Alterations in hemostasis leading to symptomatic thromboembolism have been observed in patients with acute lymphoblastic leukemia (ALL) receiving Escherichia coli asparaginase (CASP) combined with steroids. Moreover, hereditary prothrombotic risk factors are associated with an increased risk for venous thromboembolism in pediatric ALL patients treated according to the BFM 90/95 protocols (including CASP combined with prednisone during induction therapy). To assess whether the thromboembolic risk associated with established prothrombotic risk factors is modified by treatment modalities (prednisone or dexamethasone), the present analysis was performed. Three hundred thirty-six consecutively recruited leukemic children treated according to different BFM protocols (PRED group, n = 280, 60 mg/m2 prednisone; DEXA group, n = 56, 10 mg/m2 dexamethasone during induction therapy) were studied. Study end point was the onset of symptomatic vascular accidents during induction therapy. Cumulative thromboembolism-free survival was significantly reduced in children in the PRED group (thrombosis frequency, 10.4%) compared with children in the DEXA group (thrombosis frequency, 1.8%; P = .028). Although no significant difference was found in the overall prevalence of prothrombotic risk factors, 46.5% of patients in the PRED group who experienced thromboembolic events were carriers of a prothrombotic risk factor, whereas no carrier in the DEXA group had a thromboembolism. At the time of maximum CASP activity, fibrinogen and activities of antithrombin, plasminogen, and protein S were significantly reduced in the PRED group. No significant correlation could be found between CASP activity and levels of coagulation factors. In conclusion, the use of dexamethasone instead of prednisone, administered with CASP, significantly reduced the onset of venous thromboembolism.
Introduction
Alterations in hemostasis have been frequently observed in patients with acute lymphoblastic leukemia (ALL), and thromboembolic accidents are well documented in children receiving Escherichia coli asparaginase (CASP) as a single agent or in combination with vincristine or steroids, sometimes complemented by an anthracycline.1-7
Recently published data suggest that factor II (FII) G20210A and factor V (FV) G1691A mutations, deficiencies of protein C, protein S, antithrombin, and elevated lipoprotein (Lp)(a) and von Willebrand factor (VWF) concentrations are associated with venous thromboembolism in pediatric patients with ALL treated according to the BFM 90/95 protocols. In these protocols, CASP combined with prednisone was used during induction therapy.1 In addition, we have demonstrated that children with ALL treated with different leukemia protocols carried a similar rate of the aforementioned prothrombotic risk factors but had a significantly lower rate of severe thrombotic events: 11.5% symptomatic venous thromboses occurred within the BFM 90/95 protocols versus only 2.0% within the Cooperative Acute Lymphoblastic Leukemia (COALL) study 92/97 protocols.2Thus, the carrier state of inherited prothrombotic risk factors and treatment modalities have an impact on the risk for thromboembolism in children with ALL.
To assess whether the risk for vascular occlusions associated with established prothrombotic risk factors is additionally modified by specific treatment modalities, especially by prednisone or dexamethasone, the present analysis on prospectively enrolled children with ALL treated with the BFM 90/95 protocol (data published in 1999)1 and the new BFM 2000 protocols was performed.
Patients, materials, and methods
Ethics
The present multicenter study was performed in accordance with the ethical standards laid down in a relevant version of the 1964 Declaration of Helsinki and approved by the medical ethics committee at the Westfälische Wilhelms-University, Münster, Germany.
Patients
With parental consent, children 12 months of age and older who had acute onset of ALL treated according to the BFM 90/95 or the BFM 2000 induction protocols between December 1994 and January 2002 were included in this prospective multicenter analysis. Subjects without complete remission of the disease (day 33 of the induction protocol), children classified at high risk, children with concomitant chronic diseases, hepatic failure, or severe septicemia, and adolescents abusing oral contraceptives or nicotine were excluded from the survival analysis. In addition, children receiving heparin prophylactically (individual decisions by the participating centers) were excluded from the thromboembolism-free survival analysis. The participating centers were from northwestern or eastern Germany.
In total, 336 children (median age, 5.1 years [range, 1-18 years]; boys n = 179, girls n = 157) with newly diagnosed leukemia were prospectively enrolled in this study. Two hundred eighty of them were treated according to the BFM 90/95 protocols,1 and 56 were treated according to the BFM 2000 protocol (pilotized, n = 47; randomized, n = 9).
Study end points
The diagnosis of symptomatic venous thromboembolism during ALL induction therapy was defined as the end point of this study. Thrombotic events occurring outside induction therapy were recorded on an exploratory basis.
Imaging methods used
Venous thromboembolism was diagnosed if echogenic material was found within the lumen of a vein on gray scale and if partial or complete absence of flow was demonstrated by pulse-wave and color Doppler sonography in a symptomatic patient. Alternatively, venography was used in children with suspected vascular occlusion in the upper extremity,8 and cerebral venous thromboses were diagnosed with magnetic resonance imaging or computed tomography.1
Leukemia therapy
Children in the ALL-BFM 90/95 protocols (PRED group) received CASP medac (Kyowa, Hakko, Kyogo, Japan) in doses of 5000 U/m2 at 3-day intervals, starting on day 12 through day 33 (8 doses). Prednisone (60 mg/m2) on days 1 to 36 and weekly vincristine (1.5 mg/m2) as well as daunorubicin (30 mg/m2) on days 8 and 15 (standard risk) and also on days 22 and 29 (medium risk) were additional elements of therapy. Further, children received prophylactic intrathecal methotrexate on days 1, 12, 30, 45, and 59 during induction therapy. In reinduction therapy, the children received CASP medac (10 000 U/m2) on days 8, 11, 15, and 18, along with DEXA (10 mg/m2) on days 1 to 21, weekly vincristine (1.5 mg/m2), and doxorubicin (30 mg/m2) on days 8, 15, 22, and 29, respectively.1
In the BFM 2000 protocol (DEXA group), patients received 10 mg/m2 DEXA on days 8 to 29 during induction therapy (instead of PRED on days 1-36 in BFM 90/95). The administration of CASP, vincristine, and daunorubicin was no different from administration in the BFM 90/95 protocols. In reinduction therapy, children with standard risk received CASP 10 000 U/m2 on days 8, 11, 15, and 18, along with dexamethasone (10 mg/m2) on days 1 to 21 and vincristine (1.5 mg/m2) and doxorubicin (30 mg/m2) on days 1 and 8, respectively. Patients at medium risk received this reinduction protocol for a second course 10 weeks later.
Depending on the individual decisions by the participating centers, children's polychemotherapy was administered through peripheral veins or with the use of Broviac, Hickman, or Porth catheters implanted within the first weeks of therapy.
Laboratory analyses
FII G20210A and FV G1691A mutations, plasminogen activator inhibitor-1 4G/4G polymorphism, activated protein C resistance (APC-R), fibrinogen, antithrombin, plasminogen, Lp(a), protein C, and protein S were investigated with standard laboratory techniques at leukemia onset.1,2,9 Von Willebrand factor (VWF) was determined by enzyme-linked immunosorbent assay (ELISA; Asserachrom, Stago, Asnieres-sur Seine, France). Additionally, in patients with thrombosis, FVIIIC coagulant activity and FXII were assayed with deficient plasma from Dade-Behring (Marburg, Germany) in samples obtained before the thrombotic event. Plasmatic coagulation factors were again investigated in a subgroup of 57 children (PRED, n = 25; DEXA, n = 32) on days with maximum CASP activity (days 27-33 during induction therapy).10 Classification of deficiency states and risk cutoffs were performed as recently described.1 2
Statistics
All statistical analyses were performed using the StatView 5 software package (SAS Institute, Cary, NC). The probability of a symptomatic thromboembolic event as a function of time was determined by the method of Kaplan and Meier. The log-rank test was used to compare the thromboembolism-free survival in ALL patients treated with PRED or DEXA during induction therapy. χ2 analysis and Fisher exact test were used to compare the rates of prothrombotic risk factors in children treated with PRED and DEXA. Continuous data were presented as medians and ranges and were evaluated by nonparametric statistics using the Wilcoxon Mann-Whitney U test. The correlation between CASP activities and levels of coagulation factors was tested using Spearman rank analysis.
Results
Prothrombotic risk factors
No significant difference was found in the overall prevalence rate of prothrombotic risk factors in the white populations studied (P = .72). The heterozygous FII G20210A mutation was found in 2.3% of the PRED group and in 3.5% of the children treated with DEXA (P = .63); the FV G1691A mutation was present in 7.1% of both groups (P = .90); inherited protein C deficiency was found in 2.1% of the PRED group versus 1.8% of the DEXA group (P = 1.0); inherited protein S deficiency was confirmed in 1.1% of the PRED group, and antithrombin deficiency was reported only once in children treated with PRED. Elevated Lp(a) (more than 30 mg/dL) was diagnosed in 6.5% of children treated with PRED compared with 7.1% in the DEXA arm (P = 1.0).
Presence of central lines
In 249 of 280 (88.9%) children in the PRED group and in 51 of 56 (91.0%) children in the DEXA group, polychemotherapy was administered through central lines (P = .48). Compared with children in the PRED group, no symptomatic central line–associated thromboembolism was observed (PRED, n = 18; 7.2%), and no thromboembolism-associated death occurred in the DEXA group (PRED, n = 1; 0.4%). The rate of Broviac, Hickman, or Porth catheters implanted was no different between the 2 groups investigated.
Thromboembolic events during induction therapy
Compared with 29 (10.4%) patients in the PRED group who had symptomatic vascular accidents, only one 9-year-old girl of 56 (1.8%) children receiving DEXA had symptomatic cerebral venous thromboembolism on day 33 of induction therapy. In this patient, however, hypoplasia of the superior sagittal sinus had been diagnosed before leukemia onset. Although 24 of 29 thromboses in the PRED group were associated with at least one prothrombotic risk factor,1 no inherited prothrombotic risk factor could be identified in the 9-year-old girl with thrombosis in the DEXA group. Fibrinogen, antithrombin, protein C, protein S, plasminogen, and Lp(a) levels were within the normal range at the time of symptomatic thromboembolic onset. In addition, in children with thrombosis, FVIIIC activities were normal before the onset of symptomatic thrombosis.
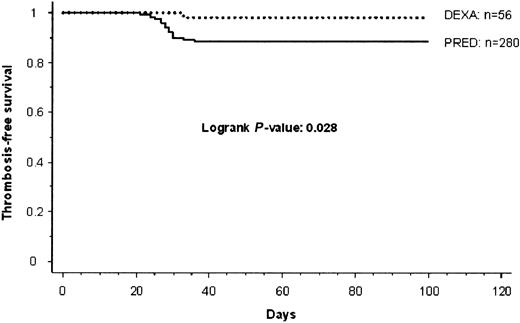
As recently reported in PRED-treated children, 15 of 29 children had cerebral venous thrombosis; in 5 of them it was associated with a central line placed in the internal jugular vein. Twelve of 29 patients showed catheter-related superior caval vascular occlusion, and thrombosis of femoral and pelvic veins was diagnosed in another 2 patients, respectively.1 Children with cerebral sinus thrombosis initially were treated for headache, coma, vomiting, or seizures. Patients with symptomatic catheter-related thrombosis in the upper venous system showed swelling of the corresponding extremities (n = 4) or neck tissues (n = 2), and superficial vein collaterals were observed in 3 children. In the remaining 3 patients, loss of catheter patency was the leading symptom of vascular obstruction. The 2 patients with thrombosis of the lower extremity initially were treated for blue and swollen legs, with superficial collateral circulation additionally visible in one patient. The 9-year-old girl in the DEXA group was treated for headache and seizures at the onset of thrombosis. The significantly reduced cumulative thrombosis-free survival in children treated with PRED compared with children in the DEXA group during ALL-induction therapy (P = .028) is shown in Figure1.
Thrombosis-free cumulative survival in children with ALL.
PRED versus DEXA during induction therapy.
Thrombosis-free cumulative survival in children with ALL.
PRED versus DEXA during induction therapy.
Thromboembolic events not occurring during induction therapy
Of all patients receiving reinduction therapy in the PRED group, symptomatic thromboembolism in the central nervous system developed in 1.7% (n = 3) of them during reinduction therapy with CASP and dexamethasone administration. In the DEXA group, 2 (3.6%) thromboembolic events were observed. A 10-year-old boy (at medium risk) acquired venous sinus thromboembolism directly after the cessation of dexamethasone application during second reinduction therapy (day 20). At a similar time point, day 27, during the second reinduction course and 3 days after intrathecal application of 12 mg methotrexate, a 13-year-old girl (medium risk) experienced methotrexate-related central nervous system toxicity mimicking a strokelike episode; symptoms lasted approximately 10 days.11 No other patient in the entire study group had a strokelike episode. Interestingly, each child had a positive family history of recurrent venous thromboembolism. Therefore, a comprehensive laboratory diagnostic work-up was performed on each. In the 10-year-old boy, elevated Lp(a), homozygous plasminogen activator inhibitor-1 4G/4G polymorphism, and FXII deficiency levels were found, and the 13-year-old girl carried the heterozygous FV Gly1691Ala mutation.
Plasmatic coagulation factors at maximum CASP activities
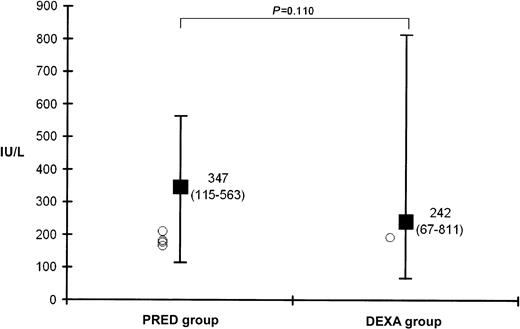
Figure 2 shows the medians of maximum CASP activities (days 27-33) and individual CASP values (circles) available in 5 children before the onset of symptomatic thromboembolism (PRED, n = 4; DEXA, n = 1). Concentrations of fibrinogen and free-protein S and activities of antithrombin, protein C, and plasminogen at days of maximum CASP activities are shown in Table 1. Significantly lower values of fibrinogen, antithrombin, plasminogen, and free-protein S antigen were found in the PRED group. VWF levels were significantly higher in children treated with DEXA. No difference was found for protein C activities. Correlation analysis between CASP activities and levels of coagulation factors revealed no significant results (fibrinogen,P = .2; antithrombin, P = .4; protein C,P = .3; protein S, P = .7; plasminogen,P = .1).
Median (range) values of maximum CASP activity (days 27-33) in children during induction therapy.
BFM 90/95 protocol (PRED group) versus BFM 2000 protocol (DEXA group) and individual CASP values (○) available in 5 children before the onset of symptomatic thromboembolism (PRED, n = 4; DEXA, n = 1). ■ indicates median; error bars, range.
Median (range) values of maximum CASP activity (days 27-33) in children during induction therapy.
BFM 90/95 protocol (PRED group) versus BFM 2000 protocol (DEXA group) and individual CASP values (○) available in 5 children before the onset of symptomatic thromboembolism (PRED, n = 4; DEXA, n = 1). ■ indicates median; error bars, range.
Plasmatic coagulation and fibrinolytic factors
| Prothrombic risk factors . | PRED group n = 25 . | DEXA group n = 32 . | P . |
|---|---|---|---|
| Fibrinogen, mg/dL | 43 (10-80) | 101 (43-481) | < .001 |
| Antithrombin activity, % | 56 (41-90) | 75 (35-111) | .002 |
| Protein C activity, % | 68 (45-136) | 72 (53-136) | .330 |
| Free-protein S antigen, % | 55 (28-90) | 64 (44-108) | .020 |
| Plasminogen activity, % | 47 (29-78) | 53 (29-101) | .021 |
| von Willebrand factor antigen, % | 108 (68-172) | 214 (100-442) | .0004 |
| Prothrombic risk factors . | PRED group n = 25 . | DEXA group n = 32 . | P . |
|---|---|---|---|
| Fibrinogen, mg/dL | 43 (10-80) | 101 (43-481) | < .001 |
| Antithrombin activity, % | 56 (41-90) | 75 (35-111) | .002 |
| Protein C activity, % | 68 (45-136) | 72 (53-136) | .330 |
| Free-protein S antigen, % | 55 (28-90) | 64 (44-108) | .020 |
| Plasminogen activity, % | 47 (29-78) | 53 (29-101) | .021 |
| von Willebrand factor antigen, % | 108 (68-172) | 214 (100-442) | .0004 |
Median (range) values of plasmatic coagulation and fibrinolytic factors during maximum CASP activity on days 27 to 33 in children during induction therapy. BFM 90/95 protocol (PRED group) versus BFM 2000 protocol (DEXA group).
Discussion
The present prospective, multicenter study in children with ALL was performed to evaluate the risk for vascular occlusions in children treated according to BFM protocols and receiving either dexamethasone or prednisone (BFM 2000, 10 mg/m2 dexamethasone [DEXA group]; BFM 90/95, 60 mg/m2 prednisone [PRED group]) during leukemia induction therapy. Besides the detection of inherited thrombophilic risk factors, the aim of this study was to investigate further coagulation proteins that might possibly be modified by the specific treatment modalities.
The recorded rate of 1.8% thromboembolic events within the DEXA group was within the lower range of published data in leukemic children during steroid and CASP administration (eg, 0.0%-2.4%).2,6,7 12 In contrast, the percentage of patients with a thromboembolic event was higher in the PRED group—10.4% of these children had venous thrombosis during induction therapy. Patients were recruited from the same living population and had a similar rate of central venous lines.
Within each patient group the rate of established prothrombotic risk factors was found within the prevalence rate reported for healthy white persons,13 and the recorded positive family history of thrombosis in the patients investigated was less than 1%. Although 46.5% of the PRED-treated children with at least one prothrombotic risk factor (FV G1691A, FII G20210A, protein S, protein C, and antithrombin deficiency, or elevated level of Lp[a]) experienced symptomatic thromboembolism,1 no child in the DEXA group with such a risk factor experienced symptomatic thromboembolism during ALL induction therapy. The reported 9-year-old girl in the DEXA group had cerebral venous thromboembolism based on venous hypoplasia of the affected sinus. In this patient, no association with further acquired risk factors (eg, decrease of coagulation inhibitors) could be identified. The cerebral venous thromboembolism that occurred during reinduction in the DEXA group is within the rate reported in the PRED group (application of dexamethasone during reinduction therapy). Thus, the administration of DEXA instead of PRED during induction therapy seems to be responsible mainly for the reduced rate of symptomatic thromboembolism in the children investigated.
Besides the involvement of the previously mentioned genetic risk factors for thrombophilia, additional factors such as endothelial cell injury or acquired coagulation imbalance, commonly described during combined steroid and CASP administration in childhood leukemia, may function as trigger mechanisms for thromboembolic manifestation during childhood ALL in children treated according to the BFM 90/95 study protocols.3-7 In the present survey, the possibility of further hemostatic alterations within the BFM protocols with the change of one drug only is described for the first time. Data presented here from the subgroup analysis clearly show that, during the time of similar maximum CASP activity, fibrinogen concentrations and activities of antithrombin (major changes), plasminogen, and free-protein S antigen (minor changes) were significantly reduced in the PRED-treated children. Moreover, correlation analysis between CASP activities and levels of coagulation factors revealed no significant results. Hence, a direct relationship between CASP based on the administration of 5000 U/m2 CASP and levels of coagulation factors and the frequency of thrombotic events is unlikely.
Glucocorticoids administered along with CASP in the children investigated have a number of inhibitory effects during inflammatory reactions. Early effects are the inhibition of developing edema, capillary dilatation, deposition of fibrin, and migration of leukocytes, whereas late effects are inhibition of capillary proliferation, proliferation of fibroblasts, and deposition of collagen. Moreover, glucocorticoids have a protective effect on the integrity of cell membranes and reduce the synthesis of prostaglandins and thromboxane through the inhibition of arachidonic acid release from phospholipids. On the one hand, dexamethasone has a stronger glucocorticoid effect than prednisone, which is why these aspects may explain a more protective role of dexamethasone concerning the development of thrombotic events.14 However, it remains unclear how far these mechanisms may alter the levels of coagulation factors and platelet function leading to thromboembolism. The data presented here are in agreement with previously published findings measured during the course of BFM reinduction therapy (90/95 protocols, DEXA and CASP).15 In the latter study values measured for fibrinogen, antithrombin, and plasminogen were found within the range reported in the present study (DEXA group), in which moderate acquired deficiency states of the coagulation proteins investigated were observed only in a minority of cases. On the other hand, we speculate that the decreased values of plasmatic coagulation proteins found in the PRED group during ALL induction therapy are possibly caused by a more pronounced synergistic effect of CASP and PRED in lowering hepatic protein synthesis compared with CASP and DEXA. Further studies, however, are necessary to clarify this issue.
Pui et al7,16 demonstrated in 1985 and 1987 that an altered von Willebrand factor molecule was found in leukemic children treated with CASP and PRED. Therefore, we have included the determination of von Willebrand factor antigen in the study presented here. VWF levels were significantly higher in children treated with DEXA, but no association was found with thromboembolic events in the patients reported here. FVIIIC coagulant activity was investigated only in children with symptomatic thrombosis from samples obtained before the onset of thromboembolism. Neither in the PRED-treated children nor in the DEXA group were elevated FVIIIC levels higher than 120% of normal found. This observation is in agreement with data shown by Pui et al.7 We conclude that increased values of FVIIIC, measured from samples before the onset of symptomatic thrombosis, do not play an important role in the etiology of chemotherapy-induced vascular accidents in German children treated according the BFM protocols.
Although no conclusion can be drawn with respect to the overall leukemic event-free survival in patients treated according to the different German BFM 90/95 and BFM 2000 treatment protocols, data from this multicenter survey suggest that the old problem of thromboembolic complications in ALL patients should be revisited. Evidence is given that the combination of inherited prothrombotic risk factors with acquired deficiencies of one or more proteins regulating thrombin and fibrin formation5 are responsible for the development of thromboembolic events in leukemic children treated with CASP and prednisone. The use of dexamethasone instead of prednisone, concomitantly administered with CASP in leukemic patients of the same living population treated according to BFM-adapted protocols during induction therapy, significantly reduced the symptomatic onset of venous thromboembolism in the children investigated. With respect to symptomatic thromboembolism, further studies are recommended to clarify the possible protective effect of dexamethasone during ALL induction therapy.
We thank Susan Griesbach and Gabriele Braun-Munzinger for assistance in editing this manuscript. In addition, we thank Susan Halimeh (Münster), Annette Laupert (Frankfurt), and Cornelia Wermes (Hanover) for data collection. We also thank Petra Schulze-Westhoff for measuring CASP activities.
Prepublished online as Blood First Edition Paper, November 27, 2002; DOI 10.1182/blood-2002-06-1901.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Ulrike Nowak-Göttl, Pediatric Hematology/Oncology, University Children's Hospital, Albert Schweitzer Str 33, D-48149 Münster, Germany; e-mail:leagottl@uni-muenster.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal