Posttranslational processing of Ras proteins has attracted considerable interest as a potential target for anticancer drug discovery. Rce1 encodes an endoprotease that facilitates membrane targeting of Ras and other prenylated proteins by releasing the carboxyl-terminal 3 amino acids (ie, the -AAX of the CAAX motif). Homozygous Rce1 mutant embryos(Rce1−/−) die late in gestation. To characterize the role of Rce1 in hematopoiesis, we performed adoptive transfers and investigated cells from the recipients. Rce1−/− fetal liver cells rescued lethally irradiated recipients and manifested normal long-term repopulating potential in competitive repopulation assays. The recipients of Rce1−/− cells developed modest elevations in mature myeloid cells (neutrophils + monocytes), but remained well. Bone marrow cells from mice that received transplants of Rce1−/− activated extracellular signal-related kinase (ERK) normally in response to granulocyte-macrophage colony-stimulating factor. These data suggest that pharmacologic inhibitors of Rce1 will have minimal effects on normal hematopoietic cells.
Introduction
Ras proteins regulate cell fates by cycling between an active guanosine 5c-triphosphate (GTP)-bound state and an inactive guanosine 5c-diphosphate (GDP)-bound state in response to extracellular stimuli, including activation of hematopoietic growth factor receptors.1-3RAS genes are mutated frequently in myeloid malignancies and in other human cancers (reviewed in Bos4 and Rodenhuis5). In addition toRAS point mutations, inactivation of the NF1tumor-suppressor, expression of the Bcr-Abl fusion, and mutations of the FLT3 receptor are thought to contribute to leukemogenesis, at least in part, by deregulating Ras signaling.6-8 While these data establish hyperactive Ras as a therapeutic target, disrupting Ras function may have adverse effects on normal tissues.
Ras proteins undergo posttranslational modification at a common C-terminal CAAX sequence (reviewed in Gibbs and Oliff,9Sebti and Hamilton,10 and Le and Shannon11). Processing is initiated by farnesyltransferase (Ftase), which attaches a farnesyl lipid to the thiol group of the cysteine (the “C” of the CAAX motif). Prenylation targets Ras to membranes, and is required for the biologic activity of normal and oncogenic Ras. Ftase inhibitors have shown promise as anticancer agents.10-13 However, K-Ras and N-Ras are substrates for geranylgeranyltransferase 1 (GGTase 1) and are processed by this alternative pathway when Ftase is inhibited. Indeed, extensive data now support the view that non-Ras CAAX proteins are critical in vivo targets of the Ftase inhibitors (reviewed in Sebti and Hamilton,10 Le and Shannon,11 and Omer et al14). After prenylation, the carboyxl terminal 3 amino acids are released by Rce1, an integral membrane endoprotease of the endoplasmic reticulum. The final step in Ras processing involves methylation of the prenylcysteine by isoprenylcysteine carboxyl methyltransferase.
The murine Rce1 gene was disrupted to elucidate its role in development and tumorigenesis.15 Homozygous mutant embryos(Rce1−/−) demonstrated late embryonic lethality with normal organogenesis.15 To define the importance of Rce1 in hematopoiesis, we have performed adoptive transfer, biochemical, and competitive repopulation experiments.
Study design
Mice
Rce1 knock-out mice have been described.15 C57Bl/6 mice and congenic B6.SJL-PtrcaPep3b/BoyJ (B6/BoyJ) mice (CD45.1+) were purchased from Jackson Laboratory (Bar Harbor, ME). B6/BoyJ mice express a variant allele of the CD45 cell surface protein (CD45.1). The experimental procedures were approved by the UCSF Committee on Animal Research.
Adoptive transfer and competitive repopulation
Heterozygous Rce1 mutant(Rce1+/−) mice were mated to produce wild-type,Rce1+/−, and Rce1−/−embryos. Fetal liver cells were isolated and injected into irradiated 8- to 12-week-old recipients as described.15,16 The competitive repopulation methodology was based on published protocols.17 Briefly, recipients received a mixture of C57Bl/6 CD45.2 Rce1−/− or wild-type fetal liver tester cells (1 × 105 to 2 × 106, per mouse) with a standard number of congenic BoyJ CD45.1 competitors (5 × 105 per mouse). Mice injected with only competitor cells consistently showed less than 5% autologous recovery.
Monitoring
Complete blood cell counts were measured in a Hemavet instrument (CDC Technologies, Oxford, CT) and differential counts were confirmed by examining blood smears stained with Wright-Giemsa. For chimerism studies, blood and bone marrow cells were stained with allele-specific antibodies to CD45.1 and CD45.2 (Pharmingen, San Diego, CA) followed by fluorescence activated cell staining (FACS) analysis. In some experiments, cells were also labeled with antibodies that recognize CD3 (T lymphocytes), B220 (B lymphocytes), Gr-1 (granulocytes), and Mac-1 (myelomonocytic cells) (Pharmingen). Using a Becton Dickinson FACScan, 10 000 events were collected and the data were analyzed using FlowJo (Tree Star, San Carlos, CA) and Cell Quest software (Becton Dickinson, Mountain View, CA).
Colony assays
Colony-forming units-granulocyte macrophage (CFU-GMs) were assayed in methylcellulose medium (Stem Cell Technologies, Vancouver, BC, Canada) containing murine granulocyte-macrophage colony-stimulating factor (GM-CSF) concentrations. Colony growth was scored on day 8.
Kinase assays
Lysates were prepared from bone marrow as described.7,18 Protein concentrations were equalized using the Bradford Colorimetric Assay (Pierce Chemical, Rockford, IL) and equal loading was confirmed by Western blotting. Extracellular signal-related kinase (ERK) was immunoprecipitated with a specific antiserum (Cell Signaling, Beverly, MA; catalog no. 9101) and kinase immune complex assays were performed as described18using an Elk1 fusion protein (New England Biolabs, Beverly, MA) as a phosphorylation substrate.
Results and discussion
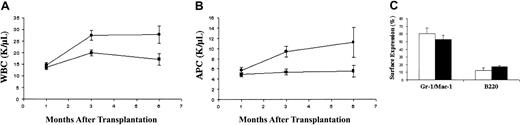
Fetal liver cells of all Rce1 genotypes efficiently rescued hematopoiesis in irradiated recipients. Because leukocyte counts in mice injected with wild-type orRce+/− cells were similar, these groups were combined for analysis. Mice engrafted withRce1−/− fetal liver cells developed leukocytosis by 3 months that persisted until they were killed at 6 months (Figure 1A). This was due to increased numbers of mature myeloid cells in theRce1−/− recipients (Figure 1B). These mice remained well, and demonstrated normal spleen sizes and splenic architecture at killing (data not shown). However, FACS analysis of marrow and spleen from recipients ofRce1−/− fetal liver cells demonstrated an increase in the percentage of myeloid cells (Gr-1 and/or Mac-1–positive) and a commensurate reduction in B lymphocytes (B220-positive) (Figure 1C). Because GM-CSF promotes the growth of myelomonocytic cells, we assayed CFU-GM growth in methylcellulose over a range of GM-CSF concentrations. Although there was some variability between individual experiments, colony growth was similar for wild-type, Rce1+/−, andRce1−/− cells.Rce1−/− CFU-GM colonies grown in saturating concentrations of GM-CSF demonstrated less spreading than wild-type colonies.15
Hematologic parameters in recipients of
Rce1−/−, Rce1+/−, and wild-type fetal liver cells. Recipients ofRce1−/− cells (n = 32) are shown as ● in panels A and B and as ■ in panel C. We observed no differences in recipients of Rce1+/− and wild-type fetal liver cells, so the data from these mice were pooled (n = 40) and are shown as ▪ in panels A and B and as ▪ in panel C. (A) White blood cell counts (WBCs). (B) Absolute phagocyte counts (APCs; monocytes + neutrophils). (C) Relative numbers of bone marrow cells expressing the myeloid markers Gr-1 and Mac-1 and the B-lymphocyte marker B220 6 months after adoptive transfer.
Hematologic parameters in recipients of
Rce1−/−, Rce1+/−, and wild-type fetal liver cells. Recipients ofRce1−/− cells (n = 32) are shown as ● in panels A and B and as ■ in panel C. We observed no differences in recipients of Rce1+/− and wild-type fetal liver cells, so the data from these mice were pooled (n = 40) and are shown as ▪ in panels A and B and as ▪ in panel C. (A) White blood cell counts (WBCs). (B) Absolute phagocyte counts (APCs; monocytes + neutrophils). (C) Relative numbers of bone marrow cells expressing the myeloid markers Gr-1 and Mac-1 and the B-lymphocyte marker B220 6 months after adoptive transfer.
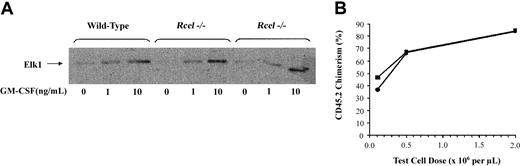
ERK activities were measured in bone marrow collected 3 to 6 months after adoptive transfer. In multiple experiments, wild-type andRce1−/− cells demonstrated equivalent basal and GM-CSF–stimulated ERK activities (Figure2A).
ERK activation and repopulating potential of
Rce1−/− cells. (A) Bone marrow was collected 3 to 6 months after adoptive transfer, incubated under low (0.1%) serum conditions for 4 hours, and then stimulated with recombinant murine GM-CSF. The cells were lysed after 10 minutes, immunoprecipitated with an antibody against ERK, and phosphorylation of an Elk1 substrate was measured. (B) Percentages of peripheral blood leukocytes derived from Rce1−/− (▪) and wild-type (●) 4 months after adoptive transfer with a standard population of CD45.1 competitor cells. Data are pooled from independent experiments and represent 3 to 6 recipients at each point.
ERK activation and repopulating potential of
Rce1−/− cells. (A) Bone marrow was collected 3 to 6 months after adoptive transfer, incubated under low (0.1%) serum conditions for 4 hours, and then stimulated with recombinant murine GM-CSF. The cells were lysed after 10 minutes, immunoprecipitated with an antibody against ERK, and phosphorylation of an Elk1 substrate was measured. (B) Percentages of peripheral blood leukocytes derived from Rce1−/− (▪) and wild-type (●) 4 months after adoptive transfer with a standard population of CD45.1 competitor cells. Data are pooled from independent experiments and represent 3 to 6 recipients at each point.
Wild-type and Rce1−/− fetal liver tester cells were injected into irradiated hosts with the same reference population of BoyJ competitor cells to directly compare their repopulating potentials. Cells of both genotypes demonstrated equivalent repopulating potentials over a dose range that produced 10% to 70% donor cell chimerism (Figure 2B). These data provide strong evidence that inactivation of Rce1 does not impair the proliferative capacity of hematopoietic cells.
As the only known CAAX protease in mammalian cells, Rce1 represents an attractive target for cancer drug discovery. Genetic ablation eliminates Ras endoproteolytic activity, which results in mislocalization of approximately 50% of Ras away from the plasma membrane.15 Importantly, Rce1-deficient cells are unable to process either farnesylated or geranylgernaylated substrates.15 Our data suggest that the amount of Ras that is correctly targeted to the plasma membrane inRce1−/− cells is sufficient for normal signal output from activated cytokine receptors, which is consistent with the low density of many receptors on hematopoietic precursors. Alternatively, hematopoietic cells may express another protease that can compensate for the loss of Rce1. It is unclear why recipients ofRce1−/− transplants demonstrated modest proliferation of mature myeloid elements; however, many cellular proteins in addition to Ras are substrates for Rce1. This modest myeloid proliferation raises the possibility that inhibiting Rce1 might paradoxically stimulate the growth of myeloid malignancies.
At first glance, these results argue that Rce1 represents a poor therapeutic target in leukemia, particularly when genetic lesions such as the BCR-ABL translocation, loss of NF1, or mutations of FLT3 lead to aberrant activation of normal Ras proteins. However, lack of toxicity to normal hematopoietic cells also represents a distinct advantage and it is noteworthy that STI-571 (Gleevec) is remarkably selective for cells that express Bcr-Abl despite fully inhibiting c-Abl and c-kit.19 Similarly, it is possible that blocking Rce1 will differentially affect cells that express normal versus mutant Ras proteins. Studies in tissue culture and in transgenic mice have shown that transformed cells select for higher levels of oncogenic Ras20,21; this may render them sensitive to a modest reduction in Ras signaling. Furthermore, because oncogenic Ras accumulates in the GTP-bound conformation, mislocalizing it away from the plasma membrane might sequester effectors such as Raf. Interestingly, somatic deletion of Rce1 was recently associated with a reduction in Hras- andKras-induced transformation in soft agar and with a competitive disadvantage of skin carcinoma cells expressing oncogenicHras.22 We are exploiting this conditional mutant allele to investigate the effects of inactivating Rce1 in genetically engineered mouse models of myeloid malignancies associated with hyperactive Ras.
Prepublished online as Blood First Edition Paper, November 14, 2002; DOI 10.1182/blood-2002-07-2250.
Supported by awards from the US Army Neurofibromatosis Research Program (Projects DAMD 17-00-1-0594 and DAMD 17-02-1-0638) and by National Institutes of Health grant CA84221 (K.M.S.), and by grants HL-41633, HL-47660, and HL-15451 (S.G.Y.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Kevin M. Shannon, University of California, San Francisco, 513 Parnassus Ave, HSE 302, San Francisco, CA 94143; e-mail: kevins@itsa.ucsf.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal