The human i and I antigens are characterized as linear and branched repeats of N-acetyllactosamine, respectively. Conversion of the i to the I structure requires I-branching β-1,6-N-acetylglucosaminyltransferase activity. It has been noted that the null phenotype of I, the adult i phenotype, is associated with congenital cataracts in Asians. Previously, the identification of molecular changes in the IGnT gene, associated with the adult i phenotype, has been reported. In the present study, we demonstrate that the human I locus expresses 3 IGnT forms, designated IGnTA, IGnTB, and IGnTC, which have different exon 1, but identical exons 2 and 3, coding regions. The molecular genetics proposed for the I locus offer a new perspective on the formation and expression of the I antigen in different cells and provide insight into the questions derived from investigation of the adult i phenotype. Molecular genetic analyses of the Iloci of the 2 adult i groups, with and without congenital cataracts, were performed, and enzyme function assays and expression patterns for the 3 IGnT transcripts in reticulocytes and lens-epithelium cells were analyzed. The results suggest a molecular genetic mechanism that may explain the partial association of the adult i phenotype with congenital cataracts and indicate that a defect in theI locus may lead directly to the development of congenital cataracts. The results also suggest that the human blood groupI gene should be reassigned to the IGnTC form, not the IGnTB form, as described previously.
Introduction
The i and I antigens are carbohydrate structures characterized as linear and branched repeats of N-acetyllactosamine, Galβ1-4GlcNAcβ1-3Galβ1-4GlcNAc-R and Galβ1-4GlcNAcβ1-3(Galβ1-4GlcNAcβ1-6)Galβ1-4GlcNAc-R, respectively, carried on glycolipids and glycoproteins.1-5They were first identified on human red blood cells (RBCs),6-10 but are known to be present also on the surface of most human cells and on soluble glycoproteins in various body fluids, including milk,11 saliva,12plasma,12 amniotic fluid, urine, and ovarian cyst fluid.4,13 The N-acetyllactosamine repeats are synthesized by the sequential action of β-1,3-N-acetylglucosaminyltransferase and β-1,4-galactosyltransferase. Conversion of the i antigen into an I-active structure requires the action of a third enzyme, the I-branching β-1,6-N-acetylglucosaminyltransferase (I β6GlcNAcT).2,14,15 It was determined that the expression of the I and i antigens reflects a reciprocal relationship that is developmentally regulated. Adult human RBCs fully express I antigens and contain only a few i antigens, which predominate in fetal and neonatal RBCs. After birth, the quantity of I antigen gradually increases as the level of i antigen falls, until the healthy adult RBC Ii status is reached at about 18 months of life.10,16 The developmentally regulated expression of Ii antigen is exhibited on RBCs and also in many other tissues. Furthermore, altered expression patterns for the I and i antigens have often been observed during oncogenesis17 and, thus, the Ii are considered oncodevelopmental antigens.18
Most adult RBCs fully express the I antigen; however, in a small percentage of individuals, the RBCs are rich in the i antigen and contain very low levels of I.9,10,16 This phenotype is called the adult i phenotype and is believed to result from lack of I-branching transferase activity.3,14 Although the adult i phenotype is rare, it has attracted considerable attention because it has been noted to be associated with congenital cataracts in Asians. Yamaguchi et al19 first reported the association of the adult i phenotype with congenital cataracts among Japanese individuals.20,21 Of 31 Japanese with the adult i phenotype, 29 had congenital cataracts, whereas none of the I-phenotype members of these families had the same eye defect, indicating that there was no recombination between the Ii phenotype and cataracts. Linkage of the adult i phenotype and congenital cataracts has been observed in 3 Taiwanese pedigrees.22,23 In these 3 families, the 5 i members all have congenital cataracts, whereas none of the other 17 members with the I phenotype are so afflicted. The association does not seem to be as pronounced in the white population, however, with only 3 individuals in 2 families formally reported as having both the adult i phenotype and congenital cataracts.24-26
The association of the adult i phenotype with congenital cataracts can be explained either by a close linkage between independent I- and cataract-related genes or by a pleiotropic effect of the gene responsible for the adult i phenotype on the development of cataracts. However, because of the reduced strength of the association in whites, it has been suggested that the former hypothesis of a close linkage between 2 independent genes is the more tenable one.24
We have previously reported molecular genetic analysis of the adult i phenotype in Taiwanese subjects who also suffered from congenital cataracts.23 The results obtained from mutation detection within the 2 I-branching enzyme-encoding genes, segregation analyses, and enzyme function assays have identified an association between molecular changes in the IGnT gene and the adult i phenotype. These findings suggest that the IGnT gene, first reported in 1993 by Bierhuizen et al,27 is the candidate for the blood group I gene. Further, it was demonstrated that 3 different molecular bases, including 2 missense mutations (1043G>A and 1148G>A) and a gene deletion, are responsible for the adult i phenotype.23 Despite the identification of the molecular bases for the adult i phenotype, the molecular genetic basis for the association of the adult i phenotype with congenital cataracts remains obscure. The molecular factors revealed indicate, however, that the association may result from a pleiotropic effect of the same gene mutation but not from a linkage of 2 independent genes, because it is unlikely that 2 different nucleotide changes would be linked to the nearby gene that has, by chance, also mutated to result in the development of cataracts.
The present investigation shows not only that the human Ilocus expresses the previously reported IGnT transcript, but also that 3 IGnT forms are expressed from the humanI locus. Comparison of molecular genetic analyses for adult i Taiwanese and white subjects, with and without congenital cataracts, respectively, reveals a molecular genetic background accounting for the partial association of the adult i phenotype with congenital cataracts and redefines the IGnT gene form responsible for the expression of the blood group I antigen on RBCs.
Patients, materials, and methods
Samples
Our use of human subjects was conducted under the tenets of the Helsinki protocol, and the program was approved by the Institutional Review Board at Mackay Memorial Hospital. Peripheral blood or genomic DNA samples were obtained from 6 unrelated whites bearing the adult i phenotype, denoted as JCC, 97013, 987U1, 944C2, 1110Y2, and 1110X3. The 5 samples from Taiwanese with the adult i phenotype were designated as W-3, W-5, S-6, S-7, and C-3, and have been described previously.23 The 5 adult i Taiwanese all have congenital cataracts, whereas the 6 adult i whites are not so afflicted. The QIAamp DNA Blood Mini Kit (Qiagen GmbH, Hilden, Germany) and the QIAamp RNA Blood Mini Kit (Qiagen) were used to purify genomic DNA and total RNA, respectively, from the peripheral blood cells. Genomic DNA from 51 randomly selected whites was prepared from their saliva using the QIAamp DNA Blood Mini Kit.
Poly A+ RNA samples from the human brain (cerebellum), heart, small intestine, kidney, and prostate were purchased from Clontech Laboratories (Palo Alto, CA).
Demonstration of the existence and structures of 3 IGnTtranscripts: IGnTA, IGnTB, and IGnTC
The 5′ and 3′ rapid amplification of cDNA end (RACE) was performed on Marathon-Ready cDNA derived from human prostate (Clontech) to establish the cDNA structures of the IGnTA, IGnTB, and IGnTC transcripts. Primers used were as follows: IR32 (ATGTACATAGTGGCCGTGGCAGCCTCC, antisense sequence, spanning the exons 2-3 junction of the 3 IGnT cDNAs), IAR34 (5′-ACGCGCTAAAAAGACAGTGCTTCCAAGAGC-3′, for IGnTA, complementary of nucleotides 8-37, codon for initiation methionine as nucleotides 1-3), IBR35 (5′-TGTGCAAACTTGAGTCAGCCTCAAAGGGTC-3′, forIGnTB, complementary of nucleotides 118-147), ICR36 (5′-CTCACAAAAAGACCACGCTGAGCAGAGTG-3′, for IGnTC,complementary of nucleotides 33-62) for 5′-RACE, IF23 (5′-GGAGGCTGCCACGGCCACTATGTACATGG-3′, spanning the exons 2-3 junction), and IFa (5′-CAGAGTGAAACTGCGATACAACCCAGCTGG-3′, 7 nucleotides upstream to the stop codon in exon 3) for 3′-RACE. Amplified DNA fragments from RACE were cloned into the pCRII-TOPO vectors by a TOPO TA Cloning Kit (Invitrogen, Groningen, The Netherlands). The DNA sequences were determined using the BigDye Terminator Cycle Sequencing Kit (Applied Biosystems, Foster City, CA). The full cDNA structures of the 3 IGnT forms were built by assembling the sequences from the longest 5′- and 3′-RACE products, and the full cDNA structures were further demonstrated by reverse transcription polymerase chain reaction (RT-PCR) using the synthetic primers that anneal to the most 5′ and 3′ sequences of the cDNAs.
RT-PCR analysis for the expression of IGnTA, IGnTB, and IGnTC
RT-PCR was used to evaluate the expression of the 3IGnT transcripts for different human tissues and cells. The first-strand cDNAs were primed by oligo-dT primer and synthesized by ThermoScript reverse transcriptase (Invitrogen). PCR amplification was then performed, using the forward primers IAF1 (5′-GGAGCCACTTCAGAAATGTGTCAC-3′, for IGnTA, nucleotides −236 through −213, codon for initiation methionine as nucleotides 1-3), IBFm (5′-GATGAAACGGAATCGATTCCCAGCGTCTCC-3′, forIGnTB, nucleotides −147 through −118), and ICF2 (5′-GCAAATTCAACCTCTCACACCGATC-3′, for IGnTC, nucleotides −76 through −52). The reverse primer, IRc (5′-AGCTGCAGTTTCCCTTCAGTCATGAGTAGC-3′, antisense sequence, 12 nucleotides downstream to the stop codon in exon 3), was common for the 3 transcripts. The PCR program included 5 minutes at 94°C followed by 40 cycles of 30 seconds at 94°C, 30 seconds at 62°C, and 1.5 minutes at 72°C.
Molecular cloning and sequence analysis for the IGnT gene of the adult i individuals
The exon 1A, 1B, 1C, 2, and 3 regions of the IGnTgene were amplified by PCR using genomic DNA as a template. The primer pairs were as follows: IAF1 and IAR5 (5′-TAGTATTGACCATGCAGTGTTTATTCG-3′, antisense sequence, 30 nucleotides downstream to exon 1A) for exon 1A, IBFm and IBRd (5′-CCTAATAAAAAGTGGCTGGTTATTCTAAAGCC-3′, antisense sequence, 50 nucleotides downstream to exon 1B) for exon 1B, ICF2 and ICR6 (5′-TGAGTCAGTTCTCTAGGCGAGCAG-3′, antisense sequence, 49 nucleotides downstream to exon 1C) for exon 1C, IFh (5′-TCCCTTCTCTCATGACTCTCATCTCTACGC-3′, 22 nucleotides upstream to exon 2) and IRk (5′-ACACCAACAGGCAGCGGCCTAGAAGCATGG-3′, antisense sequence, 14 nucleotides downstream to exon 2) for exon 2, and IFg (5′-GTCGGAGAGTACCTCTAGTATTCTGTAAGTTC-3′, 57 nucleotides upstream to exon 3) and IRc for exon 3. The PCR products were cloned into the pCRII-TOPO vectors, and the DNA sequences were determined. Multiple clones from 2 batches of PCR products were sequenced to distinguish any PCR error from actual sequence polymorphism.
PCR–restriction fragment length polymorphism (RFLP) analysis
The 505G>A mutation identified in the IGnTC gene destroys a BstNI recognition sequence (CCWGG), and thus a PCR-RFLP analysis was developed to detect the mutation. The 606–base pair (bp) fragment encompassing the 505-nucleotide position of theIGnTC gene was PCR amplified using genomic DNA as a template and primers ICF9 (5′-AGAAGAGGCTGCATTCCC-3′, nucleotides 270-287 ofIGnTC) and ICR11 (5′-GATCAATGGCCCTTTGGTCACG-3′, complementary of nucleotides 835-856 of IGnTC). The PCR program included 5 minutes at 94°C followed by 30 cycles of 30 seconds at 94°C, 30 seconds at 58°C, and 1 minute at 72°C. The PCR products were subjected to digestion by BstNI restriction endonuclease and were then analyzed by agarose gel electrophoresis. The PCR product amplified from the wild-typeIGnTC allele was cleaved into 233-, 225-, 101-, and 47-bp fragments, whereas that from the mutant IGnTC allele with the 505G>A mutation (IGnTC*505A) yielded 280-, 225-, and 101-bp fragments.
A PCR-RFLP analysis for FspI was designed to detect the 683G>A mutation identified in the IGnTC gene. The 683-nucleotide position of the IGnTC gene was sampled by PCR amplification using primers ICFm55 (5′-AGGGGTGCTGCCTCCTGACCATGCAATTGCGC-3′, nucleotides 651-682 of IGnTC) and ICR56 (5′-GATGGTCAGCTGATGTGGAGGTGAAGT-3′, complementary of nucleotides 754-780 of IGnTC). In the ICFm55 primer, 2 nucleotides (boldfaced) were changed from AA to GC, so that an FspI recognition sequence (TGCGCA) would be produced when a mutant IGnTC allele with the 683G>A mutation (IGnTC*683A) was used as a template to produce the PCR product. The PCR program included 5 minutes at 94°C followed by 30 cycles of 30 seconds at 94°C, 30 seconds at 65°C, and 30 seconds at 72°C. After digestion by FspI restriction endonuclease, the 130-bp PCR product amplified from the IGnTC*683A allele was cleaved into 100- and 30-bp fragments, whereas that from the wild-type IGnTC allele was resistant to digestion.
Functional analyses of the enzymes encoded from theIGnT cDNAs
The cDNA fragment encompassing the region of nucleotides 79 to 1206, which encodes the amino acid residues 27 to 402, of theIGnTA gene was prepared by RT-PCR and primers IAF (5′-aattggcccagccggccGAGTTATGGGAGAATAAACG-3′) and IRx (5′-ttaagggcccAAAATACCAGCTGGGTTGTATCGCAG-3′, antisense sequence). Primers ICF (5′-aattggcccagccggccCAATTGAGCCCGCCAAAAAGTTATGAG-3′) and IRx were used to prepare the cDNA fragment encompassing the region of nucleotides 76 to 1206, which encodes the amino acid residues 26 to 402, of the IGnTC gene. The primers containedSfiI and ApaI recognition sequences (underlined) at the 5′ ends, respectively. Total RNA prepared from peripheral blood cells of an individual with the common I phenotype and an individual with the adult i phenotype (W-3), who is a heterozygote with theIGnTB*1043A/IGnTB*1148A genotype, served as templates. The amplified cDNA fragments were cloned intoSfiI and ApaI sites of the mammalian expression vector pSecTaq2A (Invitrogen), which is designed for the secretion of the expressed protein by the N-terminal secretion signal from the V-J2-C region of the mouse immunoglobulin κ chain. Vectors bearing wild-type IGnTA and IGnTC, and mutantIGnTA*1049A, IGnTA*1154A, IGnTC*1049A,and IGnTC*1154A cDNAs, were selected and sequence-confirmed. Construction of the vectors bearing wild-type IGnTB cDNA has been described previously.23
The expression vectors bearing mutant IGnTC*505A andIGnTC*683A cDNAs were constructed as follows. DNA fragments were obtained from PCR amplification using ICF and ICR6 as primers and genomic DNA from the adult i individual (1110X3), who is a heterozygote with the IGnTC*505A/IGnTC*683A genotype, as a template, and were digested by SalI restriction endonuclease. The SalI-cleaved fragments, encompassing the region of nucleotides 76 to 822 of IGnTC, were selected and ligated with the fragments encompassing the region of nucleotides 823 to 1206 obtained by SalI digestion of the wild-typeIGnTC cDNA product, amplified by the ICF and IRx primers mentioned in the preceding paragraph. The ligated fragment was cloned into SfiI and ApaI sites of the pSecTaq2A vector, and vectors bearing the respective IGnTC*505A andIGnTC*683A cDNAs were selected and sequence-confirmed.
The constructed and the mock pSecTaq2A plasmids were prepared using the EndoFree Plasmid Kit (Qiagen) for transfection. Expression of the constructed and mock plasmids in COS-7 cells, and the subsequent GlcNAcT assay, were performed as described previously.23
Preparation of the RNA samples from reticulocytes and lens-epithelium cells
Pure reticulocytes were isolated from whole blood through positive isolation of cells expressing the transferrin receptor (CD71) by immunomagnetic separation.28 Eight milliliters of venous blood from a healthy adult was drawn in a tube containing anticoagulant, and, after centrifugation at 2300 g for 15 minutes, white blood cells (the buffy coat layer) were removed. Dynabeads CD71 (Dynal Biotech, Lake Success, NY), which are immunomagnetic beads coated with monoclonal antibody against human transferrin receptor, were then used to isolate the reticulocytes, following the protocol provided by the manufacturer.
Four lenses were obtained from 4 individuals who had undergone eye surgery. Total RNA samples from the isolated reticulocytes and from the lens-epithelium cells were purified using the RNeasy Mini Kit (Qiagen).
Results
IGnTA, IGnTB, and IGnTC are expressed from the human I locus
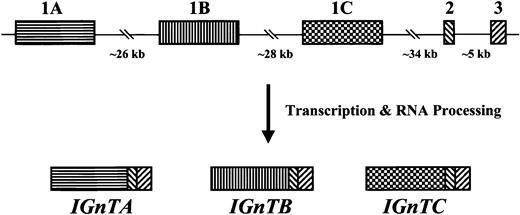
The human IGnT gene is located on chromosome 6p24. The coding sequence for the gene is divided into 3 exon regions, with respective coding nucleotides of 919, 93, and 191 bp.29Basic Local Alignment Search Tool (BLAST) analysis of the gene databases at the National Center for Biotechnology Information (National Institutes of Health, Bethesda, MD) with the exon 1 coding sequence of the IGnT gene revealed 2 novel open reading frames with significant homology. The relative positions of these 2 novel regions to exons 1, 2, and 3 of the IGnT gene were known from BAC clone RP11-360019 on human chromosome 6 (GenBank accession no. AL139039) (Figure1). In this paper, the 2 novel open reading frames are designated 1A and 1C, with the original exon 1 region of the previously reportedIGnT gene designated as 1B.
Schematic representation of the organization of the human
I locus and the structures of the expressedIGnT gene. Three IGnTtranscripts, IGnTA, IGnTB, and IGnTC,which have different exon 1 but identical exon 2 and exon 3 coding regions, are expressed from the human I locus. The coding nucleotides of exons 1A, 1B, and 1C have 925, 919, and 925 bp, respectively, and the common exon 2 and exon 3 have respective coding nucleotides of 93 and 191 bp.
Schematic representation of the organization of the human
I locus and the structures of the expressedIGnT gene. Three IGnTtranscripts, IGnTA, IGnTB, and IGnTC,which have different exon 1 but identical exon 2 and exon 3 coding regions, are expressed from the human I locus. The coding nucleotides of exons 1A, 1B, and 1C have 925, 919, and 925 bp, respectively, and the common exon 2 and exon 3 have respective coding nucleotides of 93 and 191 bp.
From the 5′-RACE and RT-PCR analyses, it was demonstrated that the homologous 1A and 1C regions are transcribed and also processed to link with the exon 2 and exon 3 regions, as for exon 1B (Figure1). Both of the intron sequences at the exon–intron junctions conform to the GT-AG consensus (data not shown). Thus, 3 transcripts with different exon 1, but identical exons 2 and 3, coding regions are expressed from the human I locus. The 3 transcripts are designated IGnTA, IGnTB, and IGnTC. Exon 1A and exon 1C consist of 925 coding nucleotides. TheIGnTB is the original IGnT gene reported by Bierhuizen et al.27
The 5′- and 3′-RACE, performed on cDNA derived from human prostate to establish the IGnT cDNA structures, yielded products of different sizes, indicating the possible existence of different transcript structures for the 3 IGnTs. The full cDNA structures of the IGnTA, IGnTB, andIGnTC transcripts obtained from prostate cDNA (supplemental data on the Blood website [see the Supplemental Figure link at the top of the online article]; GenBank/EBI DataBank accession numbers AF458024, AF458025, andAF458026, respectively) have open reading frames of 1209, 1203, and 1209 bp, respectively, which predict respective protein products of 402, 400, and 402 amino acid residues (Figure 2). The protein products predicted from the novel IGnTA andIGnTC cDNAs have potential hydrophobic transmembrane segments at N-terminals, as does the IGnTB-encoded β6GlcNAcT, and 73% sequence identity with the IGnTB β6GlcNAcT is revealed for both. Conservation of 9 Cys residues and 66% overall sequence identities are demonstrated for the 3 IGnT proteins.
Amino acid sequences deduced from the IGnTA, IGnTB, and IGnTC cDNAs.
The 9 conserved Cys residues are marked by asterisks. Dashed lines underline the hydrophobic segments at the N-terminals.
Amino acid sequences deduced from the IGnTA, IGnTB, and IGnTC cDNAs.
The 9 conserved Cys residues are marked by asterisks. Dashed lines underline the hydrophobic segments at the N-terminals.
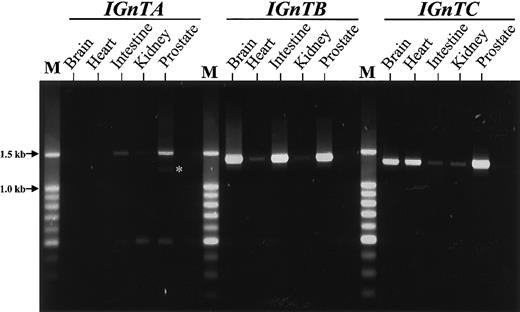
Expression profiles for the IGnTA, IGnTB, andIGnTC transcripts for different human tissues were analyzed using RT-PCR. As shown in Figure3, wide distributions were noted for the 3 IGnT transcripts in the human tissues analyzed, except that IGnTA was not detected in the RNA samples from the brain, and it appeared that different IGnT forms are expressed differentially in quantity in various human tissues, although the RT-PCR analysis was not quantitative.
Expression profiles for the
IGnT gene from various human tissues. Poly A+ RNA samples from the human brain (cerebellum), heart, small intestine, kidney, and prostate were primed using oligo-dT primer to synthesize the first-strand cDNA. Then, PCR using gene-specific forward primer and common reverse primer was performed, as described in “Patients, materials, and methods.” The RT-PCR products were analyzed using 1.5% agarose gel electrophoresis. The expected sizes of the products from the IGnTA, IGnTB, andIGnTC transcripts were 1486, 1391, and 1326 bp, respectively. From RT-PCR for the IGnTA, an additional product approximately 500 bp in size, consisting of a shorter exon 1A region conjoining with exon 2-3 regions, was observed. This smaller product is believed to result from alternative splicing and does not have a correct reading frame relative to the exon 2-3 coding sequence. The faint bands (indicated by an asterisk) below those of theIGnTA 1486-bp fragment were the hybrid complex of theIGnTA 1486-bp and the alternatively spliced 500-bp products.
Expression profiles for the
IGnT gene from various human tissues. Poly A+ RNA samples from the human brain (cerebellum), heart, small intestine, kidney, and prostate were primed using oligo-dT primer to synthesize the first-strand cDNA. Then, PCR using gene-specific forward primer and common reverse primer was performed, as described in “Patients, materials, and methods.” The RT-PCR products were analyzed using 1.5% agarose gel electrophoresis. The expected sizes of the products from the IGnTA, IGnTB, andIGnTC transcripts were 1486, 1391, and 1326 bp, respectively. From RT-PCR for the IGnTA, an additional product approximately 500 bp in size, consisting of a shorter exon 1A region conjoining with exon 2-3 regions, was observed. This smaller product is believed to result from alternative splicing and does not have a correct reading frame relative to the exon 2-3 coding sequence. The faint bands (indicated by an asterisk) below those of theIGnTA 1486-bp fragment were the hybrid complex of theIGnTA 1486-bp and the alternatively spliced 500-bp products.
The protein products encoded from the novel IGnTA andIGnTC cDNAs were expressed in mammalian cells (COS-7), and their potential GlcNAcT activity was examined and compared with that of the I β6GlcNAcT encoded from the IGnTB cDNA. The amounts of GlcNAc transferred to the acceptor substrate (LS-tetrasaccharide c [NeuNAcα2-6Galβ1-4GlcNAcβ1-3Galβ1-4Glc]) from the donor substrate (UDP-GlcNAc) by the medium concentrates harvested from the cells transfected with respective expression vectors were measured. GlcNAcT activity was demonstrated in the enzyme function assay for the expressed IGnTA and IGnTC protein products, as was the IGnTB β6GlcNAcT (Figure4); the IGnTC enzyme appeared to have the highest GlcNAc-transferring activity. It has been demonstrated that the predominant activity of the enzyme encoded from the IGnTB transcript is I-branching β6GlcNAcT activity.27 30 Compared with the IGnTB β6GlcNAcT, comparable or even higher GlcNAcT activity was demonstrated for the IGnTA and IGnTC enzymes with the same acceptor substrate, which has been shown to be a good acceptor substrate for the I β6GlcNAcT activity assay. Nevertheless, further carbohydrate structure analysis of the products generated from IGnTA and IGnTC activity is required to characterize the GlcNAcT activity and demonstrate the I β6GlcNAcT nature of the IGnTA and IGnTC enzymes.
The GlcNAcT activities of the enzymes encoded from the IGnTA, IGnTB, andIGnTC cDNAs.
The protein products encoded from the IGnTA, IGnTB, and IGnTC cDNAs were expressed in mammalian cells (COS-7). The amounts of GlcNAc transferred to the acceptor substrate (LS-tetrasacchride c [NeuNAca2-6Galb1-4GlcNAcb1-3Galb1-4Glc]) from the donor substrate (UDP-GlcNAc) by the medium concentrates harvested from the cells transfected with respective expression vectors were measured as has been described previously.23 The results of the average and standard deviation (indicated by error bars) of 4 tests are diagrammatically represented. Endogenous transfer of GlcNAc in the absence of acceptor substrate was corrected for each test. The amounts of the transferred GlcNAc in the vector control, pSecTaq2A, indicate the background levels of the assay, which are believed to result from the addition of the acceptor substrate.
The GlcNAcT activities of the enzymes encoded from the IGnTA, IGnTB, andIGnTC cDNAs.
The protein products encoded from the IGnTA, IGnTB, and IGnTC cDNAs were expressed in mammalian cells (COS-7). The amounts of GlcNAc transferred to the acceptor substrate (LS-tetrasacchride c [NeuNAca2-6Galb1-4GlcNAcb1-3Galb1-4Glc]) from the donor substrate (UDP-GlcNAc) by the medium concentrates harvested from the cells transfected with respective expression vectors were measured as has been described previously.23 The results of the average and standard deviation (indicated by error bars) of 4 tests are diagrammatically represented. Endogenous transfer of GlcNAc in the absence of acceptor substrate was corrected for each test. The amounts of the transferred GlcNAc in the vector control, pSecTaq2A, indicate the background levels of the assay, which are believed to result from the addition of the acceptor substrate.
Wild-type IGnTA and IGnTB, but mutantIGnTC, are present in the adult i whites without congenital cataracts
The exon 1A, 1B, 1C, 2, and 3 regions of the IGnT gene of the individual with the adult i phenotype (JCC) were amplified by PCR, cloned, and sequence-analyzed. The coding sequences for these exon regions and the adjacent splice sites were inspected, and the wild-type sequences of the exon 1A, 1B, 2, and 3 regions in this adult i white were demonstrated, with a G>A substitution at the 505 nucleotide position (505G>A) of exon 1C identified. All 4 of the analyzed clones bearing the exon 1C region possessed the 505G>A mutation, suggesting that the individual was homozygous for the 505G>A mutation.
A PCR-RFLP for BstNI was developed (as described in “Patients, materials, and methods”) and used to detect the 505G>A mutation for the 6 adult i whites. It was demonstrated that 5 of the 6 samples (JCC, 97013, 987U1, 944C2, and 1110Y2) were homozygous for the 505G>A mutation in the IGnTC gene, whereas the remaining individual, 1110X3, harbored one mutant IGnTC allele with the 505G>A mutation (data not shown).
The IGnT exon 1A, 1B, 1C, 2, and 3 regions of the 1110X3 individual were PCR-amplified, cloned, and analyzed. Wild-type sequences of the exon 1A, 1B, 2, and 3 regions were also demonstrated for this white i adult and, in addition to the IGnTC allele with the 505G>A mutation, another allele with the 683G>A mutation in the exon 1C region was identified. The PCR-RFLP for FspI, which was developed to detect the 683G>A mutation, proved the heterozygosity of the 683G>A mutation in this individual.
The 505G>A and 683G>A mutations in the IGnTC predict the amino acid alterations of Ala169Thr and Arg228Gln, respectively. The 2 mutated IGnTC alleles are designated IGnTC*505Aand IGnTC*683A (Figure5A). These results suggest the presence of wild-typeIGnTA and IGnTB in these adult i whites and demonstrate their carriage of double-dose mutant IGnTCalleles. Five of the 6 whites were homozygous for theIGnTC*505A allele, and one was a heterozygote with theIGnTC*505A/IGnTC*683A genotype.
Mutations identified in theIGnT gene of adult i individuals.
(A) Mutations identified in the IGnTC gene of the adult i whites. IGnTC indicates the wild-type coding sequence of theIGnTC gene. ATG and TGA correspond to the respective translation start and stop codons. The mutant alleles identified in white subjects with the adult i phenotype, designatedIGnTC*505A and IGnTC*683A, possess 505G>A and 683G>A nucleotide substitutions, respectively, which predict the respective amino acid alterations of Ala169Thr and Arg228Gln. (B) Schematic representation of the mutations identified in theIGnT gene of adult i Taiwanese with congenital cataracts. The 2 G>A changes, identified, as reported previously, at the 1043 and 1148 nucleotide positions of the IGnTB gene, locate in the common exon 3 region of the 3 IGnT forms, and, thus, the 2 mutations also contribute to form the mutant IGnTA*1049A andIGnTC*1049A as well as IGnTA*1154A andIGnTC*1154A, respectively. Exons 1A and 1C possess 6 more coding nucleotides than the exon 1B does, and thus the nucleotide numbers of the same G>A changes in the IGnTA andIGnTC transcripts are different from those in theIGnTB transcripts. The Gly-to-Glu and Arg-to-His alterations resulting from the mutations in the encoded enzyme products are shown.
Mutations identified in theIGnT gene of adult i individuals.
(A) Mutations identified in the IGnTC gene of the adult i whites. IGnTC indicates the wild-type coding sequence of theIGnTC gene. ATG and TGA correspond to the respective translation start and stop codons. The mutant alleles identified in white subjects with the adult i phenotype, designatedIGnTC*505A and IGnTC*683A, possess 505G>A and 683G>A nucleotide substitutions, respectively, which predict the respective amino acid alterations of Ala169Thr and Arg228Gln. (B) Schematic representation of the mutations identified in theIGnT gene of adult i Taiwanese with congenital cataracts. The 2 G>A changes, identified, as reported previously, at the 1043 and 1148 nucleotide positions of the IGnTB gene, locate in the common exon 3 region of the 3 IGnT forms, and, thus, the 2 mutations also contribute to form the mutant IGnTA*1049A andIGnTC*1049A as well as IGnTA*1154A andIGnTC*1154A, respectively. Exons 1A and 1C possess 6 more coding nucleotides than the exon 1B does, and thus the nucleotide numbers of the same G>A changes in the IGnTA andIGnTC transcripts are different from those in theIGnTB transcripts. The Gly-to-Glu and Arg-to-His alterations resulting from the mutations in the encoded enzyme products are shown.
The IGnTC*505A and IGnTC*683A alleles are uncommon in the general white population
The incidence of the IGnTC*505A andIGnTC*683A alleles in the white population was evaluated using PCR-RFLP analyses. Genomic DNAs obtained from 51 randomly selected whites were screened. It was determined that one individual possessed one IGnTC*505A allele and that none had the 683G>A mutation in the IGnTC (data not shown). These results indicate that the 2 G>A mutations in the IGnTC gene are uncommon in this ethnic group. The low incidence of theIGnTC*505A and IGnTC*683A alleles agrees with the observed rarity of the adult i phenotype.
All 3 IGnT forms of the adult i Taiwanese individuals with congenital cataracts were mutated
Previously, the IGnTB genes of the 5 Taiwanese with the adult i phenotype were analyzed, and 3 different molecular changes were identified: missense mutations of 1043G>A and 1148G>A and gene deletion of the exon 1B, exon 2, and exon 3 regions.23 The 1043G>A and 1148G>A mutations predict the amino acid alterations of Gly348Glu and Arg383His, respectively, in the encoded IGnTB β6GlcNAcT enzyme. Pedigree analysis and enzyme function assay further demonstrated the association between these molecular changes and the adult i phenotype.
Because 2 new exon regions, 1A and 1C, which contribute to produce 2 novel IGnT forms, were identified in the present study, the samples from the adult i Taiwanese were reanalyzed. The exon 1A and 1C regions of subject W-3, demonstrated as heterozygous for theIGnTB*1043A/IGnTB*1148A genotype, were cloned and sequence-analyzed. No abnormalities were found in the exon 1A and 1C regions. The result indicates that, except for the 2 previously identified mutations, no additional mutation is present in the exon 1A and 1C regions of the adult i Taiwanese subject, W-3.
The 2 previously identified missense mutations, 1043G>A and 1148G>A, are located in the exon 3 region; however, the exon 3 for theIGnTB is also common to the IGnTA andIGnTC. Thus, the G>A change in the IGnTB*1043AcDNA also yields the IGnTA*1049A and IGnTC*1049Atranscripts, and the G>A change in the IGnTB*1148A cDNA also produces the IGnTA*1154A and IGnTC*1154Atranscripts (Figure 5B). The IGnTA*1049A, IGnTA*1154A, IGnTC*1049A, andIGnTC*1154A, as well as the IGnTB*1043A andIGnTB*1148A, transcript structures were demonstrated in the blood cell RNA sample from i adult W-3 with the use of RT-PCR.
For i adult C-3, it was demonstrated previously that the exon 1B, exon 2, and exon 3 regions were deleted. PCR amplifications of the genomic DNA sample from C-3 were performed to inspect the exon 1A and 1C regions. As expected, the exon 1C region was absent, but the exon 1A region appeared to be intact in the C-3 individual (data not shown). However, because of the deletion of the exon 2 and exon 3 regions, it is believed that the complete IGnTA transcript, as well as the IGnTB and IGnTC transcripts, are lacking in i adult C-3.
Thus, all 3 IGnT forms are mutated in these adult i Taiwanese. Subjects W-3 and W-5 are heterozygotes withIGnTA*1049A/IGnTA*1154A, IGnTB*1043A/IGnTB*1148A, andIGnTC*1049A/IGnTC*1154A genotypes, and S-6 and S-7 are homozygous for the IGnTA*1049A, IGnTB*1043A, and IGnTC*1049A alleles, whereas the 3 IGnTs are deleted completely or partially in C-3.
Activity of the enzymes encoded from the mutant IGnTgenes
The effects of the Ala169Thr and Arg228Gln changes, resulting from the 505G>A and 683G>A mutations in the IGnTC genes, respectively, on the GlcNAc-transferring activity were examined using the enzyme function assay. Although residual activity was observed, the enzyme activity of the products encoded from theIGnTC*505A and IGnTC*683A cDNAs was dramatically reduced when compared with that of the wild-typeIGnTC-encoded enzyme (Table1).
GlcNAcT activities of the enzymes encoded from the mutant IGnTA and IGnTCcDNAs
| . | GlcNAc transferred, pmol . |
|---|---|
| Vector | 26.7 ± 8.5 |
| IGnTA | 143.0 ± 5.4 |
| IGnTA*1049A | 23.2 ± 4.9 |
| IGnTA*1154A | 24.3 ± 3.9 |
| Vector | 7.2 ± 2.3 |
| IGnTC | 210.9 ± 63.5 |
| IGnTC*505A | 24.5 ± 5.1 |
| IGnTC*683A | 15.6 ± 3.0 |
| IGnTC*1049A | 12.6 ± 2.1 |
| IGnTC*1154A | 4.7 ± 6.0 |
| . | GlcNAc transferred, pmol . |
|---|---|
| Vector | 26.7 ± 8.5 |
| IGnTA | 143.0 ± 5.4 |
| IGnTA*1049A | 23.2 ± 4.9 |
| IGnTA*1154A | 24.3 ± 3.9 |
| Vector | 7.2 ± 2.3 |
| IGnTC | 210.9 ± 63.5 |
| IGnTC*505A | 24.5 ± 5.1 |
| IGnTC*683A | 15.6 ± 3.0 |
| IGnTC*1049A | 12.6 ± 2.1 |
| IGnTC*1154A | 4.7 ± 6.0 |
Results are the averages and standard deviations of 4 tests. Endogenous transfer of GlcNAc in the absence of acceptor substrate was corrected for each test.
In our previous report, the Gly348Glu and Arg383His alterations, predicted from 1043G>A and 1148G>A, respectively, totally eliminated the original enzyme activity of the IGnTB-encoded β6GlcNAcT. The 2 G>A changes in the mutant IGnTA andIGnTC genes also led to the Gly350Glu and Arg385His alterations, respectively, in their corresponding enzymes (Figure 5B). The activities of the enzymes encoded from theIGnTA*1049A and IGnTC*1049A and theIGnTA*1154A and IGnTC*1154A transcripts were inspected. The Gly350Glu and Arg385His changes in the C-terminal segments of theIGnTA- and IGnTC-encoded GlcNAcTs effectively eliminated the original GlcNAc-transferring activity, similar to the effects of the Gly348Glu and Arg383His changes on IGnTB β6GlcNAcT (Table 1).
IGnTC is the only one of 3 IGnT transcripts expressed in reticulocytes, whereas only the IGnTBtranscript is expressed in lens-epithelium cells
The expression of the 3 IGnT transcripts in reticulocytes, the RBC precursors, was examined using RT-PCR. Pure reticulocytes were isolated from white cell–depleted whole blood cells through positive selection of CD71 marker-expressing cells by immunomagnetic separation (as described in “Patients, materials, and methods”). The RT-PCR analyses revealed that the IGnTA, IGnTB, and IGnTC transcripts were detected in the RNA purified from whole blood cells, whereas only the IGnTCtranscript was detected in the RNA purified from reticulocytes (Figure6A), indicating that the IGnTC gene is the only one of the 3IGnT forms expressed in reticulocytes.
RT-PCR analyses of
IGnT gene expression in the RNA samples purified from whole blood cells and reticulocytes and from lens-epithelium cells. Pure reticulocytes were isolated from whole blood through positive isolation of cells expressing the transferrin receptor (CD71) by immunomagnetic separation, as described in “Patients, materials, and methods.” Total RNA from whole blood cells (the buffy coat layer) and reticulocytes (A) and lens-epithelium cells (B) was prepared, and RT-PCR analysis for the IGnTA, IGnTB, andIGnTC transcripts was conducted as described in “Patients, materials, and methods” and the legend of Figure 3.
RT-PCR analyses of
IGnT gene expression in the RNA samples purified from whole blood cells and reticulocytes and from lens-epithelium cells. Pure reticulocytes were isolated from whole blood through positive isolation of cells expressing the transferrin receptor (CD71) by immunomagnetic separation, as described in “Patients, materials, and methods.” Total RNA from whole blood cells (the buffy coat layer) and reticulocytes (A) and lens-epithelium cells (B) was prepared, and RT-PCR analysis for the IGnTA, IGnTB, andIGnTC transcripts was conducted as described in “Patients, materials, and methods” and the legend of Figure 3.
By contrast, RT-PCR analysis demonstrated only the expression of theIGnTB transcript in the RNA purified from human lens-epithelium cells (Figure 6B).
Discussion
The present study has demonstrated not only that the humanI locus expresses the previously reported IGnTtranscript, but also that 3 IGnT forms (IGnTA, IGnTB, and IGnTC), which have different exon 1 but identical exon 2 and exon 3 coding regions, are expressed from the human I locus. A similar molecular mechanism has been observed in the human UDP-glucuronosyltransferase UGT1Alocus, which contains at least 12 promoters/first exons, which can be spliced and joined with common exons 2 through 5, leading to the formation of 12 different isoforms.31 The enzyme encoded from the IGnTB gene has been shown to exhibit the I-β6GlcNAcT activity.27 31 When an acceptor substrate that mimics the structure of the i precursor was used in the enzyme function assay, similar or even higher GlcNAcT activity was demonstrated for the enzymes encoded from the novel IGnTAand IGnTC in comparison with IGnTB β6GlcNAcT, suggesting that the IGnTA and IGnTC enzymes also have I-β6GlcNAcT activity. Nevertheless, further enzyme characterizations for the novel GlcNAcTs encoded from the IGnTA and IGnTC forms are required to demonstrate their I β6GlcNAcT activity. Whether these 2 novel enzymes possess any other glycosyltransferase specificity other than the I-β6GlcNAcT activity is also worthy of further investigation.
It has been demonstrated that the expression of the I antigen is developmentally regulated and altered during the process of oncogenesis. At present, it has been determined that the humanI locus expresses 3 different IGnT forms and that the 3 IGnTs are expressed differentially in different tissues and cells. The 5′-RACE analyses revealed that theIGnTA, IGnTB, and IGnTC cDNAs did not have a common 5′ region, indicating that transcription of the 3IGnT forms may be determined by different DNA regulatory regions or by different regulatory mechanisms. Thus, when attempting to understand the expression of the I antigen in different tissues and cells and the mechanisms for the appearance and disappearance of the I antigen during developmental and oncogenetic processes, one should consider the functional roles of the 3 individual IGnTforms. Further, it may be of interest to examine the expression profiles for the various IGnT transcripts during cell-differentiation stages and to elucidate the regulatory mechanisms for each of the 3 IGnT forms to understand how the differential regulation of the IGnTs may be manipulated.
Although detailed enzyme characterization of the newly revealedI gene glycosyltransferase products remains indefinite and awaits further elucidation, this molecular genetic analysis of the human I locus provides insight into the questions derived from investigation of the adult i phenotype, including the partial association of the adult i phenotype with congenital cataracts.
It has long been noted that the human I locus is correlated with congenital cataracts. Although a significant association of the adult i phenotype with congenital cataracts has been demonstrated for Asians, it is not as pronounced for the white population. Thus, it has been suggested that the association results from a close linkage of 2 independent I- and cataract-related genes, rather than from a pleiotropic effect of the gene responsible for the adult i phenotype on the development of cataracts. It was further surmised that the association may be a result of deletion of a chromosomal region that encompasses the 2 independent I- and cataract-related genes. Nevertheless, we demonstrated in our previous study that nucleotide substitutions were the molecular origins of the adult i phenotype in Taiwanese with congenital cataracts, and this result highly suggests that the association may result from the pleiotropic effect of the same mutant gene.
The IGnT genotypes for the 5 adult i Taiwanese and 6 adult i whites, with and without congenital cataracts, respectively, together with their IGnT enzyme activities as inferred from the enzyme function assays, are presented in Table2. The adult i whites without congenital cataracts have wild-type IGnTA and IGnTB gene forms but possess a double dose of the mutant IGnTC genes, which encode enzyme product with markedly low GlcNAcT activity, whereas all 3 IGnT forms are mutated and all of the activities of the encoded GlcNAcTs are abolished in the adult i Taiwanese with congenital cataracts.
I genotypes and IGnT enzyme activities of the i adults without and with congenital cataracts
| i Adult group . | Patient no. . | Igenotype . | Enzyme activity . | ||||
|---|---|---|---|---|---|---|---|
| IGnTA . | IGnTB . | IGnTC . | IGnTA . | IGnTB . | IGnTC . | ||
| Without congenital cataracts | JCC | WT | WT | 505/505 | √ | √ | X |
| 97013 | ND | ND | 505/505 | ND | ND | X | |
| 987UI | ND | ND | 505/505 | ND | ND | X | |
| 944C2 | ND | ND | 505/505 | ND | ND | X | |
| 1110Y2 | ND | ND | 505/505 | ND | ND | X | |
| 1110X3 | WT | WT | 505/683 | √ | √ | X | |
| With congenital cataracts | W-3 | 1049/1154 | 1043/1148 | 1049/1154 | X | X | X |
| W-5 | 1049/1154 | 1043/1148 | 1049/1154 | X | X | X | |
| S-6 | 1049/1049 | 1043/1043 | 1049/1049 | X | X | X | |
| S-7 | 1049/1049 | 1043/1043 | 1049/1049 | X | X | X | |
| C-3 | Deletion | Deletion | Deletion | X | X | X | |
| i Adult group . | Patient no. . | Igenotype . | Enzyme activity . | ||||
|---|---|---|---|---|---|---|---|
| IGnTA . | IGnTB . | IGnTC . | IGnTA . | IGnTB . | IGnTC . | ||
| Without congenital cataracts | JCC | WT | WT | 505/505 | √ | √ | X |
| 97013 | ND | ND | 505/505 | ND | ND | X | |
| 987UI | ND | ND | 505/505 | ND | ND | X | |
| 944C2 | ND | ND | 505/505 | ND | ND | X | |
| 1110Y2 | ND | ND | 505/505 | ND | ND | X | |
| 1110X3 | WT | WT | 505/683 | √ | √ | X | |
| With congenital cataracts | W-3 | 1049/1154 | 1043/1148 | 1049/1154 | X | X | X |
| W-5 | 1049/1154 | 1043/1148 | 1049/1154 | X | X | X | |
| S-6 | 1049/1049 | 1043/1043 | 1049/1049 | X | X | X | |
| S-7 | 1049/1049 | 1043/1043 | 1049/1049 | X | X | X | |
| C-3 | Deletion | Deletion | Deletion | X | X | X | |
The numbers 505, 683, 1043, 1049, 1148, and 1154 represent the G > A change at respective nucleotide positions.
WT indicates wild type; ND, not determined; √, normal enzymatic activity, X, decreased enzymatic activity.
The first conclusion that can be drawn from these results is that the exact gene form responsible for the expression of the blood group I antigen on RBCs should be assigned to the IGnTC, not theIGnTB, as previously described.23 This conclusion is further supported by the observation thatIGnTC is the only one of the 3 IGnT forms expressed in reticulocytes, the RBC precursors.
Second, the molecular genetics of the human I locus support the proposition that more than one I-branching enzyme exists. The proposition that different I-branching enzymes may be responsible for I-antigen synthesis in different tissues is based on the presence of normal quantities of I antigen in the saliva, milk, and plasma of i adults.4,32 33 The 3 IGnT forms display differential expression patterns in different tissues and cells, as illustrated in Figures 3 and 6. We have analyzed the expression of theIGnT gene in human mammary and salivary glands by RT-PCR, with the results demonstrating that the IGnTB transcript is the predominant form of the 3 IGnT transcripts expressed in these 2 tissues (data not shown). If the IGnTB β6GlcNAcT is responsible for I-antigen formation in these 2 tissues, normal expression of this antigen in the milk and saliva of the i adults with defective IGnTC but normal IGnTB would be expected.
The most significant conclusion that can be drawn from these results is that a molecular genetic mechanism accounting for the partial association of the adult i phenotype with congenital cataracts is suggested. Mutation events that occur in the specific exon 1 region of the IGnT gene may lead to a defect in one form of IGnT enzyme activity in certain cell types that express the specificIGnT gene form, whereas those that occur in the common exon 2-3 region may result in elimination of the activity of all 3 of the IGnT enzymes. This is precisely what was observed for the 2 adult i groups with or without congenital cataracts, and the molecular events observed for the 2 groups are consistent. The human lens cells are differentiated from the surface of a single layer of epithelium cells. RT-PCR analysis detected only IGnTC transcript in the RNA purified from reticulocytes, however, with only the IGnTBexpressed in the human lens-epithelium cells (Figure 6). A defect inIGnTC gene function leads to the absence of I antigen in RBCs, whereas congenital cataracts occur in those i adults in whom all 3 IGnT-enzyme functions are defective, but not in analogs in which only the IGnTC form is defective (Table 2).
The molecular genetics of the human I locus revealed in the present study provide a new view of the formation and expression of the I antigen. The architecture of the I-gene structures and the results obtained from the molecular analysis of the i adults answer many questions about the human I gene, the adult i phenotype, and the relationship with congenital cataracts. The most interesting deduction is that the I β6GlcNAcT activity of the humanI gene (possibly from the IGnTB gene) may play an essential role in maintaining lens transparency. It should be noted, however, that the lenses used for the RT-PCR analysis were from adults, and the question of whether the expression of IGnTB in the lens occurs during human embryonic development is left for future consideration. In addition, although the results of the present investigation are highly suggestive, direct evidence implicating anI-gene defect in the development of congenital cataracts is still lacking. The requisite evidence may be obtained through a gene knockout experiment in a mouse model. Further investigation to elucidate the functional role of I β6GlcNAcT activity for maintenance of lens transparency will be significant.
Prepublished online as Blood First Edition Paper, November 7, 2002; DOI 10.1182/blood- 2002-09-2693.
Supported in part by National Health Research Institute grant NHRI-EX90- 8601SL (M.L.) and National Science Council grant NSC 91-2314-B-002-405 and National Research Program for Genomic Medicine of National Science Council grant NSC 91-3112-B-002-028 (L.-C.Y.).
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Marie Lin, Transfusion Medicine Laboratory, Mackay Memorial Hospital, 45 Ming-San Rd, Tamshui, Taipei County 251, Taiwan; e-mail: marilin@ms2.mmh.org.tw.


![Fig. 4. The GlcNAcT activities of the enzymes encoded from the IGnTA, IGnTB, andIGnTC cDNAs. / The protein products encoded from the IGnTA, IGnTB, and IGnTC cDNAs were expressed in mammalian cells (COS-7). The amounts of GlcNAc transferred to the acceptor substrate (LS-tetrasacchride c [NeuNAca2-6Galb1-4GlcNAcb1-3Galb1-4Glc]) from the donor substrate (UDP-GlcNAc) by the medium concentrates harvested from the cells transfected with respective expression vectors were measured as has been described previously.23 The results of the average and standard deviation (indicated by error bars) of 4 tests are diagrammatically represented. Endogenous transfer of GlcNAc in the absence of acceptor substrate was corrected for each test. The amounts of the transferred GlcNAc in the vector control, pSecTaq2A, indicate the background levels of the assay, which are believed to result from the addition of the acceptor substrate.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/6/10.1182_blood-2002-09-2693/4/m_h80634017004.jpeg?Expires=1769146367&Signature=T-SGMIwc4rvcNbqxv6geDHqHySU2ZNMEiPsgNinkmZhVhMWMXHfJ~buEsa1ffWeQmIiMi2LwoqVV6DuKkwFwvRrqeM3l3Zcj1GpNREv1e1mQAyfSZlN~QW4bqXMJyspbDUsL~AsQDQN-KaXeXkRszDT3C81k3gmTRCb3E9eRwvxNySTQzdfFa9E8fXm0fM0CbtZP1cYu0a4LryJtCJ6YkVtMrvfypq29y-I~mF~DVNb6t1mM-TGTtqvsTYb0SHxsBFv7H9RiKH-sxQP84s18dnxlae9KbxYW18xtZXxiVhNoWnCFIUhrxSvph3aJGpnO~6kG54GNUojZca-1C7YjKQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal