The development of molecularly targeted treatments of adult leukemias warrants investigation of these targets in similar pediatric leukemias. The NF1 tumor suppressor gene, which encodes a GTPase activating protein for p21ras, is frequently inactivated in juvenile myelomonocytic leukemia (JMML). Other patients with JMML acquire activating RAS gene mutations. Recipient mice reconstituted with Nf1−/− fetal hematopoietic cells develop a myeloproliferative disease (MPD) that models the human disease. JMML arises from clonal expansion of a hematopoietic stem cell, and JMML cells and murineNf1−/− hematopoietic cells are hypersensitive to granulocyte macrophage-colony stimulating factor and KitL, the ligand for c-kit. We generated embryos doubly mutant for theWv allele of c-kit and Nf1 to ask if reduction of c-kit activity would delay or prevent the development of MPD. Despite a reduction in c-kit activity to approximately 10% of wild-type levels,Nf1−/−;Wv/Wvcells induced MPD in recipient mice.
Introduction
Mutations in the NF1 tumor suppressor gene cause neurofibromatosis type I (NF1). NF1encodes neurofibromin, which negatively regulates p21rasactivity by accelerating the conversion of active p21ras-GTP to inactive p21ras-GDP.1,2 Children with NF1 are at markedly increased risk of developing juvenile myelomonocytic leukemia (JMML), for which existing treatments are largely ineffective.3,4 Although Nf1 knockout mice (Nf1−/−) die in utero around day embryonic (E)13.5, recipients of transplantedNf1−/− fetal hematopoietic stem cells (HSCs) develop myeloproliferative disease (MPD) that closely models JMML.5,6 This model has been exploited for preclinical studies of therapeutics that target hyperactive p21ras.7
Murine Nf1−/− fetal liver cells form excessive numbers of myeloid progenitors in methycellulose cultures containing low concentrations of kit ligand (KitL),8 and we recently found that Nf1−/− HSCs have a self-renewal advantage in vivo.9 KitL is a potent survival and proliferative growth factor for both HSCs and myeloid progenitors (reviewed in Broudy10). Inasmuch as JMML often arises from clonal expansion of a HSC and that Nf1−/−myeloid progenitors are hypersensitive to KitL, we hypothesized that genetic inhibition of the KitL/c-kit pathway would alter the progression of MPD in recipients of Nf1−/−fetal liver cells. Evaluating c-kit as a therapeutic target is an important priority as the tyrosine kinase inhibitor (imatinib mesylate) induces regression in gastrointestinal stromal tumors (GISTs), a malignancy characterized by mutations that constitutively activate the c-kit kinase.11 To assess if impairing c-kit function would modulate the MPD induced by Nf1 inactivation, we generated fetal stem cells that were doubly mutant atNf1, and the dominant white spotting (W)locus, which encodes c-kit, performed adoptive transfers and monitored the recipient mice for development of MPD. We find that theWv mutation, which reduces c-kit kinase activity by approximately 90%, does not suppress the capacity ofNf1−/− fetal liver cells to induce MPD.
Study design
Animals and adoptive transfer procedure
Nf1+/− mice in a C57BL/6.129 background were backcrossed for 13 generations into C57BL/6J mice, which were purchased from Jackson Laboratories (Bar Harbor, ME).Wv/Wv mice were purchased from Jackson Laboratories in a C57BL/6J strain. Studies were conducted with a protocol approved by the Indiana University Animal Care and Use Committee. The Nf1 and Wv alleles were genotyped as previously described.8 12 The crosses used to generate day-E13.5 fetal livers, which contain the experimental groups, are outlined here. Filial (F)0:Nf1+/−; Wv+/+ ×Nf1+/+;Wv/Wv.F1: Nf1+/−;Wv/+ ×Nf1+/−;Wv/+. F2 experimental groups: Nf1+/+;Wv+/+,Nf1−/−;Wv+/+, Nf1+/+;Wv/Wv,Nf1−/−;Wv/Wv.
Syngeneic recipients were given transplants of 3 million day-E13.5 fetal liver cells generated from the intercrosses following irradiation with 1100 cGy as previously described.9Animals that underwent transplantation were followed for signs of MPD,9 and photomicrographs of spleen sections were taken with an Olympus DP11 (Melville, NY).
Progenitor assays
Colonies derived from myeloid progenitors were enumerated exactly as described.9 Recombinant granulocyte macrophage-colony stimulating factor (GM-CSF), interleukin-3 (IL-3), and KitL were purchased from Peprotech (Rocky Hill, NJ), and recombinant IL-1 and M-CSF were obtained from R&D Systems (Minneapolis, MN). Cells and recombinant growth factors were added to agar for growth of low proliferating potential–colony forming cells (LPP-CFCs) and high proliferating potential–colony forming cells (HPP-CFCs), and the solution was thoroughly admixed before plating. LPP-CFCs and HPP-CFCs were enumerated in 8% CO2 and 5% O2. LPP-CFCs and HPP-CFCs were scored by indirect microscopy on days 7 and 14, respectively.
Results and discussion
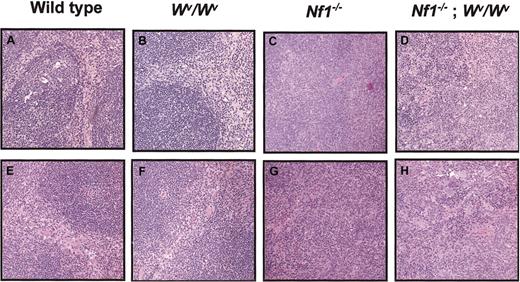
Of 300 embryos generated from the Wv ×Nf1 intercross, only 3 were doubly mutant (Nf1−/−;Wv/Wv) at day E13.5, and fetal liver cell numbers were markedly reduced. This is consistent with previous studies usingWv/Wv mice.12 Despite these obstacles, one recipient received a transplant successfully from each doubly mutant embryo; 2 of the recipients developed white blood cell (WBC) counts of 0.045 and 0.050 × 109/L ([45 000 and 50 000 per mm3] compared with an average WBC count of 0.017 × 109/L (17 000 per mm3) in recipients of wild-type fetal liver cells; n = 10). Each mouse died at 2 months of age and had gross splenomegaly upon inspection, which is consistent with MPD previously observed in recipients ofNf1−/−-deficient fetal liver cells.6,9 The third recipient of transplantedNf1−/−;Wv/Wv cells survived to 4 months of age, which allowed a more detailed analysis. The spleen weight and cellularity were markedly increased compared with the wild type and Wv/Wv controls and were comparable to mice that received transplants ofNf1−/− fetal stem cells (Table1). The MPD in mice that received transplants of Nf1−/− cells is characterized by a dramatic increase in the numbers of splenic HPP-CFCs and LPP-CFCs.9 The numbers of HPP-CFCs and LPP-CFCs were similar in the spleens of recipients injected with singly mutantNf1−/−orNf1−/−;Wv/Wv cells and were increased 30-fold (HPP-CFCs) and 10-fold (LPP-CFCs) versus mice that received transplants of wild-type orWv/Wv cells (Table 1). Finally, the spleens of the recipients of transplantedNf1−/−;Wv/Wv orNf1−/− cells demonstrated myeloid cell infiltration and effacement of splenic architecture, which is consistent with development of MPD (Figure 1A-D).
Effect of Wv and Nf1genotypes on spleen weight, splenic cellularity, WBC count, and total number of HPP-CFCs and LPP-CFCs per spleen in primary recipients
| . | Genotype . | |||
|---|---|---|---|---|
| Nf1+/+;Wv+/+ . | Wv/Wv;Nf1+/+ . | Wv+/+;Nf1−/− . | Wv/Wv;Nf1−/− . | |
| Spleen weight, mg | 73.0 | 66.0 | 294.0 | 298.0 |
| Splenic cellularity, total cells × 108 | 1.41 | 1.06 | 4.24 | 4.91 |
| WBC count, ×106 | 18.3 | 17.2 | 17.5 | 6.6 |
| Total HPP-CFC/spleen | 1300 ± 650 | 1663 ± 286 | 17 453 ± 2717* | 24 240 ± 1 171* |
| Total LPP-CFC/spleen | 6500 ± 1300 | 6995 ± 1116 | 192 610 ± 15 571* | 196 220 ± 11 953* |
| . | Genotype . | |||
|---|---|---|---|---|
| Nf1+/+;Wv+/+ . | Wv/Wv;Nf1+/+ . | Wv+/+;Nf1−/− . | Wv/Wv;Nf1−/− . | |
| Spleen weight, mg | 73.0 | 66.0 | 294.0 | 298.0 |
| Splenic cellularity, total cells × 108 | 1.41 | 1.06 | 4.24 | 4.91 |
| WBC count, ×106 | 18.3 | 17.2 | 17.5 | 6.6 |
| Total HPP-CFC/spleen | 1300 ± 650 | 1663 ± 286 | 17 453 ± 2717* | 24 240 ± 1 171* |
| Total LPP-CFC/spleen | 6500 ± 1300 | 6995 ± 1116 | 192 610 ± 15 571* | 196 220 ± 11 953* |
Spleen weight, splenic cellularity, white blood cell counts, and total numbers of HPP-CFCs and LPP-CFCs per spleen were measured in primary recipients of transplanted fetal liver cells from the 4 F2 experimental groups (outlined in “Study design”) 4 months after transplantation. Total numbers of HPP-CFCs and LPP-CFCs per spleen represent the mean number of colonies ± SEM in 3 replicate experiments from spleen cells harvested from the same primary recipient.
P < .05 for comparisons ofNf1−/−;Wv/Wv orNf1−/−;Wv+/+ versusNf1+/+;Wv+/+ primary recipients by paired Student t test.
Effects of Wv andNf1 genotypes on splenic architecture in primary and secondary recipients.
(A-D) Low-power view (original magnification × 10) of spleen sections from primary recipients of transplanted fetal liver cells from the 4 F2 experimental groups outlined in “Study design.” Recipients of transplanted Nf1+/+;Wv+/+and Nf1+/+;Wv/Wv fetal liver cells show a normal distribution of red pulp and white pulp (A-B). In contrast, recipients of transplantedNf1−/−;Wv/Wv orNf1−/−;Wv+/+ fetal liver cells showed expansion of the red pulp with loss of normal splenic architecture (C-D). (E-H ) A representative low-power view (original magnification × 10) of spleen sections from secondary recipients of transplanted low-density mononuclear cells harvested from the bone marrow of primary recipients of transplanted fetal liver cells from the 4 F2 experimental groups. Secondary recipients of transplantedNf1+/+;Wv+/+ andNf1+/+;Wv/Wv LDMNCs show a normal distribution of red pulp and white pulp (n = 7 for each genotype) (E-F). In contrast, secondary recipients of transplantedNf1−/−;Wv/Wv orNf1−/−;Wv+/+ LDMNCs showed expansion of the red pulp with loss of normal splenic architecture (n = 7 for each genotype) (G-H).
Effects of Wv andNf1 genotypes on splenic architecture in primary and secondary recipients.
(A-D) Low-power view (original magnification × 10) of spleen sections from primary recipients of transplanted fetal liver cells from the 4 F2 experimental groups outlined in “Study design.” Recipients of transplanted Nf1+/+;Wv+/+and Nf1+/+;Wv/Wv fetal liver cells show a normal distribution of red pulp and white pulp (A-B). In contrast, recipients of transplantedNf1−/−;Wv/Wv orNf1−/−;Wv+/+ fetal liver cells showed expansion of the red pulp with loss of normal splenic architecture (C-D). (E-H ) A representative low-power view (original magnification × 10) of spleen sections from secondary recipients of transplanted low-density mononuclear cells harvested from the bone marrow of primary recipients of transplanted fetal liver cells from the 4 F2 experimental groups. Secondary recipients of transplantedNf1+/+;Wv+/+ andNf1+/+;Wv/Wv LDMNCs show a normal distribution of red pulp and white pulp (n = 7 for each genotype) (E-F). In contrast, secondary recipients of transplantedNf1−/−;Wv/Wv orNf1−/−;Wv+/+ LDMNCs showed expansion of the red pulp with loss of normal splenic architecture (n = 7 for each genotype) (G-H).
Studies in which bone marrow cells from primary recipients were transferred into secondary hosts showed that Nf1inactivation is associated with enhanced proliferative potential in repopulating stem cells.9 To examine whether the reduction in c-kit activity altered this previously defined gain of function potential, we isolated low-density marrow mononuclear cells from each mouse of the 4 F2 experimental groups and transplanted these cells into secondary recipients. Mice were killed at 6 months of age, and spleens were examined for evidence of MPD. All recipients of transplantedNf1−/−;Wv/Wv orNf1−/− cells (n = 8) developed a dramatic myeloid cell infiltration of the spleen consistent with MPD as previously observed in the primary recipients and in prior studies. A representative spleen section from each experimental group is shown in Figure 1E-H.
In vitro hypersensitivity to GM-CSF in methycellulose cultures is a hallmark of human JMML blood and marrow cells, a characteristic that is also observed in Nf1−/− fetal liver cells.5,6 Experiments in which Nf1 andGmcsf mutant mice were mated to generate cells for adoptive transfer demonstrated a central role of this aberrant response to GM-CSF in the pathogenesis of MPD.13Nf1−/− hematopoietic cells are also hypersensitive to KitL in colony-forming assays, and KitL and GM-CSF act synergistically to enhance myeloid progenitor growth from wild-type and Nf1−/− cells.8 These data, the availability of imatinib mesylate for clinical trials, and the poor prognosis for patients with JMML led us to ask if reducing c-kit activity might modulate the course of MPD in recipients ofNf1−/− fetal liver cells. We pursued a genetic approach to this question because imatinib inhibits c-Abl and β chain of the platelet-derived growth factor receptor in addition to c-kit.11 Our data showing thatNf1−/−;Wv/Wv fetal liver cells induced MPD suggest that this disorder is not dependent on c-kit signaling. Alternatively, 10% of normal c-kit activity may be sufficient for leukemogenesis, whereas complete inhibition might interfere with the growth of JMML cells in vivo. However, this possibility is highly unlikely sinceW41/W41;Nf1−/−hematopoietic cells (W41 mutants have a 70% reduction in c-kit activity vs 90% for Wvstrains) are not hypersensitive to KitL in vivo as determined in both myeloid colony-forming assays and competitive repopulation assays, which measure HSC function (data not shown). It also possible that c-kit inhibitors will be ineffective as single agents in JMML, but will show synergistic activity with drugs that target GM-CSF signaling such as the peptidomimetic E12R.14 These hypotheses can be tested by treating mice that received transplants ofNf1−/− cells with imatinib mesylate or with other pharmacologic inhibitors of c-kit. Recently, a heterozygous environment was shown to contribute to the development of neurofibromas in mice in which Nf1 was selectively ablated in Schwann cells.15 Heterozygous Nf1 mutant cells infiltrate these lesions, and these cells demonstrate deregulated p21ras signaling in response to KitL.16 17Thus, although our data do not support a role for c-kit inhibitors in JMML, drugs that interfere with this pathway might prove useful for treating other complications of NF1.
We are indebted to Dr Tyler Jacks for providingNf1 mutant mice, and thank Marsha Hippensteel for exceptional administrative support.
Prepublished online as Blood First Edition Paper, October 17, 2002; DOI 10.1182/blood-2002-08-2635.
Supported by National Institutes of Health (NIH) grants 1 KO8 CA096579-01 and K12-HD00850 (D.A.I.), 2 R01 CA74177-06 (D.W.C.), NIDDK P30 DK49218 (D.W.C.), American Cancer Society (ACS) grant RSG-96-104-06 LIB (D.W.C., K.S.), Department of Defense (DOD) grant DAMD17-01-1-0711 (D.A.I., D.W.C.), and NIH grant R01 CA762714 (K.S.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
David A. Ingram, Indiana University School of Medicine, Herman B. Wells Center for Pediatric Research, 1044 W Walnut St, R4/470, Indianapolis, IN 46202; e-mail:dingram@iupui.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal