Elevated circulatory levels of many blood coagulation factors are known to be a risk factor for deep vein thrombosis in humans. Here we report the first direct demonstration of a close association between elevated circulatory factor IX levels in mice with thrombosis as well as myocardial fibrosis. Transgenic mice overexpressing human factor IX at persistently high levels died at much younger ages than their cohorts expressing lower levels, or nontransgenic control animals. The median survival age of animals was inversely related to the circulatory levels of human factor IX. Prematurely dying animals had focal fibrotic lesions predominantly present in the left ventricular myocardium, and vasculatures in these lesions showed fibrin deposition. Thromboemboli were also present in other organs, including lung and brain. These observations support the hypothesis that persistently high circulatory levels of factor IX are a risk factor not only for thrombosis and/or thromboembolism, but also for myocardial fibrosis mimicking human myocardial infarction.
Introduction
Elevated circulatory levels of blood coagulation factors have been implicated as a risk factor for deep vein thrombosis.1 More recently, factor VIII, factor XI, and factor IX (FIX) have also been shown to be significantly associated with deep vein thrombosis in humans.2-4 Furthermore, thrombosis-induced myocardial infarction has been suggested in humans.5 6 However, no prior direct evidence or animal models have demonstrated the involvement of elevated circulatory levels of specific procoagulant factors in the development of myocardial fibrosis and infarction.
FIX plays an important role in blood coagulation in that it is proteolytically activated by FXIa of the intrinsic pathway, as well as by the tissue factor–FVIIa complex in the extrinsic pathway.7,8 Deficiency of human factor IX (hFIX) results in abnormal bleeding (hemophilia B) in humans. This was recapitulated in mice where the endogenous murine FIX (mFIX) gene was inactivated.9,10 Circulatory levels of both hFIX and mFIX increase with advancing age.11 We recently established the basic genetic mechanisms responsible for age-related increase and stable patterns of hFIX and hPC gene expression, respectively.12,13 The mechanisms involve 2 critical genetic elements, ASE (age-related stability element) and AIE (age-related increase element). Presence of both ASE and AIE are responsible for the age-associated increase in the hFIX circulatory levels,12 although ASE alone is responsible for the age-associated stability of the hPC circulatory levels.13In these studies, we made the critical observation that the transgenic animals carrying hFIX minigenes resulting in persistently elevated levels of circulatory hFIX have a higher incidence of premature death.
In this paper, we report the direct evidence of a close association between persistently elevated levels of circulatory FIX and thrombosis with myocardial fibrosis in transgenic mouse models, mimicking thrombosis-mediated myocardial infarction in humans.
Study design
Transgenic mice
Construction of hFIX minigene expression vectors −416FIXm1, −802FIXm1, −2231FIXm1, −802FIXm1/1.4, and −2231FIXm1/1.4, and the generation of transgenic mice, both founders and subsequent generations, were as previously described.12 Human FIX minigenes were comprised of the promoter region including up to nucleotide (nt) −416, nt −802, or nt −2231, exon I-VIII with the middle portion–deleted intron I at its natural position and the immediate 3′ flanking region (380 base pair [bp] or 1.2 kilobase [kb]) of the hFIX gene. The genetic background of these transgenic animals was C57BL/6 × SJL. Each founderline was backcrossed with nontransgenic C57BL/6 × SJL animals, generating subsequent generations. Both male and female animals, approximately proportional in number, were subjected to systematic longitudinal observation for any signs of distress and sickness. All animal experiments were carried out in accordance with the institutional guidelines for animal use of the University of Michigan (Office for Protection from Research Risks no. A3114-01).
Determination of circulatory hFIX levels
Circulatory hFIX levels were measured by enzyme-linked immunosorbent assay (ELISA) as previously described.12Blood samples in aliquots of approximately 100 μL were collected via tail-tip snipping and serum samples prepared were used for quantification of circulatory hFIX levels by duplicated ELISA.
Histologic and immunohistochemical analyses
Animals were killed at various ages and subjected to histologic analyses. Animals were anesthetized with isoflurane and the inferior vena cava was accessed by midabdominal incision. To prevent postmortem clot formation, 340 U heparin (100 μL) was injected via vena cava into these animals. After 30 seconds, blood samples were drawn and 10% buffered formaldehyde was injected via vena cava. Organs were taken and fixed in 10% buffered fomaldehyde and paraffin embedded. Tissue was cut into 5-μm sections, deparaffinized in xylene, rehydrated in 15 mM sodium phosphate saline, and stained with hematoxilin and eosin (HE) as well as Mallory phosphotungstic acid hematoxylin (PTAH),14elastica van Gieson (EVG), and congo red. Immunohistochemistry was performed on deparaffinized tissues using monoclonal antifibrin antibody specific to fibrin (IM0541 from Immunotech, BP177-13276 Marseille, France) at 1:100 dilution15and immunoperoxidase staining using Vector M.O.M. Immunodetection Kit (Vector Laboratories, Burlingame, CA).
Measurement of tumor necrosis factor α (TNF-α), IL-6, and IL-1 levels
Murine TNF-α, interleukin (IL)–6, and IL-1 were determined by using an ELISA kit for quantification of cytokines (R&D Systems, Minneapolis, MN) according to the manufacturer's instructions.
Results and discussion
Through systematic analyses of transgenic mice (N = 223) generated from 4 independent founder animal lines carrying hFIX minigenes −802FIXm1, −2231FIXm1, −802FIXm1/1.4, or −2231hFIXm1/1.4,12 we found a striking inverse relationship between persistent circulatory hFIX levels and median age of death (Figure1). As expected from the transgene positional effects, transgenic animals expressed hFIX in a wide range of levels, from nondetectable to as high as 5000 ng/mL serum. Based on the hFIX expression level, animals were arbitrarily grouped into 4 categories. Animals producing hFIX at 1500 ng/mL serum or higher (n = 39) showed a rapid reduction in survival with a 50% death rate by 5 months of age and only 7% survival at 22 months of age. Animals persistently expressing hFIX in a range of 700 to 1500 ng/mL serum showed a 50% survival rate at 18.5 months of age, and only 25% survival at 24 months of age (n = 40). Animals stably expressing hFIX in a range of 200 to 700 ng/mL serum (n = 44) showed an overall survival rate of 62% at 24 months of age. Nontransgenic control animals (n = 22) and transgenic animals expressing hFIX less than 200 ng/mL serum (n = 20) lived normal life spans (more than 24 months). Animals carrying minigene −416FIXm1, which does not contain ASE, showed high levels of circulatory hFIX as high as 5000 ng/mL serum at the first month of life, followed by a rapid decrease through and after puberty. These animals also lived normal life spans.12 Together, these findings indicated that premature early death requires persistently high levels of circulatory hFIX (on top of their own mFIX). There was no sex effect observed on the survival rate of these animals. Male animals, producing hFIX in the null mFIX background, which were generated by cross-breeding of male transgenic animals producing hFIX at high levels (up to 2871 ng/mL serum) with mFIX-deficient female animals,9 lived healthy normal life spans, indicating that hFIX can substitute mFIX without any detrimental effects on these animals (data not shown).
Kaplan-Meier analysis of survival curves of transgenic mice expressing various levels of hFIX.
Kaplan-Meier survival analysis was performed using GraphPad Prism3 (San Diego, CA) and the log-rank test. Blue, red, light blue, and green lines represent the survival with various levels of circulatory hFIX (ng/mL serum) (P < .005).
Kaplan-Meier analysis of survival curves of transgenic mice expressing various levels of hFIX.
Kaplan-Meier survival analysis was performed using GraphPad Prism3 (San Diego, CA) and the log-rank test. Blue, red, light blue, and green lines represent the survival with various levels of circulatory hFIX (ng/mL serum) (P < .005).
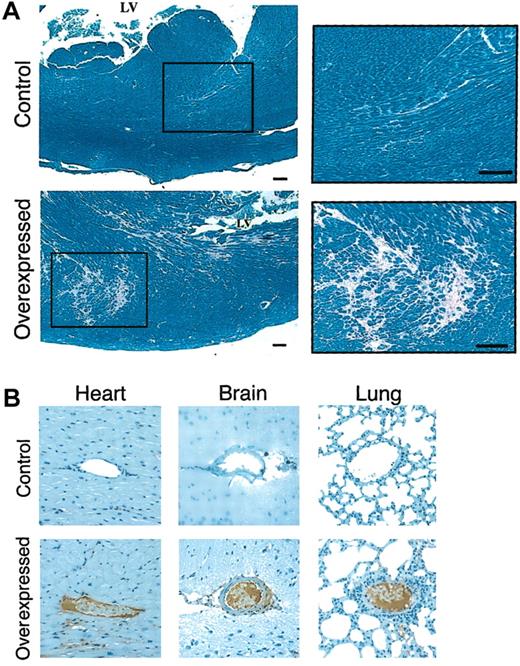
We then analyzed the etiology of the increased mortality rate among animals with persistently high hFIX levels in addition to endogenous mFIX by examining histologic sections of surviving 3- to 24-month old transgenic mice (n = 51). Regardless of the presence of the hFIX transgene, we observed dermatitis in some old age mice. Such dermatitis, however, apparently did not significantly affect animal life span. Our most important observation was that animals expressing high circulatory levels of hFIX (> 1500 ng/mL serum) had various degrees of myocardial fibrosis around cardiac vasculature, predominantly located in the myocardium of the left ventricle (Figure2A). Fibrosis replaced normal myocardium in multifocal patterns. No myocardial fibrosis, however, was found on the left ventricular endocardial surface. The observed intramyocardial distribution patterns of fibrosis may be due to ischemic injury from occlusion of small vessels and subsequent reperfusion.
Histochemical analysis of animals.
(A) Histochemical analysis of myocardium in mice expressing low and high levels of hFIX. Tissue samples were stained with PTAH.14 Control indicates the normal left ventricle in a mouse expressing low levels of hFIX. Area surrounded by a rectangle is enlarged for higher magnification in the right panel. Overexpressed indicates the corresponding left ventricle with multifocal myocardial fibrosis in a mouse expressing high levels of hFIX. Area surrounded by a rectangle is enlarged for higher magnification in the right panel. Scale bars represent 100 μm. (B) Immunohistolochemical analyses of tissues from animals expressing low and high levels of hFIX. Tissue samples were stained with fibrin-specific monoclonal antibody.15 Heart indicates fibrin-stained normal myocardial vessel of control mouse, and fibrin depositions on endothelium of a vessel in a high hFIX–expressing mouse. Brain indicates normal vessel of control mouse, and abnormal vessel occluded by thrombus of high hFIX–expressing mouse. Lung indicates normal vessel of control mouse, and fibrin-positive thrombotic material–filled abnormal vessel. Original magnification, × 200.
Histochemical analysis of animals.
(A) Histochemical analysis of myocardium in mice expressing low and high levels of hFIX. Tissue samples were stained with PTAH.14 Control indicates the normal left ventricle in a mouse expressing low levels of hFIX. Area surrounded by a rectangle is enlarged for higher magnification in the right panel. Overexpressed indicates the corresponding left ventricle with multifocal myocardial fibrosis in a mouse expressing high levels of hFIX. Area surrounded by a rectangle is enlarged for higher magnification in the right panel. Scale bars represent 100 μm. (B) Immunohistolochemical analyses of tissues from animals expressing low and high levels of hFIX. Tissue samples were stained with fibrin-specific monoclonal antibody.15 Heart indicates fibrin-stained normal myocardial vessel of control mouse, and fibrin depositions on endothelium of a vessel in a high hFIX–expressing mouse. Brain indicates normal vessel of control mouse, and abnormal vessel occluded by thrombus of high hFIX–expressing mouse. Lung indicates normal vessel of control mouse, and fibrin-positive thrombotic material–filled abnormal vessel. Original magnification, × 200.
Immunohistochemical analyses using fibrin-specific monoclonal antibodies, which do not recognize fibrinogen, identified fibrin deposits located in the myocardial vasculature of fibrotic lesions (Figure 2B, heart, overexpressed). Fibrin deposits were found with or without collapse of vasculature. Such fibrin deposits were not found at any age in the vasculature of nontransgenic control animals or animals expressing only low levels of hFIX (Figure 2B, control heart). These findings are consistent with the etiology of elevated procoagulant activity due to hFIX overexpression in these animals. The severity and multifocal distribution of myocardial fibrosis in these animals are closely correlated with persistently elevated levels of circulatory hFIX. Thrombotic occlusions of cardiac vasculature in the myocardium apparently caused local cell injury, thus inducing local fibrosis in a predominantly focal pattern. We found no evidence of atherosclerosis in the transgenic animals expressing high levels of hFIX. Elevated levels of FIX activation peptide were detected in humans during acute myocardial infarction and unstable angina.16 Futher study remains to be done regarding any elevated proteolytic activation of FIX, both hFIX and mFIX, in transgenic mice.
Histochemical analyses of various other organs of transgenic animals with myocardial fibrosis, including brain and lung, also showed thrombotic occlusion of vasculatures (Figure 2B), indicating that local thrombotic events or thromboembolisms are widespread in animals with elevated levels of circulatory hFIX. These findings suggest that increased circulatory levels of hFIX, in addition to endogenous mFIX, might have tipped the balance between procoagulant and anticoagulant activities toward a hypercoagulable state, thus causing multiple thrombosis in various organ tissues, including heart, lung, and brain. Alternatively, thrombotic occlusions of vasculatures observed in some of these organs may be due to thromboembolisms. Further studies are required to determine which of these mechanisms is primarily responsible for induction of such occlusions in systemic organs.
Inflammatory mediators such as IL-1, IL-6, and TNF are known to play roles in vascular diseases and thrombosis.17 Transgenic animals at various ages that developed myocardial fibrosis showed no significant differences in circulatory levels of these mediators in comparison with control animals or transgenic animals with normal hearts (data not shown). This may reflect in part that at the time point of animal killing for tissue analysis, transient inflammation with or without necrosis, which might have accompanied the initial thrombotic vascular occlusions, had already resolved. Further study is needed to test this possibility. Although it is very difficult to identify animals that have acute thrombotic events at early stages, those results are consistent with the above conclusion that the major etiology of the observed myocardial fibrosis is chronic accumulation of multiple local thrombi and/or thromboemboli resulting from the hypercoagulable state due to the elevated circulatory FIX levels.
We now have constructed a mouse model for age-dependent myocardial fibrosis caused by elevated hFIX levels that mimics human myocardial infarction. This is the first experimental demonstration of a tight, inverse correlation between elevated levels of circulatory FIX and life span. This animal model, together with the other animal models, such as that created from thrombomodulin mutation,18 may be valuable not only for studying the basic biology of hypercoagulable states due to the increased levels of a specific coagulation factor, but also for development of better prevention and therapy for myocardial disorders. Transgenic mouse experiments in progress focusing on overexpression of other pro- and anticoagulant factors as well as fibrinolysis factors may provide additional animal models useful for studying disorders of the cardiovascular system.
We thank Dr D. Stafford for providing FIX null mice, Dr R. Rosenberg for coaching preparation of histologic specimens, and Dr M. Levine, J. Huo, and A. Kurachi for critical reading of the manuscript.
Prepublished online as Blood First Edition Paper, October 24, 2002; DOI 10.1182/blood-2002- 05-1581.
Supported in part by National Institutes of Health (NIH) grants HL64522 and HL38644, the Multipurpose Arthritis Center of the University of Michigan (NIH grant 5P60 AR20557), the Michigan Diabetes Research and Training Center (NIH grant 5P60 DK20572), and the University of Michigan General Clinical Research Center (NIH grant MO1 RR00042).
A.A. and S.K. contributed equally to this report.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Kotoku Kurachi, Age-Dimension Research Center, National Institute of Advanced Industrial Science and Technology, AIST Tsukuba Central 4th site, 1-1-1 Higashi, Tsukuba City, Ibaraki, Japan; e-mail: kkurachi@umich.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal