The CD45 antigen is present on all cells of the hematopoietic lineage. Using a murine model, we have determined whether a lytic CD45 monoclonal antibody can produce persistent aplasia and whether it could facilitate syngeneic or allogeneic stem cell engraftment. After its systemic administration, we found saturating quantities of the antibody on all cells expressing the CD45 antigen, both in marrow and in lymphoid organs. All leukocyte subsets in peripheral blood were markedly diminished during or soon after anti-CD45 treatment, but only the effect on the lymphoid compartment was sustained. In contrast to the prolonged depletion of T and B lymphocytes from the thymus and spleen, peripheral blood neutrophils began to recover within 24 hours after the first anti-CD45 injection and marrow progenitor cells were spared from destruction, despite being coated with saturating quantities of anti-CD45. Given the transient effects of the monoclonal antibody on myelopoiesis and the more persistent effects on lymphopoiesis, we asked whether this agent could contribute to donor hematopoietic engraftment following nonmyeloablative transplantation. Treatment with anti-CD45 alone did not enhance syngeneic engraftment, consistent with its inability to destroy progenitor cells and permit competitive repopulation with syngeneic donor stem cells. By contrast, the combination of anti-CD45 and an otherwise inactive dose of total-body irradiation allowed engraftment of H2 fully allogeneic donor stem cells. We attribute this result to the recipient immunosuppression produced by depletion of CD45+ lymphocytes. Monoclonal antibodies of this type may therefore have an adjunctive role in nonmyeloablative conditioning regimens for allogeneic stem cell transplantation.
Introduction
Conventional allogeneic stem cell transplantation requires high doses of drugs, radiation, or both to produce sufficient host stem cell and immune system ablation to permit donor stem cell engraftment. These preparative regimens are associated with severe toxicity, limiting the applicability of the procedure.1Over the past few years, an alternative approach to stem cell transplantation has been developed in which lower doses of chemoradiotherapy allow progressive replacement of host lymphopoiesis and hematopoiesis with the donor's immune and hematopoietic systems.2 When used for malignant disease, this strategy exploits the alloreactivity of the donor immune system as a primary mechanism for eradicating the host's hematopoietic system and malignant clone. Although these “nonmyeloablative” transplantation regimens clearly lessen the immediate treatment-related mortality, the risk of donor marrow rejection is higher—particularly when donor and recipient are genetically mismatched at major histocompatibility complex (MHC) antigens—and delayed onset of graft-versus-host disease may cause significant morbidity and mortality.3
How could one favor donor engraftment after nonmyeloablative conditioning without resorting to toxic doses of chemoradiotherapy? One means would be to add monoclonal antibodies (MAbs) that ablate the recipient's immune system, in particular the T cells and natural killer (NK) cells responsible for graft resistance.4 If these MAbs also had activity against more primitive marrow-derived cells, they might also assist efforts to achieve myeloablation, additionally favoring donor stem cell engraftment. A particularly attractive target antigen is CD45, the common leukocyte antigen expressed by almost all nucleated white cells, including T cells, NK cells, and granulocytes, in which the level of expression generally increases as the cells mature.5 CD45 MAbs conjugated to a radionuclide have already been used in both animal models of leukemia and in human disease.6,7 Although this approach can promote disease-free survival following stem cell transplantation, the delivery of a substantial dose of radiation to some tissues can cause hypothyroidism and interstitial pneumonia in a substantial proportion of patients. A CD45 MAb has also been described that entirely ablates hematopoietic stem cells in rats and, in the absence of stem cell rescue, produces lethal aplasia.8 In the context of nonmyeloablative transplantation, particularly for nonmalignant disease, it would be preferable to use a CD45 MAb that recruits host lytic mechanisms, such as complement fixation and antibody-dependent cellular cytotoxicity, while sparing early hematopoietic stem cells.
We have recently described the use of a pair of lytic CD4 MAbs in patients with advanced leukemia.9 This treatment depletes T and B cells and mature myeloid cells, but does not destroy early progenitor cells, presumably because of their lower density of CD45 antigen.9 In the study reported here, we used 2 murine stem cell transplantation models to discover whether a lytic but nonmyeloablative CD45 MAb could indeed favor engraftment of donor marrow, and to discern whether these nonmyeloablative properties were mediated predominantly by depletion of both host hematopoiesis and lymphopoiesis or predominantly by destruction of alloreactive host lymphocytes. Our results indicate that lytic CD45 MAbs are highly immunosuppressive and produce sustained depletion of lymphoid cells. However, depletion of hematopoietic progenitor cells was transient and limited to mature subsets, so that MAb treatment significantly facilitated engraftment of fully allogeneic but not syngeneic marrow.
Materials and methods
Animals and transplantation studies
Six- to 8-week-old female mice, maintained under defined conditions, were treated on protocols approved by the Animal Protocol Review Committee of Baylor College of Medicine. In the congenic studies, C57Bl6J mice carrying the CD45.1 allele served as recipients for the bone marrow grafts from age-matched C57Bl6J mice with the CD45.2 allele. In the allogeneic setting, FVB mice with the H2-Kq haplotype4 received bone marrow grafts from male C57Bl6J mice with the CD45.2 allele and the H2-Kb haplotype. Rat antimouse CD45 MAb (30F11; BD Pharmingen, San Jose, CA), polyclonal rabbit serum against the NK cell/monocyte surface antigen GM1 (Wako, Richmond, VA), and antibodies against the T-cell epitopes CD4 and CD8 (clones GK1.5 and 5H10-1, respectively; BD Pharmingen) were given intraperitoneally after being dissolved in 400 μL phosphate-buffered saline. On the basis of preliminary experiments to determine saturating and maximally depleting doses, the anti-CD45 antibody 30F11 was given in 4 consecutive daily injections (1 μg/g body weight each), whereas the anti-GM1 antiserum (50 μL) and anti-CD4 and anti-CD8 antibodies (1 μg/g body weight) were given as single injections. The experimental groups ranged in size from 3 to 17 mice, as indicated in Figure5.
Stem cell transplants
Total-body irradiation (TBI) at a dose of 5.5 or 8 Gy was given from a 137Cs source. The grafts (2 doses of 2 × 107 freshly prepared total nucleated bone marrow cells) were suspended in 250 μL Dulbecco modified Eagle medium (DMEM) with 2% fetal calf serum (FCS) per dose and given on days 3 and 4 after CD45 treatment as retro-orbital intravenous injections.
Tissue processing
Blood sample volumes were estimated by weighing red blood cells depleted by dextran separation for 15 minutes, and the remaining red blood cells were lysed in NH4Cl solution. Nucleated cells were then counted in an improved Thomas chamber. Bone marrow mononuclear cells (MNCs) were obtained by flushing the long hind-limb bones with DMEM containing 2% FCS. Spleen cells were harvested by mechanical mincing of the spleens, followed by passage through a 70-μm mesh. For colony-forming unit (CFU) assays, 5000 whole bone marrow cells were seeded into 3 mL methylcellulose medium supplemented with murine hematopoietic growth factors (interleukin [IL]–3, IL-6, stem cell factor, granulocyte-macrophage colony-stimulating factor, and erythropoietin, M3435; StemCell Technologies, Vancouver, Canada) and plated in two 3-cm dishes. Colonies were counted on day 10 of culture.
Flow cytometry and immunohistology
We used antibodies against Thy-1 (30-H12), CD3 (17A2), and CD5 (53-7.3) to detect T lymphocytes; B220 (RA3-6B2) to detect B lymphocytes; NK1.1 to detect CD56+ NK cells; GR-1 (RB6-8C5) to detect granulocytes; and CD34, c-kit, and Sca-1 (49E8, ACK45, and E13-151.7, respectively) to detect marrow progenitor cells. The CD45.1/CD45.2 polymorphism was identified with clones A20 and 104 (Becton Dickinson, San Jose, CA). The binding of the anti-CD45 rat MAb (30F11) in vivo was detected in peripheral blood and bone marrow cell suspensions incubated with fluorescein isothiocyanin (FITC)–labeled goat antirat serum (mouse adsorbed; Caltag, Burlingame, CA) using flow cytometry and tissue sections.
A FACScan flow cytometer (Becton Dickinson) was used throughout the study, with excitation of FITC and phycoerythrin (PE) at 488 nm, and the data were analyzed with the CellQuest (Becton Dickinson) or FlowJo (Treestar, Boulder, CO) program. To determine the immunohistology of snap-frozen and Histochoice-fixed (Amresco, Solon, OH) paraffin-processed tissue sections, we used biotinylated antibodies against CD5 and B220 clones and streptavidin–horseradish peroxidase or streptavidin–alkaline phosphatase according to the manufacturer's instructions (Vector, Burlingame, CA).
Analysis of chimerism
Hematopoietic chimerism was analyzed 3 months after transplantation by flow cytometry of peripheral blood leukocytes, prepared as described in the preceding paragraph. For syngeneic transplantation of bone marrow from CD45.1+ mice into CD45.2 recipients, we calculated the percentages of CD45.1+cells among the peripheral blood MNCs (percent chimerism; Figure 5). In the allogeneic setting, flow cytometry was performed with an antibody against the H2-Kb antigen of the donor strain (Becton Dickinson), with the percentages of positive cells reported as percentage chimerism. Differences in engraftment levels induced with the various preparative regimens were statistically analyzed by 2-tailed t tests for unpaired samples. Group sizes were determined from power estimates to demonstrate significant intergroup differences and are provided on each figure or in the text.
Results
Anti-CD45–mediated cytoreduction in peripheral blood and bone marrow
In the presence of complement, the rat immunoglobulin G (IgG)2b MAb 30F11 readily lyses CD45+ murine leukocytes.10 To assess in vivo effects of the MAb, we administered it intraperitoneally for 4 consecutive days in doses of 1 μg/g body weight. The antibody injections were well tolerated and produced no obvious morbidity or early or late mortality (up to 4 months).
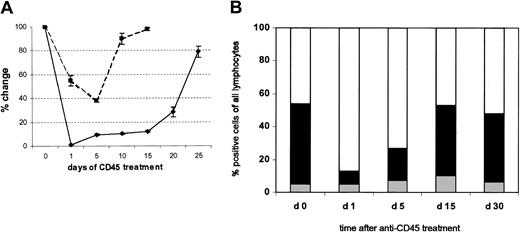
This regimen was sufficient to produce more than 95% depletion of peripheral blood leukocytes. Hence, within 24 hours after the first injection, the mean (± SD) total white blood cell count of the mice dropped to less than 5% of the baseline value, recovering to 10% by 5 days after the last injection and reaching normal levels by approximately 25 days (Figure 1A). Residual white blood cells examined on day 5 had normal morphology, and there were no discernible effects on platelets or red cells, which do not express the CD45 antigen (data not shown). The most profound effects were seen in the MNC population. The peripheral blood MNC count rapidly fell to less than 2% of its pretreatment level and recovered slowly, remaining below normal as late as day 26 after treatment (data not shown). All lymphoid lineages within the MNC compartment (T, B, and CD56+, NK) were markedly depleted (Figure 1B), with the greatest proportional effect seen on T lymphocytes. The mean (± SD) percentage of CD3+ cells in the MNC population fell from 49.1% ± 7.6% to 5.8% ± 3% (n = 5) immediately after the first injection of CD45 MAb. There was a corresponding small rise in the percentage of residual B cells (from 46.6% ± 3.4% to 65% ± 15.2%) over the first 5 days, whereas the relative proportion of NK1.1+ cells did not change (Figure 1B). The effects on peripheral blood neutrophils were much less striking. Mean (± SD) decreases from the pretreatment level did not exceed 60%, and there was rapid recovery of counts, beginning at less than 24 hours after treatment and reaching essentially normal values by day 10 (Figure 1A).
Anti-CD45–mediated reduction of total white blood cells (WBCs) and neutrophils in the peripheral blood.
(A) The numbers of WBCs in the peripheral blood of mice treated with MAb (1 μg/g body weight) daily from day 0 to day 4 were counted manually and are reported as mean (± SD) percentages of change from baseline values (♦). Results for neutrophils (▪) were determined in a similar manner. Six mice were studied in each group. (B) The proportions of B cells, T cells, and NK cells were determined before and at multiple time points after Ab infusion. ░ indicates B220; ▪, CD3; and ■, NK1.1.
Anti-CD45–mediated reduction of total white blood cells (WBCs) and neutrophils in the peripheral blood.
(A) The numbers of WBCs in the peripheral blood of mice treated with MAb (1 μg/g body weight) daily from day 0 to day 4 were counted manually and are reported as mean (± SD) percentages of change from baseline values (♦). Results for neutrophils (▪) were determined in a similar manner. Six mice were studied in each group. (B) The proportions of B cells, T cells, and NK cells were determined before and at multiple time points after Ab infusion. ░ indicates B220; ▪, CD3; and ■, NK1.1.
Consistent with these effects on the peripheral blood components, analysis of the hematopoietic stem cell compartment revealed a modest mean (± SD) reduction in whole bone marrow cellularity of 2.75 ± 0.25 × 107 total cells, compared with 3.67 × 107 cells in normal control mice. There was little change in the hematopoietic progenitor cell population: mean (± SD) 13.8% ± 2.4% CD34+ bone marrow cells versus 10.4% ± 2.7% in normal mice (n = 4 for all groups). An in vitro CFU assay of whole bone marrow cells harvested on day 5 of anti-CD45 treatment showed no reduction in clonogenic activity: mean (± SD) of 30 ± 6.9 colonies compared with 27.8 ± 2.4 colonies from untreated control mice per 5000 cells seeded (n = 4). Thus, treatment of mice with the CD45 antibody 30F11 led to marked cytoreduction in the peripheral blood, predominantly of lymphocytes. The number and functionality of bone marrow precursor cells were not reduced, allowing complete short- and long-term hematopoietic recovery without stem cell rescue (Figure 1A-B).
Targeting of lymphohematopoietic tissues with anti-CD45
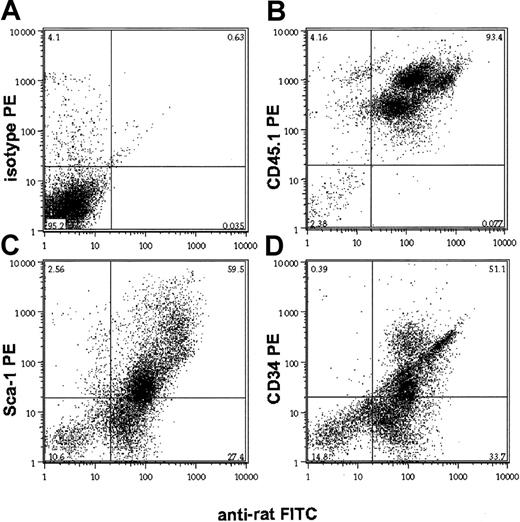
The differential depletion of mature and immature hematopoietic cell populations in peripheral blood and marrow, respectively, may reflect either the biodistribution of the CD45 MAb or the relative resistance of different subsets of CD45+ cells to receptor-saturating doses of the antibody. To distinguish between these possibilities, we analyzed the in vivo binding of the rat 30F11 CD45 MAb 24 hours after a single dose of the antibody, using a mouse-adsorbed antirat IgG antibody. In both peripheral blood and bone marrow, 30F11 bound to cells in a unimodally distributed pattern of immunofluorescence, regardless of site, indicating a uniform distribution of the antibody among all hematopoietic cells (Figure2A-B). Similarly, immunohistologic analysis of the spleen from mice 24 hours after injection revealed homogeneous and intense binding of peroxidase-labeled 30F11 to the membranes of CD45+ cells (Figure 2C). Costaining the in vivo anti-CD45–labeled bone marrow cells with antibodies against the hematopoietic progenitor cell markers Sca-1 and CD34 produced extensive in vivo labeling of these precursor cell populations (Figure3C-D), whereas costaining the bone marrow MNCs with a noncompeting, allele-specific antibody against CD45.1 revealed correlation between CD45 expression levels and the amount of anti-CD45 binding (Figure 3B). The doses of MAb given to mice were distributed to lymphoid organs and were sufficient to produce T- and B-lymphocyte depletion because the thymus and spleen were largely devoid of CD5+ T lymphocytes and B220+ B cells, respectively, with severely distorted organ architecture persisting beyond 30 days (Figure 4). Thus, the CD45-specific MAb 30F11 penetrated to marrow and lymphatic organs and achieved binding levels that were proportional to the amount of CD45 antigen expressed.
Targeting of lymphohematopoietic cells by anti-CD45 antibody in vivo.
Twenty-four hours after a single injection of MAb, the peripheral blood (A) and bone marrow (B) were incubated with an FITC-labeled antirat antibody and analyzed by flow cytometry (shaded curve). Controls consisted of peripheral blood or bone marrow from untreated mice that was stained with rat secondary FITC antibody (solid line, unshaded) and of unstained cells (dotted line). For the detection of anti-CD45 antibody to the tissues in vivo, cryostat sections of a spleen 24 hours after antibody treatment (C) and of an untreated control (D) were stained with the rat FITC antibody, and the membrane-bound fluorescence was visualized by fluorescence microscopy (original magnification × 40; enlargements [original magnification × 160] inserted).
Targeting of lymphohematopoietic cells by anti-CD45 antibody in vivo.
Twenty-four hours after a single injection of MAb, the peripheral blood (A) and bone marrow (B) were incubated with an FITC-labeled antirat antibody and analyzed by flow cytometry (shaded curve). Controls consisted of peripheral blood or bone marrow from untreated mice that was stained with rat secondary FITC antibody (solid line, unshaded) and of unstained cells (dotted line). For the detection of anti-CD45 antibody to the tissues in vivo, cryostat sections of a spleen 24 hours after antibody treatment (C) and of an untreated control (D) were stained with the rat FITC antibody, and the membrane-bound fluorescence was visualized by fluorescence microscopy (original magnification × 40; enlargements [original magnification × 160] inserted).
In vivo binding of anti-CD45 antibody 30F11 to hematopoietic progenitor cells.
Bone marrow of mice that had received a single dose (1 μg/g body weight) of anti-CD45 MAb 24 hours earlier was analyzed on a flow cytometer after double staining with a rat antibody and antibodies against the stem cell antigens Sca-1 (C) and CD34 (D). Negative and positive controls are isotype PE (A) and CD45.1 PE (B), respectively. Numbers in each quadrant represent percentages of total cells in each.
In vivo binding of anti-CD45 antibody 30F11 to hematopoietic progenitor cells.
Bone marrow of mice that had received a single dose (1 μg/g body weight) of anti-CD45 MAb 24 hours earlier was analyzed on a flow cytometer after double staining with a rat antibody and antibodies against the stem cell antigens Sca-1 (C) and CD34 (D). Negative and positive controls are isotype PE (A) and CD45.1 PE (B), respectively. Numbers in each quadrant represent percentages of total cells in each.
Distortion of thymic, splenic, and lymph node architecture by anti-CD45 treatment.
Samples of thymus, spleen, and lymph node collected on days 1 and 30 from untreated mice and from mice treated with anti-CD45 (1 μg/g body weight) were stained with antibodies against CD5 T cells (in the thymus and lymph nodes) and against B220 B cells (in the spleen). The marker-positive cells were brown diaminobenzidine [DAB] against the blue nuclear stain hematoxylin. Severe distortion of normal thymic, splenic, and lymph node histology, first seen on day 1, persisted through day 30 (magnification × 10).
Distortion of thymic, splenic, and lymph node architecture by anti-CD45 treatment.
Samples of thymus, spleen, and lymph node collected on days 1 and 30 from untreated mice and from mice treated with anti-CD45 (1 μg/g body weight) were stained with antibodies against CD5 T cells (in the thymus and lymph nodes) and against B220 B cells (in the spleen). The marker-positive cells were brown diaminobenzidine [DAB] against the blue nuclear stain hematoxylin. Severe distortion of normal thymic, splenic, and lymph node histology, first seen on day 1, persisted through day 30 (magnification × 10).
Contribution of anti-CD45–mediated cytoreduction to conditioning regimens after syngeneic and allogeneic transplantation
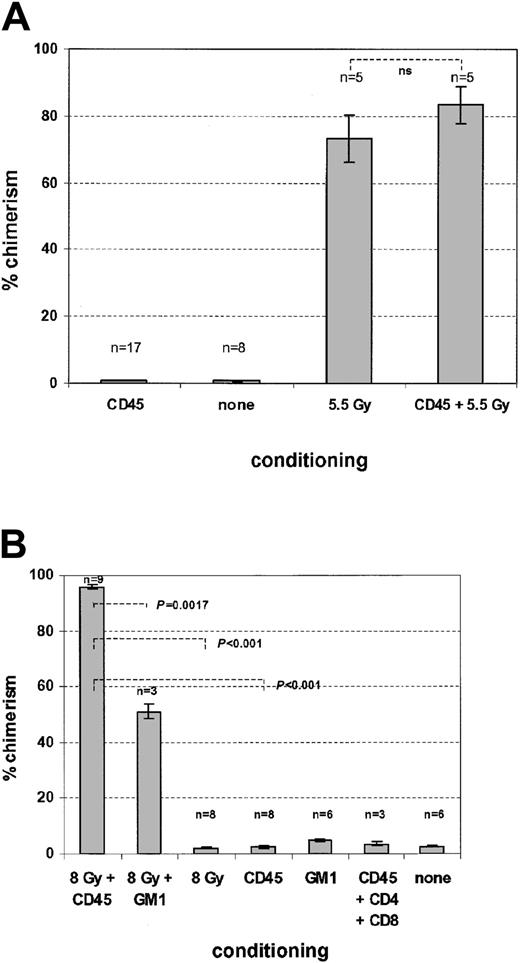
Using an allelic difference of the CD45 antigen to differentiate donor from recipient hematopoiesis in an otherwise syngeneic C57Bl6J setting, we determined whether the CD45 MAb by itself could produce sufficient depletion of recipient hematopoiesis to permit measurable competitive engraftment of donor C57Bl6J stem cells. After single injections of MAb (1 μg/g) over 4 consecutive days, we were unable to detect significant donor engraftment in either peripheral blood or marrow, even when mice received up to 4 × 107 whole bone marrow cells. By contrast, nonmyeloablative doses of TBI with 5.5 Gy allowed sufficient donor engraftment to produce mixed chimerism (Figure5A). Addition of the anti-CD45 treatment to the TBI regimen did not convert this mixed chimerism to full chimerism and produced little increase in the percentage of donor cells obtained with radiation alone. These results are consistent with a predominant effect of anti-CD45 on host lymphocytes and a lesser effect on myeloid cells, which in this essentially syngeneic model would not be expected to favor engraftment of donor stem cells.
Effects of anti-CD45 treatment on syngeneic and allogeneic hematopoietic stem cell engraftment.
Mice were prepared for transplantation with the indicated conditioning regimens and received intravenous infusion with 2 doses of 2 × 107 total bone marrow cells on days 3 and 4 after anti-CD45 treatment. The number of mice per group is shown above each column. The results are reported as mean (± SD) percentage chimerism as determined by flow cytometry on day 30 after transplantation. In the syngeneic model (A), anti-CD45 treatment alone did not allow engraftment and lacked a significant additional effect in combination with 5.5 Gy TBI alone (P = .295 by unpaired 2-tailedt test). In the allogeneic model (B), anti-CD45 treatment permitted high-level chimerism when used in conjunction with 8 Gy TBI. The chimerism induced by conditioning with the CD45 plus 8-Gy TBI combination was superior to the results obtained with either anti-CD45 or TBI alone (P < .002 by unpaired 2-tailed ttest). Neither anti-GM1 alone nor coinjection of anti-CD45 and antibodies against CD4 or CD8 induced measurable chimerism. ns indicates not significant.
Effects of anti-CD45 treatment on syngeneic and allogeneic hematopoietic stem cell engraftment.
Mice were prepared for transplantation with the indicated conditioning regimens and received intravenous infusion with 2 doses of 2 × 107 total bone marrow cells on days 3 and 4 after anti-CD45 treatment. The number of mice per group is shown above each column. The results are reported as mean (± SD) percentage chimerism as determined by flow cytometry on day 30 after transplantation. In the syngeneic model (A), anti-CD45 treatment alone did not allow engraftment and lacked a significant additional effect in combination with 5.5 Gy TBI alone (P = .295 by unpaired 2-tailedt test). In the allogeneic model (B), anti-CD45 treatment permitted high-level chimerism when used in conjunction with 8 Gy TBI. The chimerism induced by conditioning with the CD45 plus 8-Gy TBI combination was superior to the results obtained with either anti-CD45 or TBI alone (P < .002 by unpaired 2-tailed ttest). Neither anti-GM1 alone nor coinjection of anti-CD45 and antibodies against CD4 or CD8 induced measurable chimerism. ns indicates not significant.
Nonetheless, the sustained depletion of host lymphopoiesis by anti-CD45 (Figure 1) suggested that the antibody would enhance engraftment of allogeneic donor cells after a nonmyeloablative preparative regimen that was insufficient by itself to induce a significant level of donor chimerism. In this setting, the transplanted donor immune system would ablate residual recipient hematopoiesis and permit donor stem cell engraftment.2 We transplanted C57Bl6J bone marrow (H2-Kb haplotype) into FVB mice (H2-Kq haplotype) and combined a dose of TBI (8 Gy), which in this model produces only partial and transient leukodepletion,4 with CD45 MAb injections or with rabbit antimouse GM1 serum4 to enhance engraftment across the full MHC mismatch. Although administration of depleting doses of CD45 MAb alone had no effect on engraftment, the combination of antibody and low-dose radiation significantly increased the degree of chimerism over that obtained with nonmyeloablative doses of radiation or antibody treatment alone (P < .001 for each comparison). Moreover, this regimen was superior to the combination of 8 Gy TBI and anti-GM1 polyclonal serum (P < .002; Figure 5B) or anti-CD45 and anti-GM1 (not shown). Thus, treatment with anti-CD45 appears to have depleted the host immune system sufficiently to allow the engraftment of donor immune and hematopoietic systems.
Discussion
Although nonmyeloablative conditioning regimens for allogeneic transplantation have greatly reduced procedure-related mortality, an increased risk of graft rejection remains a major concern.3,11 If nonmyeloablative regimens are to be successfully extended to recipients lacking human leukocyte antigen–identical donors, additional methods to secure nontoxic host immunosuppression are highly desirable. Antilymphocyte antibodies, including antithymocyte globulin and Campath-1H (CD52), have been used to intensify the immunosuppressive properties of conventional transplantation regimens,12,13 and more recently the value of Campath-1H has been explored for the same purpose with nonmyeloablative conditioning regimens.14 We now show in a mouse model that a lytic leukocyte-depleting but nonmyeloablative CD45 MAb may also be valuable for this purpose.
CD45 is a large membrane glycoprotein whose expression and glycosylation patterns are controlled in a leukocyte-specific manner.5,15 It exists in multiple isoforms that have a molecular-weight range of 180 to 220 kDa16,17 and that result from alternative splicing of ectodomain exons 4 to 6 (also termed exons A, B, and C). T-cell expression of CD45 isoforms varies with the stage of differentiation and activation status, so that T-cell subsets with different functional properties may be defined by the CD45 isoform present on the cell surface.18 In this study, we used a MAb capable of recognizing an epitope common to all CD45 isoforms. The bulk of the CD45 molecule is located in the cytoplasm, where it acts as a tyrosine phosphatase.17 CD45 has many characteristics that favor its targeting with cytoreductive agents. It is a nonmodulatable surface antigen whose expression is apparently limited to the hematopoietic system, where it is present at high density on all white cells, erythrocyte precursors,15,19and almost all leukemic cells,20 so that lytic CD45 MAbs could deplete both normal and malignant host hematopoietic cells.
Levels of CD45 expression on lymphocytes always exceed those on myeloid cells,15 and CD45 represents up to 10% of lymphocyte cell surface protein10 (approximately 200 000 molecules per cell6). Although CD45 is present on mature leukocytes, most, if not all, human CD34+ cells express only low levels of this antigen.15,21 As CD34 is lost, CD45 expression increases significantly on lymphocytes, and to a lesser extent on myeloid cells, where it remains at constant levels.19 It is likely that this differential level of expression, rather than selective biodistribution, explains the contrasting anti-CD45 sensitivity of marrow progenitor and mature lymphoid/myeloid cells that we and others have observed.8 22
The antileukemic properties of 131I-labeled murine IgG1 CD45 MAb have been exploited in patients with advanced leukemia before stem cell transplantation. The radiolabeled antibody was combined with high-dose conventional preparative regimens, so that a localized dose of radiation would be delivered to malignant cells, contributing to the antileukemic effect of the preparative regimen without undue toxicity. Outcomes were encouraging, but one-third of the patients required thyroxin replacement despite oral iodine prophylaxis23; long-term lung fibrosis was also observed.
In the current study, we show that a nonradioactive CD45 MAb that depletes host leukocytes by recruiting host effector mechanisms may be of benefit as part of a preparative regimen for allogeneic stem cell transplantation. In general terms, the efficacy of unconjugated MAbs depends upon the range of effector mechanisms that may be recruited; the characteristics of the target antigen; and the species, isotype, and rate of catabolism of the MAb. Natural effector mechanisms include complement-mediated cytotoxicity, antibody-dependent cell-mediated cytotoxicity (ADCC), opsonization, and phagocytosis. The MAb used in this study is capable of producing both complement-mediated lysis and ADCC/opsonization, but we do not know which mechanism is dominant in vivo. Because CD45 MAb also depletes the cellular populations responsible for ADCC/opsonization, the mechanisms responsible for clearance may change during the course of treatment. It is also evident that CD45 antibodies interfere with the tyrosine phosphatase function of the CD45 molecule, producing profound changes in leukocyte function even in cells that survive the lytic process. For example, ligation and/or cross-linking of the CD45 molecule has been reported to inhibit B-cell proliferation after anti-IgM or anti-CD40 stimulation,24 reduce the human neutrophil chemotactic response to leukotriene B4 and C5a,25 inhibit the IgE-mediated release of histamine from human basophils,26 interfere with leukocyte adhesion and migration, and diminish T-cell alloreactivity, preventing acute rejection of renal allografts in mice.27 Similar effects on leukocyte function have been found after CD45 gene-disruption studies.28 29 As might be anticipated from the expression pattern of the CD45 molecule, the antibody we tested depleted both lymphoid and myeloid subsets in blood, with a lesser effect on hematopoietic precursor cells in marrow. Because the antibody does not fully deplete marrow precursor cells, production of fresh leukocytes continues, and these cells likely act to remove residual CD45 MAb once its administration is terminated. Although we did not perform formal half-life studies, the rapid normalization of the neutrophil counts at the end of treatment suggests that the half-life of nonmyeloablative CD45 MAb is therefore short. Nonetheless, the CD45 MAb also depleted lymphoid subsets in spleen and lymph nodes, so that lymphoid depletion persisted for several weeks. These distinct effects are reflected in the differential activity of the MAb in syngeneic and allogeneic transplantation. First of all, CD45 MAb treatment of mice did not facilitate engraftment of infused syngeneic marrow, even when used with a nonmyeloablative dose of radiation. Such facilitation would be expected only if there was substantial depletion of the host progenitor/stem cell compartment, because this effect would reduce the ratio of host to infused donor progenitor cells, increasing the competitiveness of the donor population. Effects on the host immune system would be less relevant because no alloreactivity exists. Second, although CD45 MAb by itself did not promote engraftment of fully allogeneic marrow, a combination of antibody and otherwise ineffectual doses of radiation (8 Gy) greatly enhanced engraftment in this setting. Like other nonmyeloablative regimens, this combination treatment probably functions by immunosuppressing the recipient sufficiently to allow the donor immune system to engraft and “reject” host stem cells, favoring engraftment of marrow stem cells from the donor. Thus, any process that enhances recipient immunosuppression would be predicted to increase allogeneic donor engraftment, even when the direct effects on recipient hematopoiesis are limited.
We attribute this beneficial effect to anti-CD45 binding of all lymphohematopoietic cells, including all T and B cells, NK cells, and the cells of the innate immunosurveillance system, such as monocytes and antigen-presenting cells. Thus, the perturbation of the lymphatic system with this agent may be more extensive than that achieved with other single MAbs. In the fully allogeneic system we used, NK cells were shown to be of likely importance in mediating the rejection mechanism,4 and these cells appear to be critical in mediating rejection when human stem cell transplantation is attempted between MHC haploidentical donor and recipient pairs.30Judging from the profound effects on circulating and lymphoid organ–based T lymphocytes, lytic CD45 MAb may be equally effective at facilitating transplantation in settings where T-cell–mediated graft rejection is a primary concern. The availability of human CD45 lytic MAbs that have been well tolerated at cytoreductive doses should allow this possibility to be tested directly in humans.
We thank Dorothy Burton for excellent animal care and maintenance.
Prepublished online as Blood First Edition Paper, November 14, 2002; DOI 10.1182/blood-2002-08-2379.
Supported in part by grants RO1 CA81179-01A1 (M.A.G.) and R21 CA82101 (M.K.B.) from the National Institutes of Health.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Malcolm K. Brenner, Center for Cell and Gene Therapy, Baylor College of Medicine, 6621 Fannin St, MC3-3320, Houston TX 77030; e-mail: mbrenner@bcm.tmc.edu.

![Fig. 2. Targeting of lymphohematopoietic cells by anti-CD45 antibody in vivo. / Twenty-four hours after a single injection of MAb, the peripheral blood (A) and bone marrow (B) were incubated with an FITC-labeled antirat antibody and analyzed by flow cytometry (shaded curve). Controls consisted of peripheral blood or bone marrow from untreated mice that was stained with rat secondary FITC antibody (solid line, unshaded) and of unstained cells (dotted line). For the detection of anti-CD45 antibody to the tissues in vivo, cryostat sections of a spleen 24 hours after antibody treatment (C) and of an untreated control (D) were stained with the rat FITC antibody, and the membrane-bound fluorescence was visualized by fluorescence microscopy (original magnification × 40; enlargements [original magnification × 160] inserted).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/6/10.1182_blood-2002-08-2379/4/m_h80633997002.jpeg?Expires=1765882317&Signature=3wndbB~H9UISo~CqzqbgCAqmtGBE8pMgtz2OOS4NU~0CxI5ua6zIg-ySGdfohsTVE6MLfns-BJq38h4LZ-SH6BzkC6rnSvi-CvnEDCmDpADQxJjqYi~zK22OImgSRc8ulhKHWLyYx32gONH53GmubnSQC~A1VVVxHcmRFLI15KOx3xtnNcfEpSdgBr2PBLXt4HbVYD~5adU2obnTPDpHTHaFwre1Lrnn81VrD4l57OSQZYJnMe7OOTrxEe0AWUSVk6r9F-fhpBtl1DnOJ9obdG1oaCjwDN9RPA3ND9zcT07X1MqR3EScUoMi44Q4VaLVF3XC~G96B4oB7aEgiKyRWw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Distortion of thymic, splenic, and lymph node architecture by anti-CD45 treatment. / Samples of thymus, spleen, and lymph node collected on days 1 and 30 from untreated mice and from mice treated with anti-CD45 (1 μg/g body weight) were stained with antibodies against CD5 T cells (in the thymus and lymph nodes) and against B220 B cells (in the spleen). The marker-positive cells were brown diaminobenzidine [DAB] against the blue nuclear stain hematoxylin. Severe distortion of normal thymic, splenic, and lymph node histology, first seen on day 1, persisted through day 30 (magnification × 10).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/6/10.1182_blood-2002-08-2379/4/m_h80633997004.jpeg?Expires=1765882317&Signature=dOOIgqsKHtGHQdpvF-Kd8f~NDI6G3x3X3w4gPLoNFIfoYcym~tKzSJD99EhuxnMvL2ogHKH4gGgMUZ9YRWgEKVEjGWo4rkzX301x49bWifj4woyY-Z4xHgEBRCPNy6oDOBydR1o~R-UU8hPwOExlCxd7QA51tKK9bthpilXpCruc0-9Cy2WHruhtr1OOwFynqfuGu-jwmd~nVV~lI413a3SLWba3i52hAqNDpiXlUtXO8h9YCltujmWwgD6LDLLmHvFSR4L1BwzSrn3AugxxfEoG1DWiUWOYj2yZNE6ipZogJ4zO5845NmpLAuQZLVtF5XJHukz91Kcz~l~b68L5mw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal