CD20 is a 33- to 36-kDa transmembrane phosphoprotein involved in the activation, proliferation, and differentiation of B lymphocytes. The predicted amino acid sequence of the CD20 suggests 4 transmembrane-spanning regions with both N- and C-termini located in the cytoplasm. We demonstrate herein that significant levels of circulating CD20 (cCD20) can be detected in the plasma of patients with chronic lymphocytic leukemia (CLL) and that cCD20 interferes with the binding of rituximab, a humanized anti-CD20 monoclonal antibody, to CLL cells. An enzyme-linked immunosorbent assay (ELISA) was developed to measure circulating cCD20 levels in the plasma. We measured cCD20 levels in the plasma of 180 patients with CLL and correlated these levels with clinical characteristics and outcome. Circulating CD20 levels correlated positively with β2-microglobulin level (p = .006) and percentage of CD38+ cells (p = .03) and negatively with platelet count (p = .004) and hemoglobin level (p = .02). Patients with advanced Rai (III/IV) or Binet (C) stage disease had significantly higher levels of cCD20 than did patients with earlier-stage disease (P = .01 and P = .006, respectively). There was no correlation between cCD20 level and age, lymphocyte count, or white blood cell count. Using a recursive classification method, we found that patients with a cCD20 level more than 1875 nM/L had significantly shorter survival than those with cCD20 1875 nM/L or below (P = .01). The prognostic value of cCD20 was independent of Rai staging or hemoglobin level. Prospective evaluation is indicated to establish whether rituximab dosing should be adjusted according to cCD20 levels.
Introduction
CD20, also called B1 (Bp35), is a phosphoprotein detected on the surface of B lymphocytes1-3 and believed to play a major role in the regulation, activation, proliferation, and differentiation of these cells.4-6 The gene for CD20 has been mapped to human chromosome 11 at position q12-q13, centromeric to the Bcl-1 locus, which is involved in the translocation t(11;14)(q13;q32).7,8 The CD20 gene codes for a protein that varies in molecular weight from 33 to 36 kDa, secondary to alternative splicing at the 5′ end and to differences in phosphorylation.1 Higher levels of CD20 phosphorylation have been reported in proliferating malignant B cells than in nonproliferating B cells.1 Although the function of the CD20 protein is not well defined, it has been suggested that CD20 regulates transmembrane calcium conductance.5 Activation of CD20 by binding to antibodies directed toward the extracellular portion of CD20 leads to tyrosine kinase pathway activation and modulation of cell cycle progression via interaction with src-related kinases.9-11 Sequence analysis of the CD20 molecule showed 4 transmembrane domains with N- and C-termini in the cytoplasm without evidence of shedding.6 It has been reported, however, that CD20 relocalizes into a detergent-insoluble membrane compartment upon binding to antibodies.12 Binding of the humanized monoclonal antibody rituximab to CD20+ cells leads to their death via complement-dependent cellular cytotoxicity or antibody-dependent cellular cytotoxicity.13-19 Use of rituximab in the treatment of B-cell malignancies has led to an improvement in outcome, and the use of this antibody is expanding rapidly.13-19 The optimization of the dosing and scheduling of rituximab could potentially increase its efficacy. Several investigators have documented variations in intensity of CD20 expression on the surface of malignant B cells in different lymphoproliferative diseases and have suggested that these variations may influence binding and efficacy of rituximab therapy.20-22 We investigated the possibility that circulating CD20 was present in plasma; binding of circulating CD20 to rituximab could potentially influence efficacy. We used immunoprecipitation, Western blot, and novel immunoassays to investigate the presence and levels of circulating CD20 (cCD20) in the plasma of healthy individuals and patients with chronic lymphocytic leukemia (CLL). Furthermore, we examined the correlation between plasma levels of cCD20 and the clinical features of the disease and prognosis.
Patients, materials, and methods
Patient and specimens
We collected plasma samples from 180 patients with CLL and from 31 healthy individuals after informed consent was obtained according to institutional guidelines. Patients requiring treatment were treated on fludarabine-based protocols; none of these protocols included rituximab. Peripheral blood samples were collected in EDTA (ethylenediaminetetraacetic acid)–treated tubes. The diagnosis of CLL was established on the basis of morphologic, immunologic, and molecular evaluation of peripheral blood and bone marrow. Immunologic evaluation was done by flow cytometric analysis of leukemic cells using CD19, CD5, CD20, CD23, CD11C, CD22, FMC-7, CD79B, CD3, CD4, CD8, κ, and λ antibodies. Molecular studies included analysis of immunoglobulin and T-cell receptor genes as well as Bcl-1 and Bcl-2 rearrangements. The diagnostic criteria for CLL were based on the history and physical examination findings recommended by the National Cancer Institute (NCI)-CLL Working Group.23 All cases were positive for CD19, CD5, and CD23. Response was evaluated according to the criteria of the NCI-CLL Working Group.23
Western blot analysis of plasma
One microliter of plasma from healthy individuals and from CLL patients was electrophoretically separated on 9.5% denaturing sodium dodecyl sulfate (SDS) polyacrylamide gels. Cell lysates from myeloid cell lines and CLL cell samples were used as negative and positive controls, respectively. The nitrocellulose membranes were blocked for 6 to 8 hours at room temperature with 5% nonfat milk in phosphate-buffered saline (PBS) containing 0.1% Tween 20 and 0.01% sodium azide. The blots were incubated overnight at 4°C with 1 μg/μL mouse anti-CD20 antibody (Ab-2 [B9E9]; Lab Vision Corporation, Fremont, CA) in 2.5% nonfat milk, 2.5 bovine serum albumin (BSA), and 0.1% Tween 20. The membrane was washed with PBS containing 0.1% Tween 20. The blot was then incubated with 1:2000 diluted antimouse horseradish peroxidase–conjugated immunoglobulin (Amersham Life Sciences, Arlington Heights, IL) in PBS containing 1% nonfat milk and 0.1% Tween 20. Immunoreactive bands were developed using the enhanced chemiluminescence (ECL) detection system (Amersham Life Sciences). To correlate the amount of protein detected on the Western blot with the levels detected by the enzyme-linked immunosorbent assay (ELISA) assay, the autoradiographs were scanned and bands were quantified using Scan Analysis software from Biosoft (Cambridge, United Kingdom). An equal amount of plasma (1 μL) was used from all samples.
Immunoprecipitation of CD20
Mouse anti-CD20 (catalog no. SC-7733, lot no. K011; Santa Cruz Biotechnology, Santa Cruz, CA) was attached to protein G beads using the immunoprecipitation kit from Pierce (catalog no. 45210; Rockford, IL) as recommended by the manufacturer. Plasma (200 μL) from each subject was mixed with 10 μL of 0.1 M dithiothreitol (DDT) and then incubated with protein G–conjugated antibody. The conjugated protein G–cCD20 was washed extensively with 25 mM Tris (tris(hydroxymethyl)aminomethane), 0.5 M NaCl buffer (pH 7.2) and then eluted from the column using 30 μL ImmunoPure solution, pH 2.8 (Pierce). The eluted protein (5 μL) was resolved by 9.5% SDS-polyacrylamide gel, stained with 0.25% Coomassie blue R-250 (Sigma-Aldrich, St Louis, MO), and destained as recommended by the manufacturer.
Plasma CD20 enzyme-linked immunosorbent assay
We used an enzyme-linked immunosorbent assay (ELISA) developed in our laboratory to measure plasma CD20 levels. Briefly, a 96-well polystyrene microplate was coated with 100 μL rabbit antigoat immunoglobulin at a concentration of 1 μg/mL in 0.05 M carbonate buffer (pH 9.5). Plates were incubated for 6 hours at room temperature and then washed with PBS containing 0.01% Tween 20. Each well was blocked with 250 μL of 2% BSA in PBS containing 0.1% Tween 20 for 2 hours at 37°C. Goat anti-CD20 (1 μL) was then added to each well, and the microplate was incubated overnight at 4°C. After a wash in PBS containing 0.1% Tween 20, 100 μL plasma was added to each of the wells, which were then incubated for 3 hours with constant shaking at room temperature. The plates were again washed in PBS containing 0.1% Tween 20 and then incubated for 3 hours with 20 μL horseradish peroxidase–conjugated humanized anti-CD20 antibody (rituximab) at a dilution of 1:400 in 2% BSA, 0.1% Tween 20. Horseradish peroxidase–enzyme conjugation of the rituximab was carried out by using standard technique. The plates were incubated for another 3 hours. The wells were then washed 6 more times with PBS containing 0.1% Tween 20. Substrate was added (100 μL) to develop the color. The plates were incubated with constant shaking for 15 to 30 minutes. The reaction was stopped with 50 μL of 2 N HCl, and the plates were read at the 450 nm wavelength. Serial dilution of the known number of molecules of synthetic CD20 peptide (catalog no. SC-7703; Santa Cruz Biotechnology) was used to generate a standard curve. For confirmation of specificity of the detected CD20, we used the same CD20 peptide or the CD20 protein precipitated from BJAB cell line in blocking experiments. Adding an increasing amount of CD20 from BJAB cells or peptide demonstrated increasing blocking of the cCD20 detection by the ELISA.
Human histocompatability class I ELISA
An established ELISA assay for the human leukocyte antigen (HLA) class I was used to analyze HLA-1 in the supernatants of tissue culture. Briefly, rabbit antimouse immunoglobulin (Sigma-Aldrich) was used for capturing the monoclonal anti-HLA class I antibodies (clone W6; Sigma). Plates were washed with PBS containing 0.01% Tween 20; 100 μL of 1:50 diluted medium in PBS containing 0.01% Tween 20 was added to each well. Horseradish peroxidase–labeled anti–β2-microglobulin (Dako, Glostrup, Denmark) was used for detection. The standard curve was prepared using purified HLA class I protein (B7 Calabratoer; SangStat Medical). Substrate was added, and plate color intensity was evaluated at 450 nm within 15 minutes.
Cell culture and quantification of cCD20
Cells from patients with CLL were maintained in RPMI 1640 medium containing 10% fetal calf serum at 5% CO2. Phorbol 12-myristate 13-acetate (PMA) (Sigma) was used to induce shedding. PMA was added to duplicate cultures at a concentration of 10 nM/L.
Competition experiments and blocking of rituximab binding
We performed several mixing studies to test the ability of circulating cCD20 to bind to rituximab and the effects of that binding on cells. We added 1 μg rituximab to 1 million cells isolated from patients with CLL or Raji cells. Flow cytometry and phycoerythrin (PE)–labeled anti-CD20 antibodies were used to monitor rituximab's effect on the CD20 binding sites on the surface of cells. The anti-Fc fragment of mouse immunoglobulin (Ig; Jackson Immuno Research Laboratories, West Grove, PA) was used to detect rituximab on the surface of cells and anti-CD19 antibody to detect B cells. Rituximab binding to cells was also analyzed after cells were mixed with 100, 300, and 500 μL of plasma or after a similar amount of buffered saline. If plasma obtained from a different patient was added to CLL cells or Raji cells, we heated the plasma for 30 minutes at 56°C to inactivate the complement pathway.
Statistical analysis
The correlations between cCD20 and other covariates were calculated using Spearman rank correlation coefficients. The Wilcoxon rank sum test or Kruskal-Wallis test was used to compare cCD20 levels among categorical variables such as Rai stage and sex. Probabilities of survival were estimated by the method of Kaplan and Meier.24
Unadjusted between-group comparisons of survival were made using the log-rank test.25 All scatter plots were smoothed using the Loess method of Cleveland, with predictive variables transformed as appropriate on the basis of these plots.26The Cox proportional hazard regression model was used to assess the ability of clinical characteristics in predicting survival, with goodness of fit assessed by Martingale residual plots and likelihood ratio statistics.27 The cut point was chosen from univariate analysis using RPART.28 This coincided with the largest change in the relative risk of death suggested by a Martingale residual plot. The procedure of RPART works as follows: It starts from a cut point that best splits the data into 2 groups. This process is then applied separately to each subgroup and continued recursively until either the subgroups reach a minimum size or no further improvement can be made.
Multivariate Cox proportional hazard models were then fitted to evaluate these predictors simultaneously. Recursive partitioning was implemented to find cut points for cCD20 or Rai staging based on Martingale residuals. All statistical analyses were carried out using Splus29 (Insightful, Seattle, WA) and CART (Classification and Regression Tree) packages (Statcon, Witzenhausen, Denmark).30
P values less than .05 were considered statistically significant.
Results
Detection of CD20 in plasma
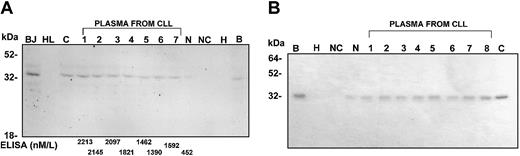
The presence of circulating CD20 in the plasma was investigated using Western blot and immunoprecipitation. As expected, CD20 was detected by Western blot in cells from patients with CLL but not in a myeloid cell line (HL60) (Figure1A). Plasma from healthy individuals showed low levels of cCD20 on Western blot, whereas plasma of patients with CLL had high levels of CD20 (Figure 1A). The cCD20 bands detected in plasma corresponded to the 35 kDa CD20 detected in the CLL cells. Additional bands seen in cell samples from patients with CLL as well as in some plasma samples may correspond to the previously reported phosphorylated and alternatively spliced CD20 protein. Immunoprecipitation also showed high levels of CD20 in plasma from patients with CLL (Figure 1B). Figure 1B shows CD20 protein precipitated from 33 μL plasma from different patients with CLL. The plasma samples were denatured and then immunoprecipitated and visualized by Coomassie stain (Figure 1B).
Western blot and immunoprecipitation demonstrating significant levels of cCD20 in the plasma of patients with CLL.
(Panel A) Western blot showing detectable CD20 protein in plasma from patients with CLL. The plasma CD20 is similar in molecular weight to CD20 detected in cell lysates from BJAB cell line (BJ) and cells from a patient with CLL (C). As expected, no CD20 is detectable in lysate from the HL60 cell line (HL). Plasma from a healthy control individual (N) shows lower levels of CD20. Immunoprecipitation products from BJAB (B) and HL60 (H) cell lysates are also shown. Precipitation from beads and antibody without plasma or cell lysate is also shown as a negative control (NC). The molecular weight marker (M) is shown. One microliter of plasma is used in lanes that contain plasma. The levels of cCD20 detected in the plasma by ELISA are shown on the bottom. (Panel B) Coomassie blue-stained gel showing immunoprecipitation of CD20 from plasma of patients with CLL. The CD20 from cell lysates from BJAB (B) and CLL (C) are shown as positive control and from HL60 (H) as a negative control. Equal amounts of plasma (33 μL) from each sample were denatured before immunoprecipitation (abbreviation as in Panel A).
Western blot and immunoprecipitation demonstrating significant levels of cCD20 in the plasma of patients with CLL.
(Panel A) Western blot showing detectable CD20 protein in plasma from patients with CLL. The plasma CD20 is similar in molecular weight to CD20 detected in cell lysates from BJAB cell line (BJ) and cells from a patient with CLL (C). As expected, no CD20 is detectable in lysate from the HL60 cell line (HL). Plasma from a healthy control individual (N) shows lower levels of CD20. Immunoprecipitation products from BJAB (B) and HL60 (H) cell lysates are also shown. Precipitation from beads and antibody without plasma or cell lysate is also shown as a negative control (NC). The molecular weight marker (M) is shown. One microliter of plasma is used in lanes that contain plasma. The levels of cCD20 detected in the plasma by ELISA are shown on the bottom. (Panel B) Coomassie blue-stained gel showing immunoprecipitation of CD20 from plasma of patients with CLL. The CD20 from cell lysates from BJAB (B) and CLL (C) are shown as positive control and from HL60 (H) as a negative control. Equal amounts of plasma (33 μL) from each sample were denatured before immunoprecipitation (abbreviation as in Panel A).
Detection of cCD20 in plasma by ELISA
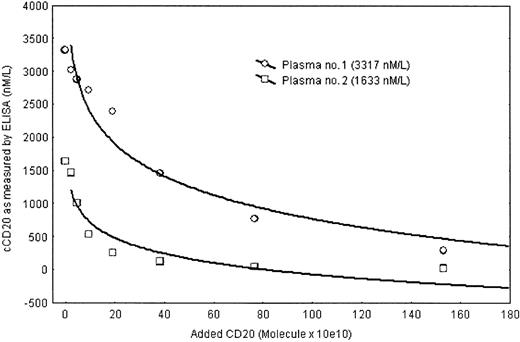
We developed an ELISA assay for detection and quantification of cCD20 in plasma and compared the levels of cCD20 detected by the ELISA assay with those detected by Western blot (Figure 1A). The intensity of the CD20 bands on the Western blot correlated with the cCD20 levels detected by ELISA (Figure 1) (Spearman, p < .0001). Dilutions and measurements of diluted samples showed almost identical values (z = 0.35 and P = .7, sign test). The specificity of the assay for CD20 was confirmed by competition experiments. CD20 isolated from the BJAB cell line or a CD20 peptide was able to block the detection of signal by ELISA when an increasing amount of CD20 was added to the wells for the detection of CD20 in a plasma sample (Figure 2).
Inhibition assay confirming the specificity of the ELISA assay in detecting CD20.
Detection of CD20 in 2 plasma samples from patients with CLL was diminished when increasing amounts of purified CD20 were added to the detecting antibody (horseradish peroxidase-labeled rituximab).
Inhibition assay confirming the specificity of the ELISA assay in detecting CD20.
Detection of CD20 in 2 plasma samples from patients with CLL was diminished when increasing amounts of purified CD20 were added to the detecting antibody (horseradish peroxidase-labeled rituximab).
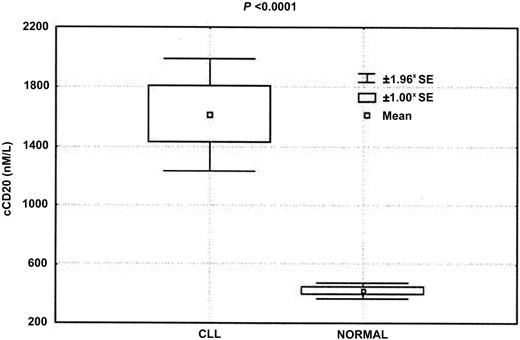
Levels of cCD20 in the plasma of patients with CLL were significantly higher than those detected in the 31 healthy individuals tested (P < .0001, Kruskal-Wallis test) (Figure3). The cCD20 levels in patients with CLL varied widely, from 52.89 to 15 740 nM (median, 776.9 nM). In contrast, the levels of cCD20 in the plasma of the 31 healthy individuals varied only from 123.55 to 547.10 nM (median, 470 nM). Additional testing demonstrated that higher levels of cCD20 could be detected in some healthy individuals who have a viral infection.
Significantly higher levels of cCD20 were detected in patients with CLL than in healthy control individuals.
Mean 1.00 and 1.96 standard errors (*SE) are shown.
Significantly higher levels of cCD20 were detected in patients with CLL than in healthy control individuals.
Mean 1.00 and 1.96 standard errors (*SE) are shown.
To test whether the detected cCD20 was the result of active shedding or breakdown of cells, we assayed levels of cCD20 in supernatants of cultured CLL cells at various time points. Peripheral blood cells from 3 different patients with CLL were cultured with and without PMA (used as a shedding agent), and samples from the supernatants of these cultures were collected at various time points. As shown in Figure4, there was no increase in cCD20 in the supernatants with time, even after 148 hours of culture. Cells cultured with PMA also showed no increase in cCD20, confirming that cCD20 does not shed from cells. As a control, levels of the HLA class I molecule, which is known to shed from cells, were analyzed before and after exposure to PMA.31 There was no significant increase in HLA levels with time in supernatants from cells cultured without PMA, but the supernatants from the same cells cultured with PMA showed significant increase in the levels of HLA class I protein, confirming the shedding of the HLA class I molecule (Figure 4A).
No evidence of ex vivo CD20 shedding.
(A) Supernatant from cultured cells of patients with CLL showed no significant increase with time regardless of whether the cells were cultured without or with a shedding agent (phorbol 12-myristate 13-acetate [PMA]). In contrast, in HLA class I, levels increased significantly in the supernatant from cells cultured with PMA. (B) Similar levels of cCD20 were detected whether plasma samples were separated from cells immediately after collection (0) or after a prolonged period at room temperature (1, 2, 4, 8, 16, 24, 36, and 48 hours).
No evidence of ex vivo CD20 shedding.
(A) Supernatant from cultured cells of patients with CLL showed no significant increase with time regardless of whether the cells were cultured without or with a shedding agent (phorbol 12-myristate 13-acetate [PMA]). In contrast, in HLA class I, levels increased significantly in the supernatant from cells cultured with PMA. (B) Similar levels of cCD20 were detected whether plasma samples were separated from cells immediately after collection (0) or after a prolonged period at room temperature (1, 2, 4, 8, 16, 24, 36, and 48 hours).
To investigate the possibility that the cCD20 detected in samples was the result of ex vivo lysis, we measured cCD20 in samples from 3 patients with CLL and high lymphocyte counts at various time points after collection. Peripheral blood samples were collected in EDTA and kept at room temperature. Circulating CD20 was analyzed in aliquots taken at various time points after collection. Figure 4B shows a representative example from a patient with high white blood cell count; there was no significant increase in the plasma cCD20 level after 48 hours at room temperature.
High levels of cCD20 correlate with advanced stage of CLL
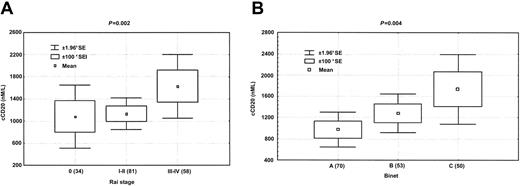
Correlation of plasma cCD20 levels with various characteristics and stage of disease in the 180 patients with CLL yielded mixed results. The patients were representative of typical populations of patients with CLL seen at M. D. Anderson Cancer Center (Table 1) and included 116 (64.4%) men. Thirty-two (17.8%) patients had Rai stage 0 disease, 81 (45.0%) had stage I-II disease, and 60 (33.3%) had stage III-IV disease. The median age was 61 years, and the median level of β2-microglobulin was 288 nM (3.4 mg/L). There was no significant difference in cCD20 levels between men and women (P = .66). Circulating CD20 levels were highly correlated with β2-microglobulin levels, platelet count, percentage of cells expressing CD19+/CD38+, and hemoglobin level (Table 2). Circulating CD20 levels did not correlate significantly with white blood cell count, lymphocyte count, or age. Levels of cCD20 correlated with both Rai and Binet stages. When cases were grouped by stages as Rai 0, Rai I-II, or Rai III-IV, patients with higher Rai stage had significantly higher levels of cCD20 (P = .01, Kruskal-Wallis test) (Figure5A). When 2 groups were used, patients with Rai stage 0-II had significantly lower cCD20 levels than those with Rai stage III-IV disease (P = .01). Similar results were obtained when Binet staging was used (P = .004) (Figure 5B). No correlation was found between cCD20 levels and presence of hepatosplenomegaly or number of sites of lymphadenopathy.
Patient characteristics (N = 180)
| Variable . | Median (range) . | No. (%) . |
|---|---|---|
| Sex | ||
| Male | — | 116 (64) |
| Female | — | 64 (36) |
| Rai stage | ||
| 0 | — | 32 (17.8) |
| I | — | 51 (28.3) |
| II | — | 30 (16.7) |
| III | — | 15 (8.3) |
| IV | — | 45 (25.0) |
| Age, y | 61 (33-84) | — |
| CD38+/CD19+, % | 7.7 (0.3-98) | — |
| Hemoglobin level, g/dL | 12.8 (4-17.8) | — |
| Platelet count, × 103/μL | 142 (4-342) | — |
| White blood cell count, × 103/μL | 55.9 (1.4-333.9) | — |
| Lymphocyte count, % | 85 (9-99) | — |
| β2-microglobulin level, mg/L | 3.4 (1.3-12.7) | — |
| cCD20, nM/L | 776.9 (52.89-15 740) | — |
| Variable . | Median (range) . | No. (%) . |
|---|---|---|
| Sex | ||
| Male | — | 116 (64) |
| Female | — | 64 (36) |
| Rai stage | ||
| 0 | — | 32 (17.8) |
| I | — | 51 (28.3) |
| II | — | 30 (16.7) |
| III | — | 15 (8.3) |
| IV | — | 45 (25.0) |
| Age, y | 61 (33-84) | — |
| CD38+/CD19+, % | 7.7 (0.3-98) | — |
| Hemoglobin level, g/dL | 12.8 (4-17.8) | — |
| Platelet count, × 103/μL | 142 (4-342) | — |
| White blood cell count, × 103/μL | 55.9 (1.4-333.9) | — |
| Lymphocyte count, % | 85 (9-99) | — |
| β2-microglobulin level, mg/L | 3.4 (1.3-12.7) | — |
| cCD20, nM/L | 776.9 (52.89-15 740) | — |
Correlation of cCD20 level with clinical parameters in patients with CLL
| Variable . | p (r value) . |
|---|---|
| Hemoglobin level | .02 (−0.18)* |
| β2-microglobulin level | .006 (0.23)* |
| RAI: 0, I-II, III-IV | .01† |
| Binet: A, B, C | .004† |
| Platelet count | .004 (−0.22)* |
| CD19+/CD38+, % | .03 (0.20)* |
| Splenomegaly | .07‡ |
| Hepatomegaly | .25‡ |
| Node enlargement | .11‡ |
| Age | .53 (0.05)* |
| Lymphocyte count | .71 (−0.03)* |
| White blood cell count | .33 (−0.07)* |
| Sex | .66‡ |
| Variable . | p (r value) . |
|---|---|
| Hemoglobin level | .02 (−0.18)* |
| β2-microglobulin level | .006 (0.23)* |
| RAI: 0, I-II, III-IV | .01† |
| Binet: A, B, C | .004† |
| Platelet count | .004 (−0.22)* |
| CD19+/CD38+, % | .03 (0.20)* |
| Splenomegaly | .07‡ |
| Hepatomegaly | .25‡ |
| Node enlargement | .11‡ |
| Age | .53 (0.05)* |
| Lymphocyte count | .71 (−0.03)* |
| White blood cell count | .33 (−0.07)* |
| Sex | .66‡ |
r value indicates correlation coefficient.
Spearman rank correlation test.
Kruskal-Wallis test.
Wilcoxon rank sum test.
Circulating CD20 levels correlate with clinical stages of CLL.
Levels of cCD20 correlated with Rai (A) and Binet (B) staging. Mean 1.00 and 1.96 standard errors (*SE) are shown.
Circulating CD20 levels correlate with clinical stages of CLL.
Levels of cCD20 correlated with Rai (A) and Binet (B) staging. Mean 1.00 and 1.96 standard errors (*SE) are shown.
Survival analysis
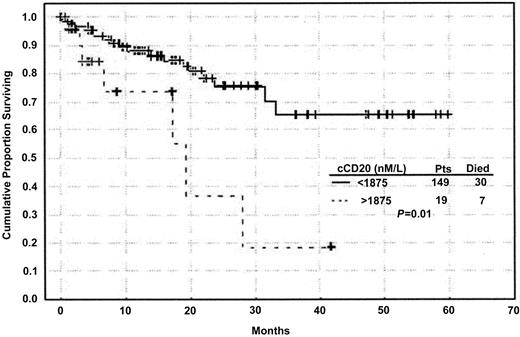
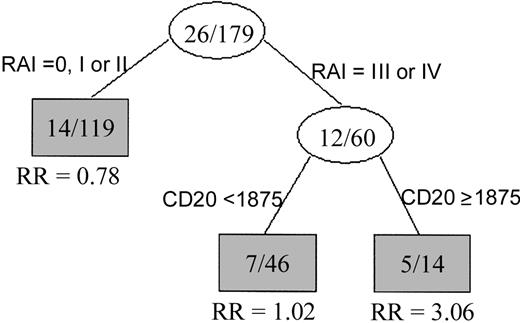
The univariate Cox proportional hazards model was used to test for variables that predict survival time in this patient group. As shown in Table 3, expression of CD38, hemoglobin level, platelet count, β2-microglobulin (β2M) levels, and Rai staging were all predictors of survival. When cCD20 was analyzed as a continuous variable in the Cox regression model, it was a strong predictor of survival (P = .002). To test for the linearity (goodness of fit) of cCD20 in predicting survival, we examined Martingale residual plots using log transformation. When the logarithm of cCD20 was used to refit the univariate Cox model, cCD20 was only marginally significant (P = .08) when used as a continuous variable. In contrast, when the cCD20 range was dichotomized using a cut point of 1875 nM/L, Kaplan-Meier plots demonstrated 2 groups of patients with significantly different survival. Patients with cCD20 levels more than 1875 nM/L had significantly shorter survival than those with cCD20 level less than or equal to 1875 nM/L (P = .01) (Figure6). Median survival time in the patients with high cCD20 levels was approximately 18 months, while the median survival in those with lower levels had not been reached at the time of analysis (Figure 6). The cut point of 1875 nM was reached using recursive partitioning procedures, a standard computer-based analysis that finds the best breakpoint for separating 2 groups. Multivariate analysis showed that the shorter survival in patients with cCD20 levels more than 1875 nM/L was independent of Rai or Binet stage or hemoglobin level. Specifically, a multivariate model including cCD20 and Rai stage indicated an increased relative risk in patients with high cCD20 level (relative risk = 3.51, P = .01) (Figure7). Similar results were observed when either Binet stage or hemoglobin level was included in the multivariate model instead of Rai stage. However, further validation with a larger number of patients is needed if this cut point were to be used for stratifying patients for therapy.
Univariate Cox proportional hazards model (N = 180)
| Variable . | Coefficient . | Relative risk . | P . |
|---|---|---|---|
| Hemoglobin level | −0.241 | 0.79 | .0005 |
| Log, β2-microglobulin level | 2 | 7.37 | .0005 |
| RAI: all stages | 0.353 | 1.42 | .018 |
| RAI: stages III and IV | 0.995 | 2.71 | .021 |
| Platelet count | −0.008 | 0.99 | .020 |
| Log, CD38+/CD19+ | 0.551 | 1.73 | .023 |
| cCD20 | 0.0002 | 1.00 | .006 |
| Log, cCD20 | 0.374 | 1.45 | .080 |
| cCD20 1875 nM or below | 1.11 | 3.03 | .020 |
| Age | 0.025 | 1.02 | .22 |
| Log, % lymphocyte count | 0.24 | 1.27 | .32 |
| Log, white blood cell count | 0.183 | 1.2 | .43 |
| Sex, male | −0.13 | 0.88 | .76 |
| Variable . | Coefficient . | Relative risk . | P . |
|---|---|---|---|
| Hemoglobin level | −0.241 | 0.79 | .0005 |
| Log, β2-microglobulin level | 2 | 7.37 | .0005 |
| RAI: all stages | 0.353 | 1.42 | .018 |
| RAI: stages III and IV | 0.995 | 2.71 | .021 |
| Platelet count | −0.008 | 0.99 | .020 |
| Log, CD38+/CD19+ | 0.551 | 1.73 | .023 |
| cCD20 | 0.0002 | 1.00 | .006 |
| Log, cCD20 | 0.374 | 1.45 | .080 |
| cCD20 1875 nM or below | 1.11 | 3.03 | .020 |
| Age | 0.025 | 1.02 | .22 |
| Log, % lymphocyte count | 0.24 | 1.27 | .32 |
| Log, white blood cell count | 0.183 | 1.2 | .43 |
| Sex, male | −0.13 | 0.88 | .76 |
Circulating CD20 levels correlate with survival.
Kaplan-Meier curve showing significantly shorter survival times in patients with high levels of cCD20 (P = .01, log-rank test).
Circulating CD20 levels correlate with survival.
Kaplan-Meier curve showing significantly shorter survival times in patients with high levels of cCD20 (P = .01, log-rank test).
Circulating CD20 is independent of Rai staging in predicting survival.
Classification tree showing that patients with advanced Rai stage disease separated into 2 groups: one with high cCD20 levels and shorter survival, the other with low cCD20 levels and relatively longer survival. The number in each node signifies the number of deaths per number of patients. RR is the relative risk (of death).
Circulating CD20 is independent of Rai staging in predicting survival.
Classification tree showing that patients with advanced Rai stage disease separated into 2 groups: one with high cCD20 levels and shorter survival, the other with low cCD20 levels and relatively longer survival. The number in each node signifies the number of deaths per number of patients. RR is the relative risk (of death).
Plasma cCD20 blocks rituximab binding to CLL cells
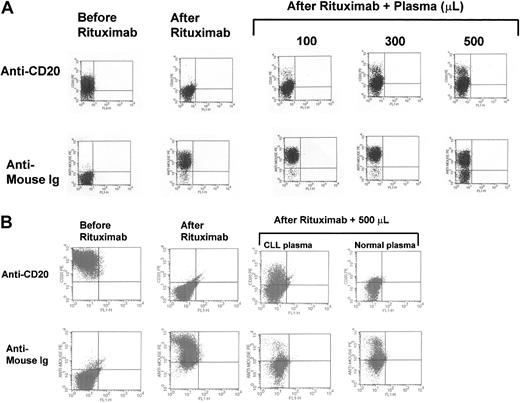
To test the significance of the levels of cCD20 and their role in rituximab therapy, we performed ex vivo mixing experiments. Increasing amounts of plasma were added to a mixture of CLL cells and rituximab. The efficacy of rituximab binding to CLL cells was evaluated by its ability to mask surface CD20 detection by conventional flow cytometry. When 1 μL rituximab was added to 1 million cells, without the addition of plasma, rituximab completely occupied all the CD20 antigen sites and the B cells became negative for CD20 (Figure8A). The rituximab on the surface of the CLL cells could be detected using PE-labeled antimouse immunoglobulin (Figure 8). The addition of 100, 300, or 500 μL of patients' plasma substantially blocked rituximab binding to cells. In other words, as shown in Figure 8, plasma cCD20 competed with cell surface CD20 for rituximab binding and significantly diminished the binding of rituximab to the surface of CLL cells. As the amount of plasma was increased, the binding of rituximab to CLL cells was further diminished (Figure 8A). This was demonstrated in samples from several patients, although the level of competition varied depending on the level of cCD20. The same effects were noted when the Raji cell line, which expresses high level of CD20, was used. The inhibition of binding of rituximab was significantly higher when plasma from patients with CLL was used as compared with plasma from healthy individuals (Figure 8B). Plasma from a patient with CD20− pre-B acute lymphoblastic leukemia with a very low level of cCD20 did not inhibit rituximab binding to Raji cells.
Plasma cCD20 sequesters rituximab.
(A) Mixing study results demonstrating the capability of plasma cCD20 to compete for rituximab and prevent it from binding to CLL cells. The top row shows an analysis of CD20 on the surface of CLL cells before and after rituximab was added alone or along with plasma. The lower row shows detection of the rituximab on the surface of CLL cells using the PE-labeled anti-Fc fragment of the mouse Ig. (B) Mixing study results using Raji cells and plasma from a patient with CLL and plasma from a healthy control individual. There is greater inhibition of rituximab binding to the Raji cells using plasma from the CLL patient with high cCD20 compared with plasma from the healthy control individual.
Plasma cCD20 sequesters rituximab.
(A) Mixing study results demonstrating the capability of plasma cCD20 to compete for rituximab and prevent it from binding to CLL cells. The top row shows an analysis of CD20 on the surface of CLL cells before and after rituximab was added alone or along with plasma. The lower row shows detection of the rituximab on the surface of CLL cells using the PE-labeled anti-Fc fragment of the mouse Ig. (B) Mixing study results using Raji cells and plasma from a patient with CLL and plasma from a healthy control individual. There is greater inhibition of rituximab binding to the Raji cells using plasma from the CLL patient with high cCD20 compared with plasma from the healthy control individual.
Discussion
CD20 is an important molecule in the maturation and proliferation of CD20+ B cells.7 The reported differences in intensity of surface CD20 expression between various B-cell malignancies suggest that CD20 may play a role in the biology of these malignancies and in their clinical behavior.13-15The sequence of the CD20 protein indicates that this molecule is tightly bound to the cell surface membrane.7 However, some very tightly attached cellular molecules such as DNA can be detected in circulation.32 The data presented here suggest that CD20 protein is detectable at significant levels in the circulation of patients with CLL. CD20 is a hydrophobic protein and likely circulates with other proteins as cell membrane fragments or large membrane complexes. We were able to immunoprecipitate CD20 in circulation only after denaturing, which supports the hypothesis that it circulates in large complexes or as fragments of cell membrane. Results from the Western blot suggest that the full-length CD20 protein, rather than a cleaved immunoreactive fragment of the protein, is in circulation. We chose to use the term circulating CD20 (cCD20) rather thansoluble CD20 to reflect our hypothesis that the circulating CD20 is a part of a membrane complex or fragment. The ex vivo data, which show that CD20 does not shed from cells activated by PMA, support the hypothesis that the cCD20 originates from cell breakdown. The variation in plasma CD20 level among patients with CLL likely reflects variations in tumor mass, rate of cell proliferation, and rate of cell turnover as well as the activity and capability of the reticuloendothelial system to remove products of the breakdown of leukemic cells. Furthermore, we have observed variation in levels among healthy individuals, and our preliminary data suggest that individuals with minor viral infection may have higher levels than other healthy individuals (data not shown).
Plasma, rather than serum, was used for measuring cCD20 to circumvent the possibility that the clotting process may damage circulating cells and influence the levels of cCD20. The finding that levels of cCD20 were similar in freshly separated plasma and in plasma separated after samples were kept 24 to 48 hours at room temperature supports the concept that the detected cCD20 is not the result of ex vivo cell lysis. Furthermore, the lack of correlation between cCD20 levels and white blood cell count supports the hypothesis that cCD20 levels are influenced by the rate of turnover of malignant cells and not related solely to tumor mass.
Levels of cCD20 could have an impact on patient care and prognosis. Circulating CD20 levels correlated positively with Rai and Binet stage and β2M levels but negatively with platelet count and hemoglobin level and can be used to further stratify patients with CLL. Circulating CD20 levels did not correlate with white blood cell count, age, splenomegaly, or lymph node enlargement. These interactions suggest that cCD20 levels reflect a specific clinical stage of the disease as well as specific biology. More important, the cCD20 level may play an important role in patients' response to rituximab. As demonstrated in the mixing experiments, cCD20 diminishes the ability of rituximab to bind to CLL cells. Accordingly, an increased dose-intensity schedule of the antibody may be needed in patients with high levels of cCD20.
Measurement of cCD20 and rituximab levels may help in designing more effective dosing and scheduling for rituximab therapy. The presence of circulating antigen that binds to rituximab raises the possibility of formation of immune complexes. These immune complexes may have other implications for therapy and result in side effects. The concept of circulating antigens can be expanded to other antibody-based therapies that are currently used in treating hematologic malignancies, including alemtuzumab (anti-CD52)33-35 and gemtuzumabozogamicin (Mylotarg; anti-CD33).36 The presence of soluble HER-2 antigens has been reported in patients with breast cancer,37 but their clinical relevance in patients receiving anti-HER-2 therapy has not yet been determined.38
In summary, cCD20 can be detected at high levels in some patients with CLL, and its level is prognostically important. Circulating CD20 may have a significant impact on the effectiveness of therapy by anti-CD20 antibodies such as rituximab. Further studies are needed to elucidate the effect of cCD20 on the pharmacokinetics and pharmacodynamics of therapies based on the use of anti-CD20 antibodies.
Prepublished online as Blood First Edition Paper, November 21, 2002; DOI 10.1182/blood-2002-06-1639.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Maher Albitar, Departments of Hematopathology and Leukemia, The University of Texas M. D. Anderson Cancer Center, 1515 Holcombe Blvd, Box 72, Houston, TX 77030-4095; e-mail: malbitar@mdanderson.org.

![Fig. 4. No evidence of ex vivo CD20 shedding. / (A) Supernatant from cultured cells of patients with CLL showed no significant increase with time regardless of whether the cells were cultured without or with a shedding agent (phorbol 12-myristate 13-acetate [PMA]). In contrast, in HLA class I, levels increased significantly in the supernatant from cells cultured with PMA. (B) Similar levels of cCD20 were detected whether plasma samples were separated from cells immediately after collection (0) or after a prolonged period at room temperature (1, 2, 4, 8, 16, 24, 36, and 48 hours).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/7/10.1182_blood-2002-06-1639/3/m_h80734070004.jpeg?Expires=1769274663&Signature=Zj3gUaN8en2Iqhb8T5WVg4wyJK3sYDBmqvHC70FSVGuDxF9WmhlaPJAAYEVA6MoDwWOvwGay08IeRIEgoyq~soXPopNtYplv2~42Fy6cbVLkdaPcwTYBiTRtnMBRHcR0HQ1Qt4e7ul5UUOgU1-Ytp8GJayJbM~NA3OSxvWhTU51Qmi7Ism35-ylNnxMIc5N794SDOO5EcLJTTYew6aSYumPzK6bj9lIilfhGzSfyHsTVcSZWuWxQJUQb5NYiEC6JklM5jNXyacM4FmcL~5LnozDwL3UYST76KX0Or1L1PtnA0L4J-YrDr9AQg9I2zo-QKGbeEJS8zNIWslETwygAcw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal