Grade 3 follicular lymphoma (FL3) is thought to have an aggressive clinical course. On the basis of possible biologic differences, the new World Health Organization (WHO) classification of lymphoma suggests further subdivision of FL3 into grades 3a and 3b and states that the percentage of involvement by diffuse large B-cell lymphoma (DLBCL) should also be reported. However, the clinical implications of these features are unclear. Therefore, we studied 190 newly diagnosed patients with lymph node–based FL3 who received anthracycline-containing combination chemotherapy. The follicular component was subclassified as grade 3a (FL3a) or grade 3b (FL3b) according to the WHO criteria, or as follicular large cleaved cell type (FLC). The percentage of a diffuse component, if present, was also recorded. Of the 190 cases, there were 107 FL3a (56%), 53 FL3b (28%), and 30 FLC (16%) cases. Diffuse areas were seen in 72 cases (31 FL3a, 28 FL3b, and 13 FLC). There were no significant differences in the clinical characteristics, overall survival, or event-free survival between patients with grades FL3a, FL3b, or FLC. However, those cases with a predominant diffuse component (> 50% diffuse) had a significantly worse overall survival (P = .0037) and event-free survival (P = .012). Therefore, we conclude that the subdivision of FL3 into cytologic subtypes does not appear to be important clinically. However, patients with FL3 having a diffuse component of more than 50% have an inferior survival that is similar to the survival of those with DLBCL.
Introduction
Grade 3 follicular lymphoma (FL3) accounts for approximately 6% of all non-Hodgkin lymphomas.1 Compared with lower-grade follicular lymphoma, FL3 is thought to be a more aggressive disease and should be treated accordingly.2-11However, there is controversy regarding the curability of FL3 with aggressive therapy.12
Both the Revised European and American Lymphoma (REAL) classification and the new World Health Organization (WHO) classification recommend subdividing follicular lymphoma into 3 grades.13,14 The REAL classification does not provide criteria for grading, but the WHO recommends using the cell counting method of Mann and Berard.15 In addition, the WHO classification suggests further subdivision of FL3 into grades 3a and 3b, based on the presence or absence of small cleaved cells (centrocytes), respectively. In FL3a, the neoplastic follicles have more than 15 centroblasts per high-power field in a background of centrocytes. In FL3b, the neoplastic follicles are composed of sheets of centroblasts without admixed centrocytes. Thus, FL3 appears to be a heterogenous disorder, and cytologic subdivision may help to define potential biologic and clinical variants.16 However, the clinical importance of this subdivision has not been investigated in a large cohort of patients with FL3.
The WHO classification also recommends the reporting of pattern in low-grade follicular lymphoma, and 3 patterns are suggested based on the percentage of the follicular component.14 The WHO classification also states that any involvement by diffuse large B-cell lymphoma (DLBCL) should be identified, estimated by percentage, and reported as a separate diagnosis. However, investigations of the prognostic value of DLBCL areas in patients with FL3 have yielded conflicting results. Although some studies have found differences in overall survival7,17 or failure-free survival,6 most studies have found no difference in survival for patients with DLBCL areas.2-4,9,10 18
The goals of this study are 2-fold: first, to evaluate the clinical significance of the subdivision of FL3 into grades 3a and 3b, and, second, to evaluate the clinical significance of DLBCL areas in FL3.
Patients and methods
Between June 1985 and December 2000, 252 patients with FL3 were treated with anthracycline-containing combination chemotherapy by the Nebraska Lymphoma Study Group. During this time, follicular lymphoma represented 22% of non-Hodgkin lymphoma diagnoses in our database, with FL3 accounting for 8.3% of non-Hodgkin lymphoma and 37% of follicular lymphoma. The University of Nebraska Institutional Review Board approved this study and informed consent was obtained in accordance with the Declaration of Helsinki. Eight cases were excluded from this study because the original biopsy slides were unavailable for review, and 10 cases could not be subclassified because of inadequate material for review (ie, needle biopsy or small sample size). An additional 31 cases were excluded because the disease was primarily extranodal, ie, not lymph node–based disease. Nine cases were excluded because they were reclassified on review (low-grade follicular lymphoma, 4; diffuse large B-cell lymphoma, 3; small lymphocytic lymphoma, 1; mantle cell lymphoma, 1). One patient was excluded because of a previous history of lymphoma, and 3 patients were excluded because documentation of informed consent was lacking. Therefore, this series consists of 190 patients with newly diagnosed, node-based FL3, all of whom underwent an excisional lymph node biopsy. Clinical features, including the components of the International Prognostic Index (IPI) and the IPI score, were recorded.19 Therapy for 108 patients (57%) consisted of combination chemotherapy, including cyclophosphamide, doxorubicin or mitoxantrone, procarbazine, bleomycin, vincristine, and prednisone or dexamethasone (CAP-BOP),20whereas 75 patients (39%) received cyclophosphamide, mitoxantrone, vincristine, prednisone (CNOP),21 and 7 patients (4%) received cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP).
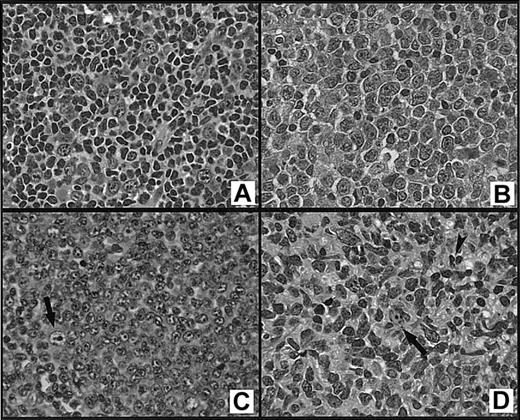
The diagnosis of FL3 was based on counting more than 15 transformed lymphoid cells (centroblasts) on average in 10 × 40 high-power fields (hpf = 0.159 mm2) of 10 neoplastic follicles.14,15 The cases were then subclassified as grade 3a (FL3a) if the follicular component contained more than 15 centroblasts/hpf in a background of small cleaved cells (centrocytes), grade 3b (FL3b) if there were solid sheets of centroblasts without small centrocytes, or grade 3b with small centroblasts (FL3bs) if there were solid sheets of small centroblasts without centrocytes (Figure 1). On review, some of our cases did not meet the WHO counting criteria for FL3. Our database includes a category of FL3 that consists predominantly of large cleaved cells, as defined in the Working Formulation.22 In these cases, the neoplastic follicles contained a predominance of large cleaved cells (large centrocytes or multilobated cells) with irregular nuclei that were 2 to 3 times the size of a small lymphocyte and fine chromatin with absent or inconspicuous small nucleoli. These cases were classified as follicular large cleaved type (FLC) if a predominance (> 50%) of the cells in the neoplastic follicles were large cleaved cells but there were less than 15 centroblasts/hpf (Figure 1D). A diffuse component was defined according to the WHO recommendation as an area lacking follicular architecture (not including interfollicular involvement).14 The percentage of a diffuse component, if present, was estimated by morphologic review of all of the slides in each case and recorded at the time of diagnosis.
Subtypes of grade 3 follicular lymphoma.
(A) Grade 3a contains more than 15 centroblasts/hpf in a background of centrocytes; (B) grade 3b consists of sheets of centroblasts with no admixed centrocytes; (C) grade 3bs consists predominantly of small centroblasts with no admixed centrocytes; a large centroblast (arrow) is shown for comparison; and (D) large cleaved cell lymphoma consisting of intermediate-sized to large cells with irregular, cleaved, or twisted nuclei, fine chromatin, and inconspicuous nucleoli; a large centroblast (arrow) and small centrocyte (arrowhead) are shown for comparison (H&E stains, original magnification, × 400).
Subtypes of grade 3 follicular lymphoma.
(A) Grade 3a contains more than 15 centroblasts/hpf in a background of centrocytes; (B) grade 3b consists of sheets of centroblasts with no admixed centrocytes; (C) grade 3bs consists predominantly of small centroblasts with no admixed centrocytes; a large centroblast (arrow) is shown for comparison; and (D) large cleaved cell lymphoma consisting of intermediate-sized to large cells with irregular, cleaved, or twisted nuclei, fine chromatin, and inconspicuous nucleoli; a large centroblast (arrow) and small centrocyte (arrowhead) are shown for comparison (H&E stains, original magnification, × 400).
For comparison purposes, a group of 530 patients with DLBCL was retrieved from the Nebraska Lymphoma Study Group Registry. These patients were treated with similar combination chemotherapy regimens (53% received CNOP, 46% CAP-BOP, and 1% CHOP) and had clinical follow-up data. In addition, a group of 186 patients with low-grade follicular lymphoma (grades 1 and 2) was retrieved from the Nebraska Lymphoma Study Group Registry. These patients were also treated with similar combination chemotherapy regimens (61% received CNOP and 39% CAP-BOP) and had clinical follow-up data. These data were used to generate survival curves that were compared with the curves for the FL3 study population.
Fisher exact test was used to compare the clinical characteristics of the various histologic groups. Median ages were compared using the Wilcoxon rank-sum test. The Kaplan-Meier method23 was used to estimate overall and event-free survival distributions. Overall survival was defined as the time from the start of treatment to the date of death or last contact. Patients alive at last contact were treated as censored for overall survival analysis. Event-free survival was defined as the time from the start of treatment to either the date of disease progression, death, or last contact. Patients who were alive at last contact and had not progressed were treated as censored for event-free survival analysis. The log-rank test was used to compare the survival distributions of the various groups.24 Cox regression was used for multivariate analysis of factors predictive of overall survival.25 26
Results
Of the 190 patients, 107 had FL3a (56%), 51 had FL3b (27%), 2 had FL3bs (1%), and 30 had FLC (16%). The 2 patients with FL3bs included a 72-year-old man with stage III disease who achieved a complete remission but died secondary to a cerebral vascular accident 5 years after diagnosis, and a 54-year-old man with stage IV disease who died of progressive lymphoma 3 years after diagnosis. Given the small number of patients with FL3bs, they were combined with the FL3b cases for all subsequent analyses. The median follow-up for the entire group was 5.9 years (range, 1-17.6) and the 5-year overall survival (OS) and event-free survival (EFS) were 59% and 38%, respectively.
There were no differences in the clinical characteristics between patients with FL3a, FL3b, or FLC (Table1). The 5-year OS was 65% for those with FL3a, 52% for FL3b, and 54% for FLC, and there were no significant differences in OS (Figure 2A) or EFS (P = .72) between patients with grades FL3a, FL3b, or FLC. There is a separation between the OS curves for FL3a and FL3b after 1 year of follow-up (Figure 2B), but this separation is of borderline statistical significance (P = .10). However, when comparing only the cases without diffuse areas (ie, cases that are 100% follicular), the difference in OS between grades FL3a and FL3b is not significant (Figure 2C). The FLC group was compared with a group of similarly treated patients with low-grade follicular lymphoma and had a worse OS (P = .054), but there was no significant difference in EFS (P = .69). The 5-year OS for the low-grade follicular lymphoma group was 74% compared with only 54% for the FLC patients.
Clinical characteristics of the 3 subtypes of follicular lymphoma, grade 3
| . | FL3a (%) . | FL3b (%) . | FLC (%) . | P . |
|---|---|---|---|---|
| Total number | 107 (56) | 53 (28) | 30 (16) | |
| Sex | ||||
| Male | 43 (40) | 30 (57) | 15 (50) | .14 |
| Female | 64 (60) | 23 (43) | 15 (50) | |
| Age, y | ||||
| Median | 61 | 60 | 66 | .33 |
| Range | 22-87 | 16-84 | 37-83 | |
| Stage | ||||
| I/II | 31 (32) | 19 (37) | 12 (43) | .52 |
| III/IV | 67 (68) | 33 (63) | 16 (57) | |
| B symptoms | ||||
| No | 79 (79) | 37 (71) | 22 (79) | .57 |
| Yes | 21 (21) | 15 (29) | 6 (21) | |
| Extranodal sites | ||||
| Fewer than 2 | 90 (90) | 50 (96) | 26 (93) | .43 |
| 2 or more | 10 (10) | 2 (4) | 2 (7) | |
| Karnofsky score | ||||
| Higher than 70 | 87 (88) | 47 (92) | 27 (100) | .14 |
| 70 or lower | 12 (12) | 4 (8) | 0 (0) | |
| LDH | ||||
| Normal | 72 (77) | 31 (65) | 17 (63) | .16 |
| High | 21 (23) | 17 (35) | 10 (37) | |
| IPI risk group | ||||
| Low (0-2) | 68 (78) | 40 (85) | 19 (76) | .57 |
| High (3-5) | 19 (22) | 7 (15) | 6 (24) |
| . | FL3a (%) . | FL3b (%) . | FLC (%) . | P . |
|---|---|---|---|---|
| Total number | 107 (56) | 53 (28) | 30 (16) | |
| Sex | ||||
| Male | 43 (40) | 30 (57) | 15 (50) | .14 |
| Female | 64 (60) | 23 (43) | 15 (50) | |
| Age, y | ||||
| Median | 61 | 60 | 66 | .33 |
| Range | 22-87 | 16-84 | 37-83 | |
| Stage | ||||
| I/II | 31 (32) | 19 (37) | 12 (43) | .52 |
| III/IV | 67 (68) | 33 (63) | 16 (57) | |
| B symptoms | ||||
| No | 79 (79) | 37 (71) | 22 (79) | .57 |
| Yes | 21 (21) | 15 (29) | 6 (21) | |
| Extranodal sites | ||||
| Fewer than 2 | 90 (90) | 50 (96) | 26 (93) | .43 |
| 2 or more | 10 (10) | 2 (4) | 2 (7) | |
| Karnofsky score | ||||
| Higher than 70 | 87 (88) | 47 (92) | 27 (100) | .14 |
| 70 or lower | 12 (12) | 4 (8) | 0 (0) | |
| LDH | ||||
| Normal | 72 (77) | 31 (65) | 17 (63) | .16 |
| High | 21 (23) | 17 (35) | 10 (37) | |
| IPI risk group | ||||
| Low (0-2) | 68 (78) | 40 (85) | 19 (76) | .57 |
| High (3-5) | 19 (22) | 7 (15) | 6 (24) |
LDH indicates lactate dehydrogenase.
Overall survival by subtypes of grade 3 follicular lymphoma.
(A) Grade 3a versus 3b versus FLC; (B) grade 3a versus 3b, all cases; (C) grade 3a versus 3b, only 100% follicular cases.
Overall survival by subtypes of grade 3 follicular lymphoma.
(A) Grade 3a versus 3b versus FLC; (B) grade 3a versus 3b, all cases; (C) grade 3a versus 3b, only 100% follicular cases.
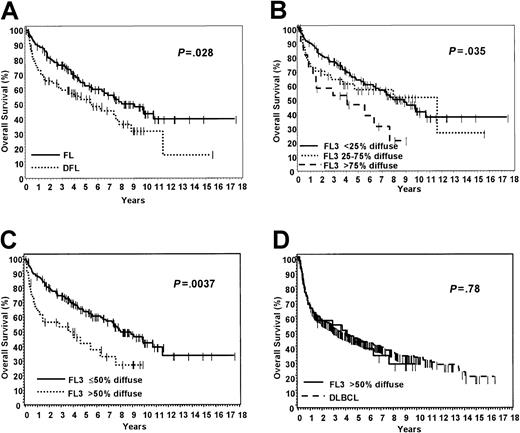
Areas consisting of DLBCL, ranging from 10% to 95%, were seen in 72 cases (38%). Twelve cases had diffuse areas of more than 80%. Of the cases with diffuse areas, 31 were FL3a, 28 were FL3b, and 13 were FLC. Diffuse areas were present in 29% of FL3a, 58% of FL3b, and 43% of FLC. Therefore, diffuse areas are more likely to be identified in FL3b. The cases that were 100% follicular (FL) were clinically similar to those with diffuse areas (DFL), with the exception of stage (Table 2). The patients with pure FL were more likely to be of higher stage than those with diffuse areas (P = .0054). The patients with DFL had a 5-year OS of 54%, which is significantly worse than the 63% OS for those with pure FL (Figure 3A). When we used the WHO recommendations for pattern reporting in low-grade follicular lymphoma,14 the patients with diffuse areas more than 75% had a significantly worse OS (Figure 3B). The cases were also analyzed by deciles and quartiles, and a cut-point of 50% was confirmed as being optimal for prediction of survival by using a survival tree method.27 The patients with a diffuse component of more than 50% diffuse had a significantly worse OS than those with a diffuse component less than or equal to 50% (Figure 3C). In addition, the EFS between these 2 groups was also significantly different (P = .012). This predominantly diffuse (> 50%) group also had an OS and EFS similar to that of a group of 530 cases of DLBCL that were treated similarly (Figure 3D).
Clinical characteristics of grade 3 follicular lymphoma patients with 100% follicular lymphoma (FL) and those with diffuse areas (DFL)
| . | FL (%) . | DFL (%) . | P . |
|---|---|---|---|
| Total number | 118 (62) | 72 (38) | |
| Sex | |||
| Male | 50 (42) | 38 (53) | .18 |
| Female | 68 (58) | 34 (47) | |
| Age, y | |||
| Median | 61 | 63 | .72 |
| Range | 33-86 | 16-85 | |
| Stage | |||
| I/II | 30 (27) | 32 (48) | .0054 |
| III/IV | 82 (73) | 34 (54) | |
| B symptoms | |||
| No | 88 (78) | 50 (75) | .72 |
| Yes | 25 (22) | 17 (25) | |
| Extranodal sites | |||
| Fewer than 2 | 106 (93) | 60 (91) | .77 |
| 2 or more | 8 (7) | 6 (9) | |
| Karnofsky score | |||
| Higher than 70 | 100 (90) | 61 (92) | .79 |
| 70 or lower | 11 (10) | 5 (8) | |
| LDH | |||
| Normal | 75 (71) | 45 (71) | 1.00 |
| High | 30 (39) | 18 (29) | |
| IPI risk group | |||
| Low (0-2) | 80 (80) | 47 (80) | 1.00 |
| High (3-5) | 20 (20) | 12 (20) |
| . | FL (%) . | DFL (%) . | P . |
|---|---|---|---|
| Total number | 118 (62) | 72 (38) | |
| Sex | |||
| Male | 50 (42) | 38 (53) | .18 |
| Female | 68 (58) | 34 (47) | |
| Age, y | |||
| Median | 61 | 63 | .72 |
| Range | 33-86 | 16-85 | |
| Stage | |||
| I/II | 30 (27) | 32 (48) | .0054 |
| III/IV | 82 (73) | 34 (54) | |
| B symptoms | |||
| No | 88 (78) | 50 (75) | .72 |
| Yes | 25 (22) | 17 (25) | |
| Extranodal sites | |||
| Fewer than 2 | 106 (93) | 60 (91) | .77 |
| 2 or more | 8 (7) | 6 (9) | |
| Karnofsky score | |||
| Higher than 70 | 100 (90) | 61 (92) | .79 |
| 70 or lower | 11 (10) | 5 (8) | |
| LDH | |||
| Normal | 75 (71) | 45 (71) | 1.00 |
| High | 30 (39) | 18 (29) | |
| IPI risk group | |||
| Low (0-2) | 80 (80) | 47 (80) | 1.00 |
| High (3-5) | 20 (20) | 12 (20) |
LDH indicates lactate dehydrogenase.
Overall survival according to diffuse areas in grade 3 follicular lymphoma.
(A) Cases with 100% follicular (FL) versus cases with diffuse areas (DFL); (B) all cases divided according to WHO-recommended pattern reporting; (C) all cases that are 50% or less diffuse versus cases that are more than 50% diffuse; (D) FL3 that is more than 50% diffuse compared with a group of 530 similarly treated DLBCL patients.
Overall survival according to diffuse areas in grade 3 follicular lymphoma.
(A) Cases with 100% follicular (FL) versus cases with diffuse areas (DFL); (B) all cases divided according to WHO-recommended pattern reporting; (C) all cases that are 50% or less diffuse versus cases that are more than 50% diffuse; (D) FL3 that is more than 50% diffuse compared with a group of 530 similarly treated DLBCL patients.
Multivariate analysis was performed using Cox regression25 26 to determine which factors were predictive of OS when controlling for other factors found to be significant in univariate analysis. The variables included were age, sex, stage, performance score, extranodal sites, LDH level, IPI score, and diffuse areas more than 50%. For OS, only a high IPI score (3-5) and diffuse areas more than 50% were independent predictors. Patients with high IPI scores had a relative risk of death of 2.2 (P = .0022) when compared with those with low IPI scores (0-2), and patients with more than 50% diffuse areas had a relative risk of death of 1.8 (P = .022) when compared with those with less than or equal to 50% diffuse areas.
Discussion
The WHO has recommended the subdivision of FL3 into grades 3a and 3b based on the number of centroblasts and the presence or absence of centrocytes. The current study is the first to analyze the clinical relevance of this subdivision in a large group of patients with nodal FL3 who received aggressive chemotherapy at the time of presentation. A recent study of follicular lymphoma suggested that this morphologic subdivision has biologic and possibly clinical significance.16 However, that study included only 27 patients with FL3, and a subset of the patients were studied at relapse. Although that study found differences in cytogenetic alterations between FL3a and FL3b, there were no differences in OS or EFS between those 2 groups.16 On the basis of our data, this subdivision does not appear to be clinically significant when patients are treated with anthracycline-containing chemotherapy. Our patients with FL3a and FL3b were clinically similar, and there were no differences in OS or EFS. Although there was a separation of the survival curves when comparing FL3a and FL3b (Figure 2B), with the FL3b cases showing a trend toward inferior survival, this separation did not reach statistical significance (P = .10). This difference is most likely due to the fact that diffuse areas were more frequent in FL3b. In fact, when evaluating only those cases that were 100% follicular, ie, cases lacking diffuse areas, there was no survival difference between FL3a and FL3b (Figure 2C).
Follicular large cleaved cell lymphoma is not recognized as an entity in the current WHO classification. However, the Working Formulation22 did recognize large cleaved cells in defining follicular large cell lymphoma. The 30 cases of FLC in our study had a clinical presentation and survival similar to the FL3a and FL3b cases, suggesting that FLC is a morphologic subtype of FL3. Furthermore, when we compared our FLC group with a group of similarly treated patients with low-grade follicular lymphoma, the FLC group had a significantly worse OS. A study of follicular large cell lymphomas by Horning et al18 also found no difference in OS when comparing cases composed of large noncleaved cells versus large cleaved cells. Many FLC cases may actually be misclassified as low-grade follicular lymphoma because they usually do not meet the strict WHO criteria for FL3. Although FLC represents a minority subtype (16%) of FL3, it is important to recognize this category because it appears to behave similarly to FL3, and, therefore, the diagnosis has important treatment implications. However, additional studies to further define this subtype of FL3 and confirm our findings are necessary.
In the Working Formulation,22 follicular lymphoma composed of small centroblasts (small noncleaved cells) had a more aggressive course. Nathwani et al28 also found that follicular lymphomas with more than 10 small noncleaved cells per hpf had a significantly worse overall survival. However, we identified only 2 cases with FL3 having a predominance of small centroblasts in our study. Because this type of grade 3b follicular lymphoma is quite rare, multi-institutional studies will be needed to delineate whether such cases are more aggressive.
Studies evaluating the significance of diffuse areas in follicular large cell or FL3 have yielded conflicting results. In most studies, the presence of diffuse areas had no effect on OS or EFS.2-4,9,10,18 One study6 found that diffuse areas predicted for EFS but not OS, and 2 studies7,17found that diffuse areas were associated with a worse OS. In the study by Bartlett et al,7 increasing percentages of diffuse areas were associated with a significantly worse OS in univariate analysis. In the study by Warnke et al,17 the cases with more than 25% diffuse areas had a significantly worse OS. In the current study, diffuse areas of more than 50% predicted for inferior OS and EFS in both univariate and multivariate analysis. Previous studies2-4,6,7,9,10,17 18 had populations ranging from 16 to 107 patients, and the median follow-up ranged from 47 to 67 months. However, our current study is considerably larger (190 patients) and also has the longest median follow-up (71 months). In addition, the current study is restricted to patients with node-based disease. It is not clear if other studies excluded primary extranodal cases. Finally, 17% of our study population had diffuse areas that were more than 50%. If the cases with diffuse areas included in the other series were predominantly those with less than or equal to 50% diffuse areas, the survival difference may not have been evident because cases with less than or equal to 50% diffuse areas behave similarly to those without diffuse areas. These factors may also account for our ability to find a difference between cases with diffuse areas versus those that are purely follicular.
It is difficult to compare our current study with previous studies because most used the Rappaport Classification or the Working Formulation. Only 3 studies6,7,10 have used the counting criteria of Mann and Berard.15 Bartlett et al7 also found a significantly worse OS for patients with areas of diffuse histology. Interestingly, this was an expansion of an earlier study18 that did not find any differences in OS in cases with diffuse areas. Apparently, the inclusion of additional patients and longer follow-up resulted in a significant survival difference. In the study by Anderson et al,6 the researchers found that diffuse areas predicted for a worse EFS but did not predict OS. However, that study did not include patients that had diffuse areas more than 80%, which may account for their inability to detect a difference in OS. A third study10 found no difference in OS for cases with diffuse areas. Although the percentage of patients with diffuse areas (30%) in that study is similar to the current study (38%), the researchers did not quantitate the diffuse areas, thus making it difficult to compare the current study with their findings.
In conclusion, the subdivision of FL3 into the cytologic subtypes of 3a, 3b, and FLC does not appear to be clinically important. However, to prevent its misclassification as a low-grade follicular lymphoma, FLC should be recognized and considered as a morphologic subtype of FL3 for clinical purposes. Finally, patients with FL3 with a significant diffuse component (> 50%) have an inferior survival that is similar to the survival of those with DLBCL. Future studies focusing on the molecular genetic events associated with these differences would be of great interest.
Prepublished online as Blood First Edition Paper, November 7, 2002; DOI 10.1182/blood-2002-07-2298.
Supported by grant CA36727 from the National Cancer Institute, Department of Health and Human Services.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Dennis D. Weisenburger, Department of Pathology and Microbiology, 983135 Nebraska Medical Center, Omaha, NE 68198-3135; e-mail: dweisenb@unmc.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal