Antigen presentation by activated human and rat CD4+ T cells has long been known to induce hyporesponsiveness due to a combination of anergy and apoptosis. It has been assumed that no such phenomenon occurs in mice due to the inability of mouse T cells to synthesize major histocompatibility complex (MHC) class II molecules. There have been several recent descriptions of the transfer of molecules, including MHC molecules, from antigen-presenting cells (APCs) to T cells. Here, we describe the acquisition of MHC class II molecules by T-cell receptor (TCR)–transgenic T cells and T-hybridoma cells following culture with APCs. Acquisition was markedly enhanced by T-cell activation either due to cognate recognition of antigen or anti-CD3 activation. When activation was induced by antigen recognition, preferential acquisition of complexes of class II molecules displaying cognate peptide was observed; in contrast, following activation by anti-CD3 the acquisition of class II molecules was MHC unrestricted. T cells that had acquired MHC class II:peptide complexes were able to act as APCs and induced proliferation and interleukin-2 secretion by resting T cells. However, when activated T cells that had acquired MHC class II:peptide complexes engaged in T:T interactions, this led to an increase in apoptosis and the induction of hyporesponsiveness. These results raise the possibility that the acquisition of MHC class II:peptide complexes by T cells during an immune response may serve to limit clonal expansion, including that induced by alloantigen following tissue or stem cell transplantation.
Introduction
Major histocompatibility complex (MHC) class II molecules are constitutively expressed on specialized antigen-presenting cells (APCs) such as dendritic cells (DCs) and macrophages. Antigen presentation by such cells to T cells results in T-cell proliferation and differentiation toward effector function. However, MHC class II expression can be induced on multiple cell types under the influence of cytokines such as gamma interferon.1-3 The consequences of antigen presentation by such nonspecialized APCs remain unclear. Most of the cell types on which MHC class II expression can be induced do not express the major costimulatory molecules CD80 and CD86. Using gamma interferon–treated primary cultures of human epithelial cells, we have previously reported the induction of antigen-specific unresponsiveness in human CD4+ T cells.2 In most species, T cells themselves express MHC class II molecules following activation.4-6 Antigen presentation by activated T cells to activated T cells has been reported by several groups to induce either T-cell anergy or apoptosis.7-12 Indeed, the first report of antigen-induced unresponsiveness resulted from the culture of a human T-cell clone with its cognate peptide in the absence of added APCs.7 Mechanistically, this is a conundrum in that activated T cells also express substantial levels of B7 family costimulatory molecules.13-15 However, the regulatory effects of T:T antigen presentation may have a biologic role in limiting clonal expansion at the later stages of an immune response. One species in which T cells do not synthesize MHC class II molecules is the mouse.16,17 It has been assumed therefore that CD4+ T:T presentation does not apply in this species. However, there have been several recent reports describing the acquisition of molecules by T cells from APCs. These have included the transfer of MHC class I molecules,18,19 B7 family molecules,20,21 and MHC class II molecules in a series of rat experiments.22-24 The earliest description of MHC class II molecule transfer came from electron microscopy studies of thymocytes in irradiation chimeras25 and studies with T-cell clones in vitro.26
In this study we have examined the capacity of T-cell receptor–transgenic and T-hybridoma cells to acquire MHC II:peptide complexes from APCs and subsequently present them to other T cells. The functional consequences of these events suggest that T:T antigen presentation may serve to limit T-cell clonal expansion. The relevance of these findings to alloimmunity following tissue and stem cell transplantation is discussed.
Materials and methods
Animals
T-cell receptor (TCR)–transgenic DO11.10 mice were bred in the Biological Services Unit of the Imperial College Faculty of Medicine. BALB/c and CBA mice were obtained from Harlan.
Cell lines
The 3A9 murine CD4+ T-cell hybridoma is specific for hen-egg white lysozyme (HEL) peptide46-61restricted by H2-Ak. A DO11.10 cell line was generated from DO11.10 TCR-transgenic mice and is specific for ovalbumin (OVA) peptide323-339 restricted by H2-Ad. DAP.3-H2-Ak transfectants have been generated in our laboratory. CTLL2 is a murine interleukin-2 (IL-2)–dependent CD8+ cell line for the detection of IL-2 production. T cells were maintained in RPMI 1640 supplemented with 10% fetal calf serum (FCS), 2 mM l-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin, and 5 × 10−5 M 2-ME. DAP.3 transfectants were maintained in Dulbecco modified Eagle medium supplemented with 10% FCS, 2 mM l-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin, and 5 × 10−5 M 2-ME with appropriate drug selection to maintain expression of the transfected genes.
Antibodies and flow cytometry
Antibodies used for the T-cell analysis are listed: fluorescein isothiocyanate (FITC)–conjugated anti–H2-Ak(clone 14V.18; Serotec, Oxford, United Kingdom); cy-chrome–conjugated DO11.10 TCR clonotypic antibody (clone KJ1-26; Caltag Lab, Burlingame, CA); FITC-conjugated annexin V, FITC-conjugated CD44 (clone Ly-24), FITC-conjugated CD3 (clone 145-2C11), phycoerythrin (PE)-conjugated anti–H2-Ak (clone 10.2.16), PE-conjugated anti–H2-Ad (clone AMS-32.1), PE-conjugated anti–TCR Vβ 8.2 (clone MR5-2), PE-conjugated anti-CD4 (clone GK1.5), and Cy-conjugated anti-CD4 (clone GK1.5) were obtained from Pharmingen (Heidelberg, Germany). FITC-conjugated sheep anti–mouse IgG was from Sigma (Dorset, United Kingdom). Anti-HEL (46-61)-H2-Ak complex (clone C4H3, kindly provided by Caetano Reis e Souza, Immunobiology Laboratory, Cancer Research UK, London Research Institute, United Kingdom) was purified from hybridoma culture.27 All flow cytometric analyses were conducted on a Becton Dickinson FACScalibur running CellQuest software (Becton Dickinson, Heidelberg, Germany).
Generation of dendritic cells
The protocol of Inaba et al28 was used to generate DCs from bone marrow. Briefly, bone marrow cells (5 × 105/mL) were cultured with 10% FCS RPMI 1640 containing 5% (vol/vol) supernatant from a granulocyte-macrophage colony-stimulating factor (GM-CSF)–secreting transfected cell line. On day 3, nonadherent cells were removed. Fresh medium with GM-CSF was added to the adherent fraction for continuing culture. Cells were used from day 8 to day 10.
Purification of CD4+ T cells
Lymph node and spleen cells were treated with a mixture of anti-CD8 and anti–H2-Ad supernatants (M5/114, YTS169, and YTS191) for 30 minutes. After antibody treatment, the cells were washed and incubated with goat anti-mouse IgG and goat anti-rat IgG dynal beads (Dynals, Oslo, Norway) for 30 minutes. MHC class II positive cells and CD8+ T cells bound to the dynal beads were removed with a magnet. Purified CD4+ T cells were recovered from the unbound cell suspension. To purify naive CD4+ T cells, anti-CD44 supernatant (Ly-24) was added to the antibody supernatant cocktail.
In vivo stimulation of DO11.10 CD4+ T cells
5 × 106 DO11.10 CD4+ T cells were transferred into BALB/c mice. After 3 days, the mice were immunized either with complete Freund adjuvant (CFA; Sigma) or CFA plus 300 μg OVA peptide via footpad injection. The DO11.10 CD4+ T cells were recovered from draining lymph node 1 day after injection.
T-APC coculture and their subsequent separation
APCs (DAP.3-H2-Ak transfectants or BALB/c DCs) were incubated with 5 μM CFSE (5-carboxy-fluoroscein diacetate succinimidyl ester) in phosphate-buffered saline (PBS) for 15 minutes in 37°C and then washed with PBS 3 times. T-cell responders (3A9 cells or DO11.10) were cocultured with CFSE-labeled APCs either pulsed with or without peptide overnight. CD4+cells were isolated from APCs by cell sorting and fixed with 1% (wt/vol) paraformaldehyde or γ-irradiated before being used as APCs in T-cell hybridoma or proliferation assays.
T-cell hybridoma assay
1 × 105 fresh responder 3A9 cells were cocultured with 2 × 105 APC-preincubated 3A9 cells in 96 flat-bottom micrometer plates. After 24 hours 50 μL supernatant was harvested and IL-2 activity determined by addition to the IL-2–dependent CTLL-2 cell line. CTLL-2 proliferation was measured by pulsing [3H]thymidine after a further 24 hours. Cells were harvested 18 hours later, followed by liquid scintillation spectroscopy. As a positive control, 5 × 104DAP.3-H2-Ak transfectants pulsed with HEL46-61were used instead of fixed 3A9 APCs.
T-cell proliferation assays
Responder CD4+ T cells (1 × 104cells/well) were stimulated with irradiated (30 Gray) APCs (DC- or APC-preincubated T cells) in 96-well plates for 3 days. Proliferation was assessed by [3H]thymidine incorporation during the last 18 hours of 72-hour assays.
Three-stage culture for bidirectional T:T presentation in vitro
DO11.10 CD4+ T cells were cocultured with CFSE-labeled BALB/c DCs with or without OVA peptide at a ratio of 4:1 for 4 hours to allow acquisition of MHC II or MHC II:peptide complexes from DCs. Afterward, the T cells were purified from the DCs by cell sorting. The purified DO11.10 CD4+ T cells that had either acquired H2-Ad:OVA complexes or H2-Ad alone were cultured in fresh medium overnight to allow T:T presentation to occur. DO11.10 CD4+ T cells without coculture served as a control. After the second stage culture, the cells were washed 3 times with PBS and then rested in fresh medium containing 10 μg/mL of anti–H2-Ad (M5/114) for 5 days to prevent any further cognate T:T interaction. After the rest culture, the cells were tested for proliferative responses to antigen restimulation by splenic APCs.
Results
CD4+ T cells acquire substantial levels of MHC class II molecules and MHC II:peptide complexes from antigen-presenting cells
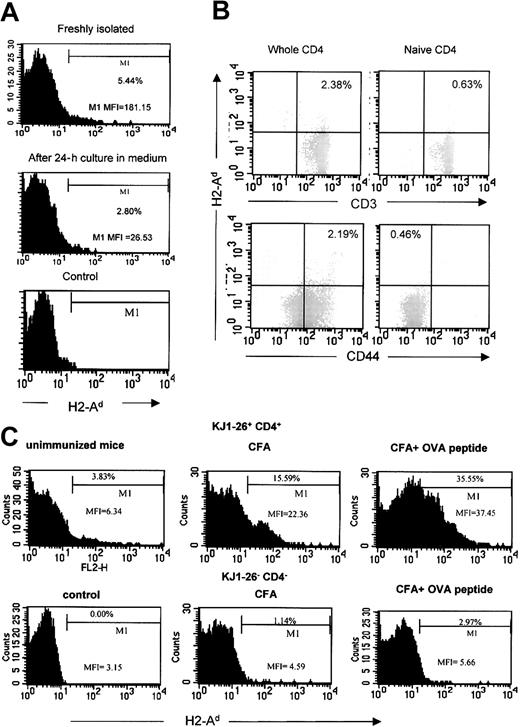
To explore MHC class II molecule acquisition by mouse CD4+ T cells, TCR-transgenic DO11.10 T cells were cocultured with BALB/c dendritic cells with increasing concentrations of cognate peptide. After overnight culture the cells were harvested and stained with FITC-conjugated anti-CD4 and PE-conjugated anti–H2-Ad antibodies. The level of H2-Adexpression on gated CD4+ T cells is shown in Figure1A. An antigen dose–dependent increase in MHC class II expression on the T cells was seen such that the majority of cells were MHC class II positive at the highest peptide concentration. These data indicate that the acquisition of MHC class II molecules was influenced by cognate recognition of the APCs. However, significant MHC class II expression also was seen on the T cells cocultured with DCs in the absence of added peptide. The kinetics of MHC class II acquisition is illustrated in Figure 1B and Table1. As can be seen, readily detectable expression of H2-Ad on T cells was detectable within 3 hours of coculture with the peptide-pulsed dendritic cells.
CD4+ T cells can acquire MHC II/peptide complexes from antigen-presenting cells.
(A) CD4+ T cells purified from DO11.10 transgenic mice were cocultured with bone-marrow–derived BALB/c DCs pulsed with different concentration of OVA323-339 peptide. After overnight coculture, the cells were harvested and stained with PE-conjugated anti–H2-Ad and FITC-conjugated anti-CD4 (solid line). The level of H2-Ad expression on DO11.10 CD4+ T cells was measured by flow cytometry. Only the CD4+ population was gated for analysis. (DO11.10 CD4+ T cells in medium control [shaded area]: mean fluorescence intensity [MFI] = 4.08; % in M1 gate = 3.04%.) (B) CD4+ T cells from DO11.10 transgenic mice were cocultured with BALB/c DCs pulsed with 10 μg/mL OVA peptide (Table 1). Cells were harvested at different time points. The level of H2-Ad on DO11.10 CD4+cells is presented by histograms. The shaded portion indicates the level at 0 h; the solid line, 3 h; and the dotted line, 20 h. (C) 3A9 CD4+ T-cell hybridoma were cocultured with DAP.3-H2-Ak transfectant prepulsed with or without 10 μg/mL HEL46-61 peptide. The level of H2-Ak expression and H2-Ak:HEL46-61complex on peptide-pulsed DAP.3 transfectant was detected by staining with FITC-conjugated anti–H2-Ak or C4H3 monoclonal antibodies specific for H2-Ak:HEL46-61 complex followed by FITC-conjugated antimouse IgG (shaded area). After overnight coculture, 3A9 cells were harvested and stained for H2-Ak and complex expression as mentioned, finally with PE-conjugated anti-CD4. The levels of H2-Ak expression and C4H3 epitope on 3A9 cells (solid line) were detected by flow cytometry. Only CD4+ populations were gated for analysis. (DAP.3 control [solid line] for C4H3 staining: MFI = 4.41, % in M1 gate = 1.29%; for H2-Ak staining: MFI = 3.44, % in M1 gate = 0.35%; 3A9 cells in medium control [shaded area] for C4H3 staining: MFI = 2.96; % in M1 gate = 0.66%; for H2-Akstaining: MFI = 3.23; % in M1 gate = 0.81%.)
CD4+ T cells can acquire MHC II/peptide complexes from antigen-presenting cells.
(A) CD4+ T cells purified from DO11.10 transgenic mice were cocultured with bone-marrow–derived BALB/c DCs pulsed with different concentration of OVA323-339 peptide. After overnight coculture, the cells were harvested and stained with PE-conjugated anti–H2-Ad and FITC-conjugated anti-CD4 (solid line). The level of H2-Ad expression on DO11.10 CD4+ T cells was measured by flow cytometry. Only the CD4+ population was gated for analysis. (DO11.10 CD4+ T cells in medium control [shaded area]: mean fluorescence intensity [MFI] = 4.08; % in M1 gate = 3.04%.) (B) CD4+ T cells from DO11.10 transgenic mice were cocultured with BALB/c DCs pulsed with 10 μg/mL OVA peptide (Table 1). Cells were harvested at different time points. The level of H2-Ad on DO11.10 CD4+cells is presented by histograms. The shaded portion indicates the level at 0 h; the solid line, 3 h; and the dotted line, 20 h. (C) 3A9 CD4+ T-cell hybridoma were cocultured with DAP.3-H2-Ak transfectant prepulsed with or without 10 μg/mL HEL46-61 peptide. The level of H2-Ak expression and H2-Ak:HEL46-61complex on peptide-pulsed DAP.3 transfectant was detected by staining with FITC-conjugated anti–H2-Ak or C4H3 monoclonal antibodies specific for H2-Ak:HEL46-61 complex followed by FITC-conjugated antimouse IgG (shaded area). After overnight coculture, 3A9 cells were harvested and stained for H2-Ak and complex expression as mentioned, finally with PE-conjugated anti-CD4. The levels of H2-Ak expression and C4H3 epitope on 3A9 cells (solid line) were detected by flow cytometry. Only CD4+ populations were gated for analysis. (DAP.3 control [solid line] for C4H3 staining: MFI = 4.41, % in M1 gate = 1.29%; for H2-Ak staining: MFI = 3.44, % in M1 gate = 0.35%; 3A9 cells in medium control [shaded area] for C4H3 staining: MFI = 2.96; % in M1 gate = 0.66%; for H2-Akstaining: MFI = 3.23; % in M1 gate = 0.81%.)
The kinetics of MHC class II requisition by T cells
| Time after coculture with 10 μg/mL OVA peptide-pulsed BALB/c DC, h . | MFI . | % in M1 gate . |
|---|---|---|
| 0 | 11.12 | 5.44 |
| 3 | 72.02 | 45.39 |
| 20 | 96.51 | 73.17 |
| Time after coculture with 10 μg/mL OVA peptide-pulsed BALB/c DC, h . | MFI . | % in M1 gate . |
|---|---|---|
| 0 | 11.12 | 5.44 |
| 3 | 72.02 | 45.39 |
| 20 | 96.51 | 73.17 |
To further define the nature of the acquired MHC class II molecules, we made use of a monoclonal antibody specific for a defined MHC II:peptide complex. This antibody, C4H3, is specific for a complex comprising H2-Ak:HEL46-61.The 3A9 murine CD4+ T-cell hybridoma is specific for the same class II peptide complex. DAP.3-H2-Ak transfectants were pulsed with HEL peptide leading to expression of high levels of the C4H3 epitope (Figure 1C). When the peptide-pulsed transfectants were incubated with 3A9 cells overnight, the majority of 3A9 cells stained positively for H2-Ak expression, and a significant proportion also displayed the C4H3 epitope (Figure 1C, bottom panels). In contrast, when the 3A9 cells were cultured with the transfectants in the absence of peptide, low-level H2-Ak expression was detected and no staining was observed with C4H3. It is interesting to note that the fraction of H2-Ak molecules occupied by the HEL peptide was significantly increased on the 3A9 T cells, compared with the transfectant APCs, as judged by the ratio of H2-Akto C4H3 fluorescence (7.6 on the APCs versus 3.7 on the T cells). This suggests 1 of 2 possibilities, either the TCR contributes to the transfer by the physical capture of MHC II:peptide complexes or the transfer involves molecules that are enriched in the molecular synapse between T cells and APCs such as the MHC II:peptide complexes for which the T cell is specific.
If the acquired MHC II:peptide complexes were to be of any functional significance in vivo, they would need to be stably expressed. The rate of decay of serologically detectable MHC class II molecules was assessed by culturing the sorted CD4+ T cells after MHC class II acquisition for varying time periods. As shown in Table 2, the percentage of positive cells and the mean fluorescence intensity did decay over time. However, readily detectable MHC class II expression was observed as much as 18 hours after in vitro culture.
MHC class II molecules acquired by T cells are stable in culture
| H2-Ak expression on 3A9 cells isolated from 3A9-HEL46-61 pulsed DAP3 H2-Ak coculture . | ||
|---|---|---|
| Time after sorting, h . | MFI . | % in M1 gate . |
| 0 | 23.46 | 27.05 |
| 3 | 17.14 | 32.86 |
| 18 | 11.99 | 17.07 |
| H2-Ak expression on 3A9 cells isolated from 3A9-HEL46-61 pulsed DAP3 H2-Ak coculture . | ||
|---|---|---|
| Time after sorting, h . | MFI . | % in M1 gate . |
| 0 | 23.46 | 27.05 |
| 3 | 17.14 | 32.86 |
| 18 | 11.99 | 17.07 |
3A9 cells were cocultured with CFSE-labeled DAP.3 H2-Ak pulsed with HEL46-61 peptide. To isolate 3A9 cells from the coculture, 3A9 cells, which were CFSE negative, were sorted. The sorted 3A9 cells, devoid of any APCs, then were cultured in medium. The sorted 3A9 cells were stained simultaneously with cy-chrome–conjugated anti-CD4 and PE-conjugated anti–H2-Ak at different time intervals. The level of H2-Ak on 3A9 was analysis by flow cytometry. Only the CD4+ population was gated for analysis. 3A9 control: MFI = 5.15; % in M1 gate = 0.09.
CD4+ T cells acquire MHC class II molecules in vivo
When staining freshly isolated ex vivo CD4+ T cells from DO11.10 mice, we had consistently noticed a low level of staining with anti–MHC class II antibody, which we interpreted as nonspecific background activity. However, having observed the above results, we tested the possibility that this was genuine MHC II expression on the T cells due to acquisition in vivo. In order to address this, the T cells were analyzed either immediately following purification or after 24 hours of culture in the absence of APCs. As shown in Figure2A, the percentage of positive cells and the mean fluorescence intensity both diminished after 24 hours of culture, suggesting that this was genuine MHC class II expression due to transfer of molecules in vivo.
CD4+ T cells can acquire MHC II in vivo.
(A) Freshly isolated CD4+ T cells from DO11.10 TCR-transgenic mice were stained with PE-conjugated anti–H2-Ad and FITC-conjugated anti-CD4 immediately following isolation or after 24 hours in medium. The level of H2-Ad on DO11.10 cells gated for CD4 expression is shown. These data have been reproduced in 3 animals. (MFI of whole CD4+ T cells: freshly isolated = 11.12; after 24 hours' culture = 5.59.) (B) CD4+ T cells, either the whole preparation or purified naive cells, from BALB/c mice were triple-stained with PE-conjugated anti–H2-Ad, cy-chrome–conjugated anti-CD4, and FITC-conjugated anti-CD3 or anti-CD44. Only the CD4+ population was gated for analysis. (C) CD4+ T cells from DO11.10 transgenic mice were transferred to BALB/c mice. Some mice were immunized with CFA plus OVA peptide or with CFA alone. T cells were recovered from the draining lymph node one day after immunization. The cells were then triple-stained with PE-conjugated anti–H2-Ad, FITC-conjugated anti-CD4, and cy-chrome–conjugated DO11.10 TCR clonotypic antibody (KJ1-26). Included in each group were 3 mice. CD4+ KJ1-26+ and CD4+KJ1-26− cells were analyzed separately as indicated on the figure. Representative mice from each group are shown.
CD4+ T cells can acquire MHC II in vivo.
(A) Freshly isolated CD4+ T cells from DO11.10 TCR-transgenic mice were stained with PE-conjugated anti–H2-Ad and FITC-conjugated anti-CD4 immediately following isolation or after 24 hours in medium. The level of H2-Ad on DO11.10 cells gated for CD4 expression is shown. These data have been reproduced in 3 animals. (MFI of whole CD4+ T cells: freshly isolated = 11.12; after 24 hours' culture = 5.59.) (B) CD4+ T cells, either the whole preparation or purified naive cells, from BALB/c mice were triple-stained with PE-conjugated anti–H2-Ad, cy-chrome–conjugated anti-CD4, and FITC-conjugated anti-CD3 or anti-CD44. Only the CD4+ population was gated for analysis. (C) CD4+ T cells from DO11.10 transgenic mice were transferred to BALB/c mice. Some mice were immunized with CFA plus OVA peptide or with CFA alone. T cells were recovered from the draining lymph node one day after immunization. The cells were then triple-stained with PE-conjugated anti–H2-Ad, FITC-conjugated anti-CD4, and cy-chrome–conjugated DO11.10 TCR clonotypic antibody (KJ1-26). Included in each group were 3 mice. CD4+ KJ1-26+ and CD4+KJ1-26− cells were analyzed separately as indicated on the figure. Representative mice from each group are shown.
To further characterize the freshly isolated ex vivo MHC II–positive T cells, whole CD4+ T cells were purified from BALB/c mice and compared with the naive CD4+ cells prepared by depleting of CD44hi cells. The cells were then triple-stained with anti-CD4, anti–H2-Ad, and other markers. The H2-Ad+ CD4+ cells were found only in the whole CD4+ population and not in the naive population. They were also CD3+, confirming that they are CD4+ T cells. Moreover, they were confined to the activated/memory population as shown by the anti-CD44 staining (Figure2B).
To examine whether activation can amplify the acquisition event in vivo, DO11.10 CD4+ T cells were transferred into BALB/c mice. After 3 days, the mice were immunized with CFA plus OVA peptide. Mice immunized with CFA or without any immunization served as controls. For mice immunized with CFA plus peptide, DO11.10 CD4+ T cells showed higher expression of MHC class II as compared with the nonimmunized animal, or animals immunized with CFA alone (Figure 2C), indicating that transfer of MHC class II can occur in vivo and is augmented by T-cell activation.
MHC II molecule acquisition is markedly enhanced by T-cell activation
The mechanisms whereby T cells acquire molecules from APCs are unclear. The levels of acquired complexes were markedly augmented following cognate recognition of MHC class II–presented antigen by APCs, implying a role for the TCR in the capture of MHC II:peptide complexes. To distinguish between a direct involvement of TCR in the capture of complexes and TCR-dependent T-cell activation, DO11.10 CD4+ T cells were cocultured with BALB/c DCs or CBA DCs in the presence or absence of OVA peptide or in the presence of anti-CD3 antibody. Low levels of MHC class II molecules were acquired by DO11.10 T cells irrespective of the types of DCs used (Figure3, top panels). This suggests that the cognate recognition independent mechanism of MHC class II capture is MHC unrestricted. However, addition of OVA peptide markedly increased acquisition of H2-Ad from BALB/c DCs, but made no difference to the levels of acquired H2-Ak, again suggesting the involvement of a cognate interaction. To determine whether the influence of cognate interaction reflected physical capture of complexes by the TCR or merely the need for T-cell activation, anti-CD3 antibody was added to cocultures of T cells with APCs in the absence of antigen, or even with APCs expressing irrelevant MHC class II molecules. In the presence of 1 μg/mL anti-CD3, DO11.10 T cells acquired comparable levels of MHC class II molecules from CBA DCs, as from BALB/c DCs in the presence of either peptide or anti-CD3. This makes it less likely that the TCR is physically involved in the capture of complexes. It is not clear whether the effect of T-cell activation is mediated by some alteration in the properties of the T-cell membrane that make it more acquisitive, or that T-cell activation merely leads to increased intimacy of T-cell:APC conjugate formation. The enrichment of cognate MHC II:peptide complexes on the T-cell surface following antigen-dependent activation, as seen with the 3A9 hybridoma, probably reflects the fact that transfer of molecules is concentrated at the interface between the T cell and the APC where cognate MHC II:peptide complexes are concentrated within the “molecular synapse” that is instrumental in T-cell activation.
MHC class II molecule acquisition is marked enhanced by T-cell activation.
DO11.10 CD4+ T cells were cocultured overnight with bone marrow–derived BALB/c DCs or CBA DCs either without peptide, with 10 μg/mL OVA323-339 peptide, or 1 μg/mL soluble anti-CD3. Cells were then harvested and stained with PE-conjugated anti–H2-Ad or anti–H2-Ak, and FITC-conjugated anti-CD4 (bold lines). The levels of H2-Ad and H2-Ak on DO11.10 CD4+ cells were analyzed by flow cytometry. Only the CD4+ population was gated for analysis. (DO11.10 CD4+ T cells in medium control [shaded areas] for H2-Ad staining: MFI = 10.64; % in M1 gate = 1.66%; H2-Ak staining: MFI = 18.76; % in M1 gate = 3.22%.)
MHC class II molecule acquisition is marked enhanced by T-cell activation.
DO11.10 CD4+ T cells were cocultured overnight with bone marrow–derived BALB/c DCs or CBA DCs either without peptide, with 10 μg/mL OVA323-339 peptide, or 1 μg/mL soluble anti-CD3. Cells were then harvested and stained with PE-conjugated anti–H2-Ad or anti–H2-Ak, and FITC-conjugated anti-CD4 (bold lines). The levels of H2-Ad and H2-Ak on DO11.10 CD4+ cells were analyzed by flow cytometry. Only the CD4+ population was gated for analysis. (DO11.10 CD4+ T cells in medium control [shaded areas] for H2-Ad staining: MFI = 10.64; % in M1 gate = 1.66%; H2-Ak staining: MFI = 18.76; % in M1 gate = 3.22%.)
MHC II:peptide complexes acquired by T cells can be recognized efficiently by other T cells
To investigate whether the acquired MHC II:peptide complexes on T cells can be presented to fresh responder T cells with the same antigen specificity, T cells were isolated from the T-APC cocultures and used as APCs in T-cell hybridoma or T-cell proliferation assays. More than 99% purity of isolated T cells was obtained after depletion of the CFSE-labeled APCs by cell sorting as shown for DO11.10 T cells in Figure 4D.
The acquired MHC II/peptide complexes by T cells can be recognized by fresh responder T cells with the same antigen specificity.
(A) DAP.3-H2-Ak transfectant either pulsed with (DAP3[H2-Ak/HEL]) or without (DAP3[H2-Ak]) HEL46-61 were used to stimulate IL-2 production by fresh 3A9 responder cells. (B) 3A9 cells were cocultured with CFSE-labeled DAP.3 H2-Ak transfectants pulsed with or without HEL46-61 peptide. 3A9 populations that had acquired H2-Ak (3A9II) or H2-Ak:HEL46-61 complexes (3A9Iip) and 3A9 cells cultured in medium only (3A9) were isolated as described in “Materials and methods,” fixed, and used to stimulate IL-2 production by fresh responder cells. 2 × 105 fixed 3A9-APCs were cocultured with fresh 3A9 responders. After 24 hours' incubation, 50 μL supernatant was harvested. IL-2 activity in the supernatant was measured by CTLL2 bioassay. (C) DO11.10 CD4+ T cells were cocultured with CFSE-labeled BALB/c DCs that had been pulsed either with or without OVA323-339peptide to allow MHC class II acquisition. After overnight coculture, CFSE-negative DO11.10 CD4+ T cells were sorted and used as APCs. The CFSE-negative DO11.10 CD4+ T-cell populations that had acquired H2-Ad (■) or H2-Ad/OVA323-339 complexes (▴) were γ-irradiated to stimulate proliferation of fresh responder cells. DO11.10 CD4+ T cells cultured in medium only (♦) and peptide-pulsed DCs (○), cell numbers corresponding to 1% of the number of DO11.10IIp cells used in the same experiment, were used as controls. (D) The purity of the isolated cells was assessed by flow cytometry. CPM indicates counts per minute. Error bars indicate SD of triplicates in the same experiment.
The acquired MHC II/peptide complexes by T cells can be recognized by fresh responder T cells with the same antigen specificity.
(A) DAP.3-H2-Ak transfectant either pulsed with (DAP3[H2-Ak/HEL]) or without (DAP3[H2-Ak]) HEL46-61 were used to stimulate IL-2 production by fresh 3A9 responder cells. (B) 3A9 cells were cocultured with CFSE-labeled DAP.3 H2-Ak transfectants pulsed with or without HEL46-61 peptide. 3A9 populations that had acquired H2-Ak (3A9II) or H2-Ak:HEL46-61 complexes (3A9Iip) and 3A9 cells cultured in medium only (3A9) were isolated as described in “Materials and methods,” fixed, and used to stimulate IL-2 production by fresh responder cells. 2 × 105 fixed 3A9-APCs were cocultured with fresh 3A9 responders. After 24 hours' incubation, 50 μL supernatant was harvested. IL-2 activity in the supernatant was measured by CTLL2 bioassay. (C) DO11.10 CD4+ T cells were cocultured with CFSE-labeled BALB/c DCs that had been pulsed either with or without OVA323-339peptide to allow MHC class II acquisition. After overnight coculture, CFSE-negative DO11.10 CD4+ T cells were sorted and used as APCs. The CFSE-negative DO11.10 CD4+ T-cell populations that had acquired H2-Ad (■) or H2-Ad/OVA323-339 complexes (▴) were γ-irradiated to stimulate proliferation of fresh responder cells. DO11.10 CD4+ T cells cultured in medium only (♦) and peptide-pulsed DCs (○), cell numbers corresponding to 1% of the number of DO11.10IIp cells used in the same experiment, were used as controls. (D) The purity of the isolated cells was assessed by flow cytometry. CPM indicates counts per minute. Error bars indicate SD of triplicates in the same experiment.
3A9 cells that acquired MHC II:peptide complexes (3A9IIp) were fixed and cocultured with fresh responder 3A9 cells. The ability of 3A9 cells to present the acquired MHC II:peptide complexes to another T cell with the same antigen specificity was determined by their ability to induce IL-2 production by the fresh responder cells using the CTLL-2 bioassay. Figure 4A shows the positive control results of culturing 3A9 cells with the DAP.3 H2-Ak transfectant as APC. This led to peptide-dependent IL-2 production. When 3A9IIp were used, comparable levels of IL-2 secretion were induced, albeit at a higher APC/T-cell ratio (Figure 4B). This demonstrates that 3A9 cells cannot only acquire MHC II:peptide complexes from APC, but the acquired complexes can be presented to other T cells with the same antigen specificity.
Parallel observations were made with T-cell lines established from DO11.10 mice (specific for OVA323-339:H2-Ad complexes) and peptide-pulsed BALB/c DCs. DO11.10 CD4+ T cells were isolated from the T-DC coculture using the method described in “Materials and methods” and used as APCs. The cells were γ-irradiated and used to stimulate resting DO11.10 T cells. DO11.10 T cells that acquired the MHC II:peptide (DO11.10IIp) isolated from cocultures with peptide-pulsed BALB/c DCs stimulated proliferation of resting DO11.10 T cells (Figure 4C), and the presentation could be blocked by adding anti–H2-Ad antibody (M5/114) (data not shown). Given the potency of dendritic cells as APCs, it was important to exclude the possibility that contaminating DCs accounted for the observed proliferation. The level of DC contamination following T-cell purification is shown in panel D of Figure 4. A dose response was performed with DO11.10 T cells and DC numbers corresponding to 1% of the number of DO11.10IIp cells used in the same experiment. The lower level of proliferation induced by this number of DCs indicates that contamination is highly unlikely to account for the response induced by DO11.10IIp T cells.
Antigen presentation by T cells that acquired MHC II:peptide complex induces apoptosis and hyporesponsiveness
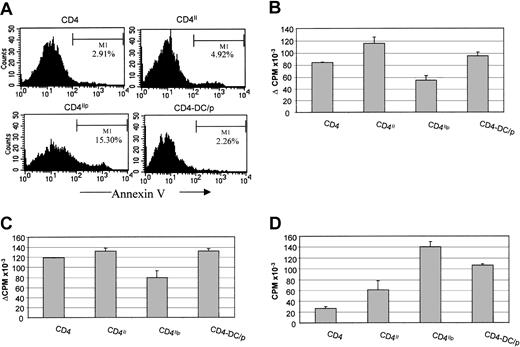
Human and rat data have demonstrated that T:T antigen presentation, involving activated T cells, induces anergy. To determine whether the presentation of acquired MHC II:peptide complexes by activated mouse T cells to activated T cells would have a similar outcome, the DO11.10 T-cell line (composed of activated T cells) was cultured with peptide-pulsed BALB/c DCs to allow MHC II acquisition, and the DCs were then removed and the T cells allowed to engage in T:T interactions. The T cells were then rested, by the addition of a blocking anti–H2-Ad antibody for 5 days. Finally, the T cells were rechallenged with peptide-pulsed BALB/c DCs. Annexin V staining of the T cells immediately after the period of T:T interaction revealed an increase in the fraction of the T cells undergoing apoptosis (15%) compared with the T cells exposed to DC antigen presentation (2%) (Figure 5A). As shown in Figure 5B, the cells that had engaged in T:T interactions were hyporesponsive compared with those that had been cultured with DCs, T cells that had acquired only MHC class II, or medium. The reduced proliferation could not be due to TCR:CD3 down-regulation or premature T-cell stimulation, because after 5 days resting, the levels of CD3:TCR had fully returned to normal, and T cells that had been stimulated with DCs had comparable proliferation to T cells in medium alone. The reduced proliferation also was accompanied by reduced IL-2 production (Figure 5C) and hyperresponsiveness to exogenous IL-2 (Figure 5D), a cardinal feature of T-cell anergy.
Bidirectional T:T presentation by the T cells that acquired the MHC II/peptide complexes can induce T-cell hyporesponsiveness and apoptosis.
DO11.10 CD4+ T cells after coculture with BALB/c DCs either pulsed with or without OVA323-339 peptide were purified after 4 hours' coculture. The purified CD4+ T cells that had acquired H2-Ad (CD4II) or H2-Ad:OVA323-339 complexes (CD4Iip) were cultured for 24 hours to allow them to engage in T:T interaction. (A) Some cells were harvested and stained with annexin V and propidium iodide to test for apoptosis. DO11.10 CD4+ T cells cultured before in medium only (CD4) and DO11.10 CD4+ T cells cultured with OVA323-339 peptide-pulsed BALB/c DCs for 24 hours (CD4-DC/p) were used as controls. The remaining cells were then rested in the presence of blocking anti–H2-Ad antibody for 5 days. (B) The proliferative response to antigen rechallenge with BALB/c DCs was measured by T-cell proliferation. (Background cpm: CD4 = 208.7, CD4II = 269.7, CD4IIP = 5619.2, CD4-DC/P = 6027.1.) (C) IL-2 production by the DO11.10 CD4+ T cells upon antigen rechallenge was measured by CTLL2 bioassay. (Background cpm: CD4 = 726.3, CD4II = 1191.9, CD4IIP = 7203.1, CD4-DC/P = 2986.8.) (D) The response of the DO11.10 CD4+ T cells to exogenous IL-2 was measured by T-cell proliferation assay. Error bars indicate SD of triplicates in the same experiment.
Bidirectional T:T presentation by the T cells that acquired the MHC II/peptide complexes can induce T-cell hyporesponsiveness and apoptosis.
DO11.10 CD4+ T cells after coculture with BALB/c DCs either pulsed with or without OVA323-339 peptide were purified after 4 hours' coculture. The purified CD4+ T cells that had acquired H2-Ad (CD4II) or H2-Ad:OVA323-339 complexes (CD4Iip) were cultured for 24 hours to allow them to engage in T:T interaction. (A) Some cells were harvested and stained with annexin V and propidium iodide to test for apoptosis. DO11.10 CD4+ T cells cultured before in medium only (CD4) and DO11.10 CD4+ T cells cultured with OVA323-339 peptide-pulsed BALB/c DCs for 24 hours (CD4-DC/p) were used as controls. The remaining cells were then rested in the presence of blocking anti–H2-Ad antibody for 5 days. (B) The proliferative response to antigen rechallenge with BALB/c DCs was measured by T-cell proliferation. (Background cpm: CD4 = 208.7, CD4II = 269.7, CD4IIP = 5619.2, CD4-DC/P = 6027.1.) (C) IL-2 production by the DO11.10 CD4+ T cells upon antigen rechallenge was measured by CTLL2 bioassay. (Background cpm: CD4 = 726.3, CD4II = 1191.9, CD4IIP = 7203.1, CD4-DC/P = 2986.8.) (D) The response of the DO11.10 CD4+ T cells to exogenous IL-2 was measured by T-cell proliferation assay. Error bars indicate SD of triplicates in the same experiment.
Taken together, these data suggest that mouse CD4+ T cells acquire MHC II:peptide complexes from APCs and can present these to each other, and that such T:T antigen presentation can induce both apoptosis and hyporesponsiveness.
Discussion
The first observation of T-cell anergy was made when a human T-cell clone was incubated with cognate peptide in the absence of APCs. The induction of anergy required the presentation of antigen by activated T cells to activated T cells. If activated human T cells were used as APCs for resting human T cells, anergy did not occur, and strong proliferation was observed (G. Lombardi and R.L., unpublished observations, 1994). Two considerations have cast doubt as to the likely significance of these findings. First, T cells are inefficient at antigen internalization and processing, and second, mouse T cells do not synthesize MHC class II molecules, thereby challenging the generality of this phenomenon. The observations made here address these 2 issues.
There have been numerous reports of the transfer of molecules from APCs to T cells in mouse and rat systems. The first such observations were made using immunoelectron microscopic analysis of mouse thymic sections, in which murine thymocytes were noted to express MHC class II molecules, presumably acquired from thymic epithelium.25More recently, the capture of MHC class I and class II molecules by mouse and rat T cells from APCs has been described.18,19,22-24 Additional reports of the transfer of costimulatory molecules from APCs to T cells have been made.20,21 Indeed, such acquired MHC:peptide complexes appeared to be functional, in that CD8+ T cells became sensitive to peptide-specific lysis by neighboring T cells after acquisition of MHC I:peptide complexes from APCs.18,19Also, naive T cells that had acquired CD80 from APCs were capable of inducing IL-2 production by responder T cells.21
The results described extend these findings by showing that the presentation of acquired MHC II:peptide complexes has the same functional effects that have been observed using human T cells that express endogenously synthesized MHC class II molecules. In previous studies in a human system we have described heterogeneity among T-cell clones, such that T:T antigen presentation by some clones induces apoptosis, whereas for other clones the dominant effect is the induction of anergy. Using a T-cell line derived from DO11.10 TCR-transgenic mice, the presentation of acquired MHC class II:peptide complexes induced both apoptosis and anergy.
The mechanisms whereby T cells acquire molecules from APCs are unclear. The levels of acquired complexes were markedly augmented by T-cell activation. This was seen following cognate recognition of MHC class II–presented antigen by APCs, implying a role for the TCR in the capture of MHC II:peptide complexes. However, the need for cognate recognition could be circumvented by the addition of anti-CD3 antibody to cocultures of T cells with APCs in the absence of antigen, or even with APCs expressing irrelevant MHC class II molecules. This makes it less likely that the TCR is physically involved in the capture of complexes. It is not clear whether the effect of T-cell activation is mediated by some alteration in the properties of the T-cell membrane that make it more acquisitive, or that T-cell activation merely leads to increased intimacy of T-cell:APC conjugate formation. Although the levels of MHC class II on T cells were increased in the context of activation, significant MHC class II acquisition was observed in the absence of activation. This activation-independent acquisition of MHC molecules appeared to be influenced by B7 family molecules, in that the acquisition of H2-Ad by DO11.10 T cells from Chinese hamster ovary transfectants was observed only, in the absence of antigen, when the transfectants coexpressed CD86 (data not shown). This result is consistent with the findings described previously by Hwang et al20 and may reflect the capacity of B7:CD28 interactions to induce intracellular signaling events independently of TCR:CD3-transduced signals.29
The in vivo significance of these observations remains a matter of speculation. We have argued previously that cognate interactions between T cells may provide an additional mechanism to limit T-cell clonal expansion during the later stages of an immune response in lymphoid tissue. As T cells clonally expand and move away from the DCs that initiated their activation, they may engage in T:T interactions of the kind described here, thereby limiting the final clone size. The involvement of acquired complexes from the APCs makes this a more credible mechanism of immunoregulation in that it obviates the need for T cells to internalize and process soluble antigen that is likely to be in very limited supply in the lymph node. The fact that acquired complexes were readily detectable 18 hours after removal of the “donor” APCs suggests that the acquired complexes may be sufficiently stable for T:T interactions to occur long after the T cell has moved away from the local APCs. The detection of low-level expression of MHC class II molecules on freshly isolated T cells ex vivo that decayed after 24 hours in culture and the amplification of these events in vivo following antigen-induced activation suggest that transfer of molecules from APCs to T cell may indeed occur in vivo. The effects of T:T antigen presentation of acquired MHC:peptide complexes may play an important role in diminishing alloimmunity following hematologic stem cell transplantation. Donor T cells specific for minor or major histocompatibility antigens are likely to acquire MHC:antigen complexes from DCs. Presentation of these complexes to recently activated antirecipient T cells may serve to diminish the antirecipient alloresponse.
An alternative interpretation of these findings in an MHC class I–restricted system has been offered recently in the context of investigating competition between T-cell populations with high and low T-cell receptor affinity in vivo.30 In this study it was argued that the greater ability of the higher affinity T cells to remove MHC:peptide complexes from APCs deprived the lower affinity T cells of adequate levels of cognate ligand to be activated. This was proposed as a mechanism of affinity maturation of T cells. Whichever interpretation is correct, the transfer of MHC:peptide complexes from APCs to T cells appears to provide a further mechanism of immunoregulation, the nature of which will require elucidation by carefully designed in vivo experiments.
We thank A. George and H. Stauss for critical reading of the manuscript.
Prepublished online as Blood First Edition Paper, November 14, 2002; DOI 10.1182/blood-2002-04-1230.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Robert Lechler, Department of Immunology, Division of Medicine, Hammersmith Campus of Imperial College Faculty of Medicine, Du Cane Road, London W12 0NN, United Kingdom; e-mail:r.lechler@ic.ac.uk.

![Fig. 1. CD4+ T cells can acquire MHC II/peptide complexes from antigen-presenting cells. / (A) CD4+ T cells purified from DO11.10 transgenic mice were cocultured with bone-marrow–derived BALB/c DCs pulsed with different concentration of OVA323-339 peptide. After overnight coculture, the cells were harvested and stained with PE-conjugated anti–H2-Ad and FITC-conjugated anti-CD4 (solid line). The level of H2-Ad expression on DO11.10 CD4+ T cells was measured by flow cytometry. Only the CD4+ population was gated for analysis. (DO11.10 CD4+ T cells in medium control [shaded area]: mean fluorescence intensity [MFI] = 4.08; % in M1 gate = 3.04%.) (B) CD4+ T cells from DO11.10 transgenic mice were cocultured with BALB/c DCs pulsed with 10 μg/mL OVA peptide (Table 1). Cells were harvested at different time points. The level of H2-Ad on DO11.10 CD4+cells is presented by histograms. The shaded portion indicates the level at 0 h; the solid line, 3 h; and the dotted line, 20 h. (C) 3A9 CD4+ T-cell hybridoma were cocultured with DAP.3-H2-Ak transfectant prepulsed with or without 10 μg/mL HEL46-61 peptide. The level of H2-Ak expression and H2-Ak:HEL46-61complex on peptide-pulsed DAP.3 transfectant was detected by staining with FITC-conjugated anti–H2-Ak or C4H3 monoclonal antibodies specific for H2-Ak:HEL46-61 complex followed by FITC-conjugated antimouse IgG (shaded area). After overnight coculture, 3A9 cells were harvested and stained for H2-Ak and complex expression as mentioned, finally with PE-conjugated anti-CD4. The levels of H2-Ak expression and C4H3 epitope on 3A9 cells (solid line) were detected by flow cytometry. Only CD4+ populations were gated for analysis. (DAP.3 control [solid line] for C4H3 staining: MFI = 4.41, % in M1 gate = 1.29%; for H2-Ak staining: MFI = 3.44, % in M1 gate = 0.35%; 3A9 cells in medium control [shaded area] for C4H3 staining: MFI = 2.96; % in M1 gate = 0.66%; for H2-Akstaining: MFI = 3.23; % in M1 gate = 0.81%.)](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/7/10.1182_blood-2002-04-1230/3/m_h80734100001.jpeg?Expires=1765887698&Signature=i34KsjjxLOHhZ5w4JSIJugO1uyQuZOvzQvlB6LcmAdQXLTrma~07g-t4i46EdMLocRsGcnPn5sg5DC9AspAjyocGA4dzbzYROBfC21RHEJB2WfAx5SXNfBNtokirM5AT3WMAWNlKD1UmCfXGo71qb9z0ToVbPT1MBRTziG8Pc8XL-eYiBFwa-DNhOpavHC1O6jgkCXM6~C7Px51eWkLp3rryp7KBJITI5pWfYtZaPcVLdP2rkAHYviBqCSA1j6t9ceurUaFCuOApN0TNP2dRQ8f2VXu9Y8JH7ypRXKX8vUEa6GHy6slv9WZfinBotEfVer8Zusrdip0yp2Dw~OuotA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. MHC class II molecule acquisition is marked enhanced by T-cell activation. / DO11.10 CD4+ T cells were cocultured overnight with bone marrow–derived BALB/c DCs or CBA DCs either without peptide, with 10 μg/mL OVA323-339 peptide, or 1 μg/mL soluble anti-CD3. Cells were then harvested and stained with PE-conjugated anti–H2-Ad or anti–H2-Ak, and FITC-conjugated anti-CD4 (bold lines). The levels of H2-Ad and H2-Ak on DO11.10 CD4+ cells were analyzed by flow cytometry. Only the CD4+ population was gated for analysis. (DO11.10 CD4+ T cells in medium control [shaded areas] for H2-Ad staining: MFI = 10.64; % in M1 gate = 1.66%; H2-Ak staining: MFI = 18.76; % in M1 gate = 3.22%.)](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/7/10.1182_blood-2002-04-1230/3/m_h80734100003.jpeg?Expires=1765887698&Signature=E-tcfrbGRw2RddcjIKqVKAeBAYCNWoovhd8hLr7AEnILD7bocvZUqF3Xa6ejUI09c6Qilj4QDsi-d6EdESOirBrIdqAC8ftXsdc3GC0fyVtHrEjMNBUB-kxW5d8iWrJw3sAazBAczhfHewKAiNXHY5T9uVca0kOSZi8uUzDYwndFtsbG-d5Agq0PAeNXqDDdKCaeGLEMSb8BG9mP5bysNSrozl7xVohfjDDKriFLaq04XqRNWJN-a4vBprwhFTBt1aDrZTq7ASs-KfMW7bAXu7G9D5ZWqu1VKlD6O5xQQnWHPeBPm1dMJz0Bukp-olpeUywGw~i5lO9ImCEGmR-FAw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. The acquired MHC II/peptide complexes by T cells can be recognized by fresh responder T cells with the same antigen specificity. / (A) DAP.3-H2-Ak transfectant either pulsed with (DAP3[H2-Ak/HEL]) or without (DAP3[H2-Ak]) HEL46-61 were used to stimulate IL-2 production by fresh 3A9 responder cells. (B) 3A9 cells were cocultured with CFSE-labeled DAP.3 H2-Ak transfectants pulsed with or without HEL46-61 peptide. 3A9 populations that had acquired H2-Ak (3A9II) or H2-Ak:HEL46-61 complexes (3A9Iip) and 3A9 cells cultured in medium only (3A9) were isolated as described in “Materials and methods,” fixed, and used to stimulate IL-2 production by fresh responder cells. 2 × 105 fixed 3A9-APCs were cocultured with fresh 3A9 responders. After 24 hours' incubation, 50 μL supernatant was harvested. IL-2 activity in the supernatant was measured by CTLL2 bioassay. (C) DO11.10 CD4+ T cells were cocultured with CFSE-labeled BALB/c DCs that had been pulsed either with or without OVA323-339peptide to allow MHC class II acquisition. After overnight coculture, CFSE-negative DO11.10 CD4+ T cells were sorted and used as APCs. The CFSE-negative DO11.10 CD4+ T-cell populations that had acquired H2-Ad (■) or H2-Ad/OVA323-339 complexes (▴) were γ-irradiated to stimulate proliferation of fresh responder cells. DO11.10 CD4+ T cells cultured in medium only (♦) and peptide-pulsed DCs (○), cell numbers corresponding to 1% of the number of DO11.10IIp cells used in the same experiment, were used as controls. (D) The purity of the isolated cells was assessed by flow cytometry. CPM indicates counts per minute. Error bars indicate SD of triplicates in the same experiment.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/7/10.1182_blood-2002-04-1230/3/m_h80734100004.jpeg?Expires=1765887698&Signature=KVATug4yLu8ohgC3LYOMZfLQ87UryCDm--lWbl5Dg0zkPOyVyiR7jcaGgmgNz~d9Y0IMI736s6rApisPq2H3ixRbZ264Hdsz92hWMy0TFV3SvPeocMNJHA1ktrOLScfciXv8GPHHk9O5dYMzKGYVXL4ourJkV1bHTZpZLep13aEAxkhn95ObeBiLPBx42V1NotvNM8uTP9xngMxHLPH2uX3pQBq~eSNjbdUGmtMmHmy6SQRxozZ3L32d7QB09Gn7JI0r6kQ5wjwhHN85W8GyWU1aNlblUWrMjgti36AiG9F-DVNYOcuJ~H7e0uK1ZOaXWC6lq0bqKijGPtzmULpD-A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal