrafted with WT or opg−/− hematopoietic cells and was associated with increased OPG levels in plasma and decreased osteoclastogenesis. In contrast,opg−/− hosts exhibited an osteoporotic phenotype and a growth of bone trabeculae was rarely seen. These findings suggest that osteosclerosis in mice with TPO overexpression occurs predominantly via an up-regulation of OPG in host stromal cells leading to disruption of osteoclastogenesis.
Introduction
Bone remodeling depends on the tightly integrated activity of 2 distinct cell types, the osteoblasts, which construct bone, and the osteoclasts, which resorb bone (for reviews, see Ducy et al1 and Teitelbaum2). Studies of spontaneous mutations and the development of genetically manipulated mice have improved the understanding of the complex processes involved in bone remodeling. Formation of bone is dependent on the number, maturation, and functions of the osteoblasts. Osteoblasts derive from mesenchymal progenitors through the regulatory action of cell-cell and cell-matrix interactions3 and by the actions of growth factors produced locally or present in the circulation.4 Among these growth factors, the most important are insulin-like growth factor I (IGF-I), which increases the differentiation of osteoblasts,5 members of the fibroblast growth factor (FGF) family, which indirectly stimulate their proliferation,6 and members of the transforming growth factor β (TGF-β) multigene family (for a review, see Centrella et al7). Bone morphogenetic proteins (BMPs) have the ability to induce osteoblast differentiation, whereas the actions of TGF-β may be stimulatory or inhibitory.7-10 On the other hand, bone resorption is controlled by monocyte-derived multinucleated, giant osteoclasts.2 Extensive, recent studies have demonstrated that the differentiation of osteoclasts requires 3 main factors. Macrophage colony-stimulating factor/colony-stimulating factor 1 (M-CSF/CSF-1) controls the survival and proliferation of monocytic progenitors.11,12 RANKL (also known as OPGL/ODF/TRANCE) binds to the RANK receptor (receptor activator of nuclear factor κB) expressed on the membrane of osteoclast progenitor cells13 and promotes osteoclast differentiation and maturation.14 Osteoprotegerin (OPG) is a secreted molecule that binds RANKL and strongly inhibits osteoclastogenesis by blunting the interaction RANKL/RANK.15 Genetically manipulated mice have elegantly demonstrated complementing skeletal phenotypes of the OPG/RANKL system. Overexpression of OPG in transgenic mice15 and targeted ablation of RANKL16 or RANK17 in knockout mice resulted in impaired osteoclastogenesis and severe osteopetrosis, whereas administration of soluble RANKL18 or creation of OPG knockout mice14 resulted in enhanced osteoclastogenesis and osteoporosis. Within the bone environment, both OPG and RANKL are synthesized by osteoblast/stromal cells. Recent in vitro studies using bone and bone marrow stromal cell lines have demonstrated that TGF-β1 was a negative regulator of osteoclastogenesis, in part, through a direct stimulation of the transcription and secretion of OPG and a down-regulation of RANKL.19,20 On the other hand, it is also reported that TGF-β with a combination of soluble RANKL and M-CSF strongly promotes the differentiation of osteoclast progenitors.21 Thus, the TGF-β family appears to have opposite functions in bone remodeling with direct stimulatory effects on bone-forming and bone-resorbing cells and with indirect inhibitory effects on bone resorption.
Myelofibrosis and, more occasionally, osteosclerosis are major complications occurring during the evolution of human idiopathic myelofibrosis (IM; also known as agnogenic myeloid metaplasia). IM is a chronic myeloproliferative disorder with a clonal stem cell disorder characterized by a trilineage myeloproliferation, splenomegaly, and extramedullary hematopoiesis.22 The molecular mechanisms underlying the clonal abnormal proliferation of hematopoietic cells23 are still unknown, but the involvement of a number of fibrogenic cytokines derived from the megakaryocyte or monocyte hyperplasia has been repeatedly discussed in relation to the stromal reactive secondary response that leads to myelofibrosis.24-30 Although less extensively studied, it has also been suggested that elevated levels of TGF-β1 and basic fibroblastic growth factor (bFGF) could be implicated in the osteogenic process.31
Overexpression of thrombopoietin (TPO), the physiologic regulator of platelet production, in rodents has provided an experimental model that recapitulates several characteristic features of human IM. High systemic levels of TPO in mice invariably cause a myeloproliferative syndrome associated with marked megakaryocyte and granulocytic hyperplasia, splenomegaly, extramedullary hematopoiesis, splenic and medullary fibrosis, and osteosclerosis.32-34 Recently, we examined the role of TGF-β1 in the promotion of myelofibrosis by engrafting mutant TGF-β1−/− hematopoietic cells, infected with a retrovirus encoding TPO, into lethally irradiated wild-type (WT) hosts. Although myelofibrosis was systematically observed in hosts repopulated with WT cells, no increase in reticulin deposition was seen in mice reconstituted with TGF-β1−/− donor cells, demonstrating that TGF-β1 was essential for the promotion of myelofibrosis.35 In addition to the absence of myelofibrosis, we did not observe significant neotrabecular bone growth in mice engrafted with TGF-β1−/− cells at 4 months after transplantation, at a time when osteopetrosis was systematic and prominent in the marrow cavity from mice repopulated with WT cells. However, although myelofibrosis did not occur at later times, bone growth in the femur lumen was noted after 6 months in animals repopulated with TGF-β1−/− cells.
To understand the mechanisms involved in the osteogenic response, we investigated whether this was due to an impaired osteoclastogenesis through the OPG/RANKL axis. To that end, bone marrow stem cells fromopg knockout mice (opg−/−) or WT littermates were infected with a retrovirus encoding the murine TPO protein and engrafted into lethally irradiatedopg−/− or WT mice for reconstitution in the long term. The 4 combinations of graft/host were studied (WT/WT,opg−/−/opg−/−,opg−/−/WT, and WT/opg−/−). We report here that, whatever the graft/host combination, all the mice developed a comparable myeloproliferative syndrome ending with severe myelofibrosis associated with elevated levels of latent TGF-β1 in the plasma. Severe osteosclerosis was observed when transplantations were performed in a WT background (WT/WT or opg−/−/WT) and was correlated with a marked elevation of OPG in plasma and a decrease in the number of osteoclasts in femurs. In contrast, only rare bony trabeculae merging from the cortical region were seen in hosts lacking OPG in the microenvironment (WT/opg−/−or opg−/−/opg−/−). Together, these observations suggest that the aberrant bone growth seen in mice overexpressing TPO may be the consequence of an inhibition of osteoclastogenesis via an increased production of OPG by stromal cells.
Materials and methods
Animals
Heterozygote opg+/− breeders back-crossed on the C57Bl/6 background were kindly provided by E. Clark (Seattle, WA).36 Mice were bred at the Institut Gustave Roussy animal facility under specific pathogen-free conditions. Genotyping was performed on DNA extracted from distal tail segments from 15-day-old pups using standard techniques. An opglocus-specific primer (primer A: 5′-GGTCCTCCTTGATTTTTCTAGCC-3′) was used in combination with primers specific forneor (primer B: 5′-TGACCGCTTCCTCGTGGCTTT AC-3′) or opg (primer C: 5′-TGCCCTGACCACTCTTATACGGGGAC-3′). Primers A and B amplify a 500-bp product from the targeted allele; primers A and C amplify a 200-bp product from the WT allele. Polymerase chain reaction (PCR) conditions were as described.36
Transduction of BM cells and transplantation
Eight- to 10-week-old male and femaleopg−/− and WT littermates were used as bone marrow (BM) donors or recipients. Four groups of 10 animals each were constituted: opg−/− BM engrafted intoopg−/− hosts (opg−/−/ opg−/−),opg−/− BM into WT hosts (opg−/−/WT), WT BM intoopg−/− hosts (WT/opg−/−), and WT BM into WT hosts (WT/WT). The infection procedure was performed as previously described.34 Briefly, 4 days after 5-fluorouracil treatment (150 mg/kg administered intraperitoneally), marrow cells were cocultured with 1 × 106 MPZenTPO virus-producing GP+E-86 cells in Dulbecco Modified medium (DMEM; Sigma Aldrich, Saint Quentin Fallavier, France) containing 10% heat-inactivated fetal bovine serum (FBS; Gibco BRL, Paisley, United Kingdom), penicillin (100 U/mL), streptomycin (100 μg/mL), glutamine, (2 mM) and supplemented with murine FLT3 ligand (muFLT3-L; 20 ng/mL), murine interleukin 3 (muIL-3; 100 U/ml), muIL-6 (20 ng/ml), and murine stem cell factor (muSCF; 20 ng/mL). All cytokines were purchased from R & D Systems (Oxon, United Kingdom). After 4 days, nonadherent cells were harvested. An aliquot was used immediately in clonogenic progenitor assays to determine the percentage of infected colony-forming cells (CFCs). The remaining were inoculated intravenously via the retro-orbital sinus into lethally irradiated hosts (9.5 Gy, x-ray apparatus, single dose) in a ratio of one donor per one recipient.
In vitro progenitor assay for transduction efficiencies
At the end of the infection protocol, cells were seeded in standard methylcellulose culture (Methocult M3134; Stem Cell Technologies, Vancouver, BC, Canada) supplemented with 1 mMl-glutamine (Gibco BRL) and 10−4 M 2β-mercaptoethanol. Medium contained 20% FBS and a combination of recombinant growth factors including muIL-3 (100 U/mL); pegylated human recombinant megakaryocyte growth and differentiation factor (PEG-rHuMGDF; 10 ng/mL), muSCF (50 ng/mL), and human erythropoietin (huEPO; 2 U/mL). Seeding densities were 2 × 104 cells/mL. Cultures were plated in triplicate and incubated at 37°C in a humidified incubator containing 5% CO2 in air. Seven days following initiation of culture, colonies (> 50 cells) were scored under an inverted microscope and 30 colonies were picked at random. The integrated retroviral sequence was detected by PCR analysis. Primer sets corresponding to the TPO cDNA were sense 5′-ACTTTAGCCTGGAGAATGGAAA-3′ and antisense 5′-CCAGGAGTAATCTTGACTCTGA-3′ allowing the amplification of a 499-bp product. Actin was used as an internal control: sense 5′-GTACCACAGGCATTGTGATG-3′ and antisense 5′-GCAACATAGCACAGCTTCTC-3′. PCR conditions were previously described.35
Hematologic evaluation and histopathology
Orbital plexus blood was collected in citrated tubes at monthly intervals from the anesthetized mice. Nucleated blood cells and differential cell counts, hematocrit level, and platelet counts were determined using an automated blood coulter calibrated for mouse blood (MS9, Schloessing Melet, Cergy-Pontoise, France). Platelet-poor plasma (PPP) was prepared and stored at −20°C for determination of TPO, OPG, and TGF-β1 levels. Twelve weeks after transplantation, 3 mice in each group were humanely killed under anesthesia. Femurs were excised, cleaned of soft tissue, fixed in Glyo-Fixx fixative (CML, Nemours, France), decalcified, and embedded in paraffin. Sections (4-5 μm) were stained with hematoxylin and eosin or Gomori stain for overall cytology and according to Gordon-Sweet for reticulin.
Adenovirus encoding biologically active TGF-β1
WT mice were injected intravenously with 2 × 108plaque-forming units (pfu) of a recombinant adenovirus vector encoding a mutated TGF-β1 cDNA. Cysteines at amino acids 223 and 225 (Ad-TGF-β1s223/s225) were changed to serine resulting in the secretion of a fraction of biologically active TGF-β1 forms.37 38 The empty vector was used as a control. Blood was collected 1 month after the injection and plasma was used for the determination of TGF-β1 and OPG levels.
Cytokine enzyme-linked immunosorbent assay
TPO and OPG levels in plasma were determined with the murine TPO or murine OPG Quantikine Kits from R & D Systems, according to manufacturer's instructions. The sensitivity limits of the assays were 62.5 pg/mL and 31.2 pg/mL, respectively. The human TGF-β1 immunoassay (R & D Systems), which detects only active forms of TGF-β1, was used for determination of circulating TGF-β1 levels. Samples were assayed before (spontaneously active TGF-β1) and after acidification (active and latent forms). For acidification, the protocol recommended by the manufacturer was followed without modification. The sensitivity of the assay was 31.2 pg/mL active TGF-β1.
Statistical analysis
The results are presented as mean ± SD. The data were analyzed with the 2-tailed Student t test.
Results
Hematopoietic changes in WT and opg−/−hosts repopulated with TPO-overexpressing hematopoietic cells
To discern whether OPG was directly implicated in osteosclerosis that develops in mice overexpressing TPO, BM cells from WT or mutant opg−/− littermates were infected with the MPZen-TPO retrovirus and engrafted into lethally irradiated WT or opg−/− recipients. Transduction efficiency in progenitor cells was evaluated at the end of the infection protocol by a colony assay as described in “Materials and methods.” No significant differences were observed between the 2 cellular genotypes with more than 80% transduced progenitor cells (range, 80%-92%; 2 repeated experiments with WT and opg−/− BM cells). Lethally irradiated hosts were engrafted with 2 to 4 × 106 cells and peripheral blood was analyzed at monthly intervals. Whatever the graft-to-host combination, TPO levels in plasma were more than 3000-fold increased 1 month after transplantation (1667 ± 125 ng/mL, n = 24, as compared to 0.464 ± 0.053 ng/mL in normal controls, n = 6) and these values remained elevated during the follow-up (Figure1A). Accordingly, platelet counts rose rapidly peaking at approximately 3.5 × 109/mL 1 and 2 months after transplantation. Although TPO levels remained high, platelet counts slowly declined in each group at 3 months (Figure 1B). Nucleated blood cells were 5- to 7-fold augmented over normal values at 2 months after transplantation and these high numbers were maintained at 3 months (Figure 1C). Whatever the time of examination or the group of mice, no major differences were seen in the differential cell counts. Leukocytosis was mainly due to an increase in polymorphonuclear granulocytes representing about 46% ± 8% of the total nucleated cell population at 3 months (n = 6 mice in each group), monocytes making up 9% ± 3% of white blood cells, and immature myeloid precursor and blast cells reaching values of 12% ± 6% as compared to 12% ± 3%, 1.2% ± 0.3%, and 0% in control mice, respectively (data not shown). In contrast to platelet and nucleated cell counts, hematocrit values in mice that received transplants in the 4 groups rapidly declined and animals became severely anemic with hematocrit values below 25% ± 3% at 3 months after transplantation (Figure 1D). These changes were comparable to those previously described in C57Bl/6 mice overexpressing TPO.34
TPO overexpression causes a comparable elevation in platelet and nucleated blood cell numbers and an anemia in hosts engrafted with TPO virus–infected BM cells from WT or
opg−/− donors. Results are presented as the mean ± SD of 7 to 10 animals per experimental group. WT hosts engrafted with WT BM cells are shown with open triangles (▵); WT hosts engrafted withopg−/− BM cells are shown with filled triangles (▴); opg−/− hosts engrafted with WT BM cells are shown with open squares (■); andopg−/− hosts engrafted withopg−/− BM cells are shown with filled squares (▪). (A) TPO in plasma was measured with an enzyme-linked immunosorbent assay (ELISA) at 1, 2, and 3 months after transplantation. Values in normal WT or opg−/−controls were 0.46 ± 0.05 ng/mL (n = 12). (B) Platelet numbers during the 3 months of follow-up. (C) Total numbers of nucleated blood during the follow-up. (D) Hematocrit values.
TPO overexpression causes a comparable elevation in platelet and nucleated blood cell numbers and an anemia in hosts engrafted with TPO virus–infected BM cells from WT or
opg−/− donors. Results are presented as the mean ± SD of 7 to 10 animals per experimental group. WT hosts engrafted with WT BM cells are shown with open triangles (▵); WT hosts engrafted withopg−/− BM cells are shown with filled triangles (▴); opg−/− hosts engrafted with WT BM cells are shown with open squares (■); andopg−/− hosts engrafted withopg−/− BM cells are shown with filled squares (▪). (A) TPO in plasma was measured with an enzyme-linked immunosorbent assay (ELISA) at 1, 2, and 3 months after transplantation. Values in normal WT or opg−/−controls were 0.46 ± 0.05 ng/mL (n = 12). (B) Platelet numbers during the 3 months of follow-up. (C) Total numbers of nucleated blood during the follow-up. (D) Hematocrit values.
Elevated levels of latent TGF-β1 in plasma
As previously reported,34 35 only insignificant amounts of spontaneously immunoreactive TGF-β1 were measured in PPP at any time during the follow-up and samples were acidified to determine levels of latent TGF-β1 forms. One month after transplantation, latent TGF-β1 levels were augmented 3- to 4-fold over baseline levels measured in controls (15.8 ± 1.2 ng/mL versus 4.1 ± 0.3 ng/mL, respectively). These levels remained 3 times higher than in controls during the 3 months of follow-up. No significant difference was seen between the 4 groups of reconstituted mice (Figure 2).
Sustained elevation in plasmatic TGF-β1 levels in hosts repopulated with WT or
opg−/− TPO virus–infected BM cells. TGF-β1 was measured in PPP by ELISA after acidification of the samples. Each bar represents the mean ± SD of 7 to 10 animals per experimental group as indicated under the x-axis. The mice receiving transplants were analyzed at 1 month (open bars), 2 months (hatched bars), and 3 months (dotted bars), except for the WT/opg−/− group where samples were lost accidentally. Independently of the graft/host combination, levels of latent TGF-β1 were significantly (P < .01) increased over control levels (black bars) in the 4 experimental groups.
Sustained elevation in plasmatic TGF-β1 levels in hosts repopulated with WT or
opg−/− TPO virus–infected BM cells. TGF-β1 was measured in PPP by ELISA after acidification of the samples. Each bar represents the mean ± SD of 7 to 10 animals per experimental group as indicated under the x-axis. The mice receiving transplants were analyzed at 1 month (open bars), 2 months (hatched bars), and 3 months (dotted bars), except for the WT/opg−/− group where samples were lost accidentally. Independently of the graft/host combination, levels of latent TGF-β1 were significantly (P < .01) increased over control levels (black bars) in the 4 experimental groups.
Development of myelofibrosis in WT andopg−/−hosts
Three mice in each group that received transplants were humanely killed 3 months after transplantation. All mice displayed splenomegaly with spleen weights increased up to 10-fold over controls (1000 ± 220 mg versus 122 ± 35 mg, respectively). Examination of histologic sections stained with hematoxylin and eosin revealed that the spleen red pulp was markedly expanded by a massive proliferation of often dysmorphic or apoptotic megakaryocytes found in large clusters associated with numerous neutrophil granulocytes displaying all stages of maturation. Eosinophils were rarely seen. Erythroid cells were poorly represented. Similar cytologic changes were seen in femur sections with an accumulation of megakaryocytes displaying nuclear and cytologic alterations. When sections were stained with silver to reveal reticulin deposition, a prominent densification of the reticulin network was seen in spleen (Figure 3) and marrow (not shown) from all the mice. Spleen sections stained according to Gomori revealed occasional accumulation of collagen fibers (not shown).
Development of myelofibrosis in the spleen of TPO-overexpressing mice.
Representative spleen sections from WT andopg−/− hosts hematologically repopulated with TPO virus–infected BM cells from WT or opg−/−donors at 3 months after transplantation. Sections were stained according to Gordon-Sweet to reveal reticulin fiber deposition. (A) WT host engrafted with WT BM cells. (B) opg−/−host engrafted with opg−/− BM cells. (C) WT host engrafted with opg−/− BM cells. (D)opg−/− host engrafted with WT BM cells. Sections show severe accumulation of reticulin fibers in the 4 experimental groups. Original magnification for all panels, × 200.
Development of myelofibrosis in the spleen of TPO-overexpressing mice.
Representative spleen sections from WT andopg−/− hosts hematologically repopulated with TPO virus–infected BM cells from WT or opg−/−donors at 3 months after transplantation. Sections were stained according to Gordon-Sweet to reveal reticulin fiber deposition. (A) WT host engrafted with WT BM cells. (B) opg−/−host engrafted with opg−/− BM cells. (C) WT host engrafted with opg−/− BM cells. (D)opg−/− host engrafted with WT BM cells. Sections show severe accumulation of reticulin fibers in the 4 experimental groups. Original magnification for all panels, × 200.
Osteosclerosis in WT hosts engrafted with WT oropg−/−BM cells
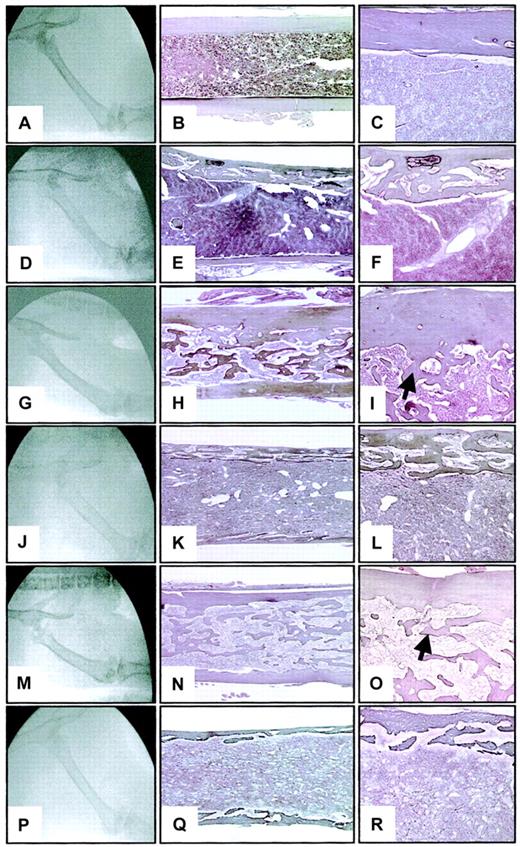
At 3 months after transplantation, femurs of WT hosts reconstituted with TPO virus–infected hematopoietic cells from either WT or opg−/− donors appeared radiographically abnormal. Femurs were usually more radiodense than WT controls (Figure 4A) and showed a disappearance of the distinct cortical margin with a blurring of the medullary compartment (Figure 4G,M). Osteosclerosis was confirmed by histologic examination. The femoral cortical region was thickened and the medullary cavity was filled with interconnecting newly formed bone trabeculae merging from the endosteal side of the cortical region (compare Figure 4B-C to H-I and N-O). Osteoclasts were rarely seen in any sections examined on the endosteal or periosteal bone surfaces.
Radiographic and histologic sections of the femurs of WT and
opg−/− hosts engrafted with WT oropg−/− TPO-overexpressing BM cells.Representative photographs from a WT control (A-C) and a 3-month-oldopg−/− control (D-F) are shown for comparison. The middle column shows longitudinal sections of femurs stained according to Gordon-Sweet (original magnification × 25). Details of the femoral cortical area are illustrated in the right column (original magnification × 60). Histologic sections are stained according to Gordon-Sweet. Note decreased bone mineral radiodensity (D) and severe cortical bone porosity in theopg−/− control (E-F). (G) Radiograph of the femur of a WT host engrafted with WT donor cells. Note significant radiodensification of the bone. (H-I) Representative histologic section of the femur shows the presence of numerous bone trabeculae (arrow) occluding the femoral cavity. (J) Radiograph of the femur of anopg−/− host engrafted withopg−/− donor cells shows poorly defined cortical region and significant loss of radiodensity. (K-L) Severe osteoporosis is confirmed histologically. (M) Radiograph of the femur of a WT host engrafted with opg−/− donor cells. Increased bone density is seen. (N-O) Representative histologic section of the femur shows osteosclerosis with accumulation of bone trabeculae in the shaft (arrow). (P) Radiograph of the femur of anopg−/− host engrafted with WT donor cells. Note thinning of the cortical region and decreased bone density. (Q-R) Histologically, the femur is profoundly osteoporotic with a porous cortical region. Radiography and histology were from the same animals examined 3 months after transplantation. Similar results were observed in 3 mice per experimental groups.
Radiographic and histologic sections of the femurs of WT and
opg−/− hosts engrafted with WT oropg−/− TPO-overexpressing BM cells.Representative photographs from a WT control (A-C) and a 3-month-oldopg−/− control (D-F) are shown for comparison. The middle column shows longitudinal sections of femurs stained according to Gordon-Sweet (original magnification × 25). Details of the femoral cortical area are illustrated in the right column (original magnification × 60). Histologic sections are stained according to Gordon-Sweet. Note decreased bone mineral radiodensity (D) and severe cortical bone porosity in theopg−/− control (E-F). (G) Radiograph of the femur of a WT host engrafted with WT donor cells. Note significant radiodensification of the bone. (H-I) Representative histologic section of the femur shows the presence of numerous bone trabeculae (arrow) occluding the femoral cavity. (J) Radiograph of the femur of anopg−/− host engrafted withopg−/− donor cells shows poorly defined cortical region and significant loss of radiodensity. (K-L) Severe osteoporosis is confirmed histologically. (M) Radiograph of the femur of a WT host engrafted with opg−/− donor cells. Increased bone density is seen. (N-O) Representative histologic section of the femur shows osteosclerosis with accumulation of bone trabeculae in the shaft (arrow). (P) Radiograph of the femur of anopg−/− host engrafted with WT donor cells. Note thinning of the cortical region and decreased bone density. (Q-R) Histologically, the femur is profoundly osteoporotic with a porous cortical region. Radiography and histology were from the same animals examined 3 months after transplantation. Similar results were observed in 3 mice per experimental groups.
Osteoporosis in opg-deficient hosts repopulated withopg−/−or WT BM cells
In contrast to WT repopulated hosts, radiographic analysis ofopg−/− recipients reconstituted with TPO virus–infected hematopoietic cells fromopg−/− (Figure 4J) or WT (Figure 4P) donors showed an overall decrease in bone density comparable to controlopg−/− mice (Figure 4D) of a matched age.18 Histologic analysis of the femurs at 3 months after transplantation showed severe osteoporosis of the femoral cortical margin (Figure 4K,Q) as displayed in agingopg−/− hosts (Figure 4E-F). The cortical region appeared porous (Figure 4L,R) with numerous vessels filled with hematopoietic cells and several resorption pits on the bone surface. When compared with control unmanipulatedopg−/− mice of a matched age, the main difference noticed was the presence of small lamellar structure growing in the marrow space. Abundant osteoblasts and multinucleated osteoclasts lining the endosteal bone surface were seen inopg−/− hosts that received transplants of either WT or opg−/− hematopoietic cells.
OPG levels in plasma
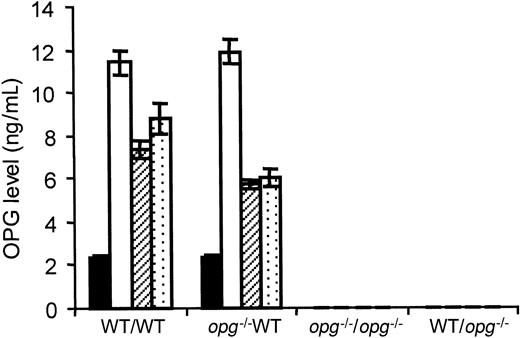
The amount of OPG protein in plasma was measured at monthly intervals in each experimental group. The average OPG level in WT controls from this colony was 2.3 ± 0.2 ng/mL (n = 8) and undetectable in the control opg−/− mutants. One month after engraftment, levels of circulating OPG were about 6-fold augmented (11.6 ± 0.6 ng/mL, n = 12) in WT repopulated hosts. No significant difference was seen between WT mice repopulated with WT or opg−/− cells (P < .1). At 2 and 3 months, levels were decreased but remained 3 to 4 times more elevated than in controls (Figure5). Whatever the time of examination, no correlation was found between TGF-β1 and OPG levels (data not shown). In contrast, no circulating OPG was detected in theopg−/− hosts reconstituted with a WT or anopg−/− transplant at any time.
OPG levels in plasma from hosts repopulated with WT or
opg−/− TPO virus–infected BM cells. OPG levels were measured in PPP by ELISA. Each bar represents the mean ± SD of 7 to 10 animals per experimental group as indicated under the x-axis. The mice receiving transplants were analyzed at 1, 2, and 3 months, except for the WT/opg−/− group where samples were lost accidentally. The level of circulating OPG in WT controls (n = 8) is shown by black bars. One month after transplantation (open bars), OPG levels were 6-fold increased in WT hosts engrafted with TPO virus–infected BM cells from WT or opg−/−donors. Levels remained about 3-fold elevated at 2 months (hatched bars) and 3 months (dotted bars) with no significant difference (P < .5) between the 2 groups. OPG was undetectable in plasma from control and engrafted opg−/−hosts.
OPG levels in plasma from hosts repopulated with WT or
opg−/− TPO virus–infected BM cells. OPG levels were measured in PPP by ELISA. Each bar represents the mean ± SD of 7 to 10 animals per experimental group as indicated under the x-axis. The mice receiving transplants were analyzed at 1, 2, and 3 months, except for the WT/opg−/− group where samples were lost accidentally. The level of circulating OPG in WT controls (n = 8) is shown by black bars. One month after transplantation (open bars), OPG levels were 6-fold increased in WT hosts engrafted with TPO virus–infected BM cells from WT or opg−/−donors. Levels remained about 3-fold elevated at 2 months (hatched bars) and 3 months (dotted bars) with no significant difference (P < .5) between the 2 groups. OPG was undetectable in plasma from control and engrafted opg−/−hosts.
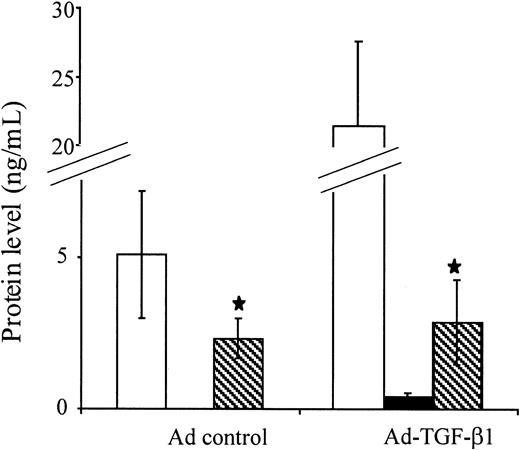
TGF-β1 has been demonstrated to be a potent cytokine-regulating OPG transcription and secretion factor in the in vitro stromal/osteoblastic cell lines.19,20 To investigate whether the increased OPG secretion was related to the elevation in systemic TGF-β1 levels in an animal context, WT mice were inoculated with an adenovirus vector encoding spontaneously bioactive forms of TGF-β1 or the empty vector.38 One month after infection, detectable levels of transgene-encoded bioactive TGF-β1 was measured in plasma (0.41 ± 0.13 ng/mL) in association with a marked elevation of latent forms (21.5 ± 6.2 ng/mL versus 5.1 ± 2.1 ng/mL in controls inoculated with the empty vector). Under these conditions, immunodetectable levels of OPG remained at baseline (Figure6). These data suggest that the rapid onset of osteosclerosis in mice with TPO overexpression is mediated through an up-regulation of endogenous stroma-derived OPG by a mechanism independent from TGF-β1.
Effect of bioactive TGF-β1 on OPG secretion in vivo.
Immunoreactive TGF-β1 (▪), latent TGF-β1 (■), and OPG (▧) were measured in PPP from WT mice infected with 2 × 108 pfu of an adenovirus encoding a mutated TGF-β1 cDNA (Ad-TGF-β1s223/s225) or the empty adenovirus vector as a control. Plasma was collected 1 month after infection. Each bar represents the mean ± SD of 6 animals. For TGF-β1 determination, samples were assayed prior to acidification (bioactive transgene-encoded, TGF-β1) and after acidification (active + latent forms). Black bar indicates spontaneously active TGF-β1; open bar, latent + active TGF-β1; hatched bar, OPG. Levels of OPG in mice treated with the empty or Ad-TGF-β1 vector are not statistically different (★P < .15).
Effect of bioactive TGF-β1 on OPG secretion in vivo.
Immunoreactive TGF-β1 (▪), latent TGF-β1 (■), and OPG (▧) were measured in PPP from WT mice infected with 2 × 108 pfu of an adenovirus encoding a mutated TGF-β1 cDNA (Ad-TGF-β1s223/s225) or the empty adenovirus vector as a control. Plasma was collected 1 month after infection. Each bar represents the mean ± SD of 6 animals. For TGF-β1 determination, samples were assayed prior to acidification (bioactive transgene-encoded, TGF-β1) and after acidification (active + latent forms). Black bar indicates spontaneously active TGF-β1; open bar, latent + active TGF-β1; hatched bar, OPG. Levels of OPG in mice treated with the empty or Ad-TGF-β1 vector are not statistically different (★P < .15).
Discussion
The systematic development of myelofibrosis and osteosclerosis in mice that received transplants of marrow cells infected with a TPO-encoding retrovirus represents a suitable model to study the underlying causes of the pathologic stromal reaction and the abnormal bone growth.32-34 We have previously documented the pivotal role of TGF-β1 secreted by hematopoietic cells in the promotion of myelofibrosis.35 In this report, we present evidence that the aberrant bone construction is mainly mediated by an up-regulation of stroma-derived OPG leading to an inhibition of bone resorption.
Hematopoietic stem cells from mutant homozygoteopg-deficient mice (opg−/−) and WT littermates36 were infected with a retrovirus to overexpress TPO and engrafted into lethally irradiated hosts. OPG is a secreted protein highly expressed in stromal/osteoblastic cells15 and in various immune and hematopoietic tissues from adult mice.39-42 To discriminate the effects of OPG derived from the host microenvironment and the hematopoietic transplant, the 4 different combinations of graft/host were analyzed. Whatever the combinations, all the repopulated mice showed comparable TPO elevation in plasma and rapidly developed the typical myeloproliferative syndrome characterized by thrombocytosis, leukocytosis, severe anemia, splenomegaly with an accumulation of dysmorphic megakaryocytes, and extramedullary hematopoiesis.32,34 In accordance with previous reports,32,35,43 TGF-β1 levels in plasma were 3- to 4-fold elevated over controls (Figure 2) and each recipient displayed prominent myelofibrosis in spleen and femurs within 3 months. Femoral radiographs and histologic analysis of WT hosts engrafted with either WT or opg−/− hematopoietic cells revealed severe osteosclerosis and a rarity of multinucleated osteoclasts. In contrast, rare bone trabeculae, porous bone cortical areas, and active osteoclastogenesis were seen in opg−/−recipients whether they were engrafted with cells originating from WT or opg−/− donors. These observations suggested a role for endogenous host-derived OPG in the induction of osteosclerosis. Because osteosclerosis developing in WT reconstituted hosts was reminiscent of the phenotype of mice overexpressing OPG,15 44 we evaluated OPG plasmatic levels during the time course. Independently of the graft origin (WT oropg−/−), OPG levels in WT hosts were 6-fold increased over controls at 1 month and remained more than 3 times elevated during the follow-up (Figure 5). In contrast, OPG was undetectable in the circulation of opg−/−hosts, even when the animals received transplants of WT bone marrow cells. These findings indicate that an up-regulation of stroma-derived OPG is implicated in the pathogenesis of osteosclerosis developing in mice with TPO overexpression.
The mechanism of OPG up-regulation in this in vivo model is not defined. Previous reports have demonstrated a stimulatory effect of TGF-β1 on OPG secretion in primary osteoblasts and stromal/osteoblastic cell lines.19,20,45 Because TGF-β1 levels are increased in mice with osteomyelofibrosis, it was tempting to postulate a TGF-β1–dependent mechanism. Our findings are not in favor of this hypothesis. First, inoculation of WT mice with an adenovirus encoding biologically active TGF-β1 failed to stimulate OPG secretion (Figure 6). Second, in WT mice developing osteosclerosis, no relationship between levels of OPG and TGF-β1 was found (not shown). Third, in WT mice repopulated with hematopoietic cells originating from TGF-β1−/− donors,35TGF-β1 levels remained at baseline, whereas significant elevations in plasmatic OPG levels were found (data not shown). These data suggest that regulation of OPG secretion might occur in vivo via a mechanism independent of TGF-β1. Cells involved in the pathogenesis of osteomyelosclerosis secrete and release platelet-derived growth factor (PDGF) and IL-1,28,46 2 cytokines that have the ability to stimulate OPG synthesis by stromal cells.47-50 Although we cannot formally exclude a possible role for TGF-β1 bound in the bone extracellular matrix, the present data suggest that the mechanisms leading to in vivo stimulation of OPG secretion still remain to be clarified.
A high level of endogenous TGF-β1 may lead to defective osteoclastogenesis through an inhibition of RANKL expression.19,51 We did not investigate the expression level of RANKL in our model, but such a mechanism appears unlikely because osteoclastogenesis remained active in theopg−/− hosts. On the other hand, it is known that TGF-β family has both positive and negative impacts on osteoblast proliferation and differentiation.7,8,10 The low growth of bone trabeculae in the marrow lumen ofopg−/− hosts that received transplants of WT or opg−/− suggests that TGF-β1 may exert direct stimulatory effects on bone construction.9Nevertheless, stimulation of bone construction appears to be far less potent that inhibition of resorption because none of theopg−/− hosts showed osteosclerosis at 3 months.
Collectively, the data confirm the prominent impact of TGF-β1 in the development of myelofibrosis in mice hyperstimulated with TPO35 and demonstrate the important role of OPG in the pathogenesis of osteosclerosis. The cellular types involved in the increased and sustained secretion of TGF-β1 are still a matter of debate.28,30 Nevertheless, the observation that osteomyelofibrosis develops at a slow rate in GATA-1lowmutant mice, whereas expression levels of megakaryocyte-derived cytokines are reduced,52 further highlights the major implication of megakaryocytes in the pathogenesis of myelofibrosis/osteosclerosis. In patients with IM, accumulation of dysplastic megakaryocytes in marrow and increased TGF-β1 levels have been linked to the pathogenesis of myelofibrosis.27 Future studies aiming to analyze plasmatic levels of TGF-β1 and OPG during the progression of IM are needed to evaluate whether development of osteosclerosis correlates with increased OPG secretion, as demonstrated by our data. Experimentally induced myelofibrosis/osteosclerosis represent valuable models for understanding the nature of the pathologic events leading to the complex stroma and bone modifications and for devising therapeutic strategies for preventing the more severe manifestations of the disease.
We are grateful to Dr Edward Clark for kindly providing theopg+/− breeders and to Dr J.-L. Villeval for the MPZenTPO virus-producing GP+E-86 cells. We acknowledge A. M. Hagnere for expert assistance with the histopathologic studies and A. Rouchès for help with the animals.
Prepublished online as Blood First Edition Paper, December 27, 2002; DOI 10.1182/blood-2002-09-2839.
Supported by grants from the Institut National de la Santéet de la Recherche Médicale, the Institut Gustave Roussy, the Ministère de la Recherche, and the Ligue Nationale contre le Cancer (Equipe labellisée 2000). H.C. and E.K. are supported by a fellowship from the Ministère de la Recherche and the Ligue Nationale contre le Cancer, respectively.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
William Vainchenker, LIPA, IFR 54, Institut Gustave Roussy, Villejuif, France; e-mail:verpre@igr.fr.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal