Dendritic cells (DCs) are potent antigen-presenting cells that likely play multiple roles in human immunodeficiency virus type 1 (HIV-1) pathogenesis. We used the simian immunodeficiency virus (SIV)/macaque model to study the effects of infection on homeostatic chemokine expression and DC localization directly in secondary lymphoid tissues. SIV infection altered the expression of chemokines (CCL19/MIP-3β, CCL21/ 6Ckine, and CCL20/MIP-3α) and of chemokine receptors (CCR7 and CCR6) that drive DC trafficking. CCL19/MIP-3β, CCL20/MIP-3α, CCR6, and CCR7 expression increased in lymph nodes during the early systemic burst of viral replication (acute infection), whereas CCL21/6Ckine expression progressively decreased throughout disease to AIDS. Parallel with the SIV-induced perturbations in chemokine expression were changes in the expression of the DC-associated markers, DC-SIGN, DC-LAMP, and DECTIN-1. During AIDS, DC-LAMP mRNA expression levels were significantly reduced in lymph nodes and spleen, and DC-SIGN levels were significantly reduced in spleen. These findings suggest that the disruption of homeostatic chemokine expression is responsible, in part, for alterations in the networks of antigen-presenting cells in lymphoid tissues, ultimately contributing to systemic immunodeficiency.
Introduction
Dendritic cells (DCs) are professional antigen-presenting cells that potently initiate immune responses and have become recognized for their roles in controlling these responses.1-3 Immature DCs (iDCs) in peripheral tissues are highly proficient at capturing and processing antigens.4,5 On receiving an activation signal, iDCs undergo a maturation program and specifically migrate from the periphery to secondary lymphoid tissues,3 alter their cell surface expression profiles,3 and up-regulate costimulatory molecules necessary to activate naive T lymphocytes.6 Different DC subsets display unique chemotactic sensitivities to different chemokines. iDCs have been reported to respond to CCL20/macrophage inflammatory protein (MIP)–3α, which guides CC chemokine receptor 6 (CCR6) expressing iDCs in their migration to peripheral sites in the skin and mucosa.7,8 Mature DCs (mDCs) respond to CCL19/MIP-3β and CCL21/6Ckine as a consequence of CCR7 up-regulation.7,9mDCs entering draining lymph nodes are driven into paracortical areas in response to the production of CCL21/6Ckine by cells spread throughout the T-lymphocyte zone.7,10 These models are supported by studies in mice that are mutant for CCL21/6Ckine or that lack CCR7, both of which have deficiencies in cellular homing to lymph nodes.11-13
The proper localization of DCs in peripheral tissues and their maturation and trafficking to secondary lymphoid tissues are critical for generating optimal immune responses. Despite their importance in controlling immune induction, the effects of HIV-1 or simian immunodeficiency virus (SIV) infection on the distributions of DCs in lymphoid tissues remain incompletely understood. Changes in the numbers, types, or distributions of DCs could contribute to progressive HIV-1– and SIV-associated immunodeficiency because of impairment of antigen processing and presentation or to increased levels of infectious virus trafficking in association with the DCs.
Interactions between HIV-1 or SIV and DCs have been demonstrated at the cellular and the systemic levels. CD11c+ and CD11c− blood DCs and monocyte-derived DCs express CD4 and CCR5, albeit at levels that are considerably lower than on T lymphocytes,14,15 and there is evidence that they can be productively infected in vitro by HIV-1.16,17 iDCs cultured from peripheral sites, including skin and mucosa, can be infected with HIV-1,18-20 possibly through a CD4-independent mechanism.16,21 Recent studies have demonstrated that HIV-121-24 and SIV22,23 can bind to DCs through association with DC-specific ICAM-3–grabbing nonintegrin (DC-SIGN), thereby potentially facilitating the transport of virus from peripheral tissues to secondary lymphoid tissues and the infection of T-lymphocytes. Regardless of whether DCs are infected by HIV-1, a number of studies have identified alterations in DC populations in HIV-1– and SIV-infected hosts. The alterations include reduced numbers of DCs in peripheral blood,25-28 reduced CD83 expression,29,30 and increased numbers in lymphoid tissues during acute infection.30 31 The mechanisms by which these alterations arise are not yet understood, but they might provide insight into areas for novel therapeutic interventions.
The overall goal of the present study was to determine the effects of pathogenic SIV infection on secondary lymphoid tissues. To do this, we have examined the expression of 3 DC-recruiting homeostatic chemokines, the 2 cognate chemokine receptors, and 3 DC-associated markers. We found that in lymph node and spleen, the expression of chemokines CCL19/MIP-3β and CCL20/MIP-3α and chemokine receptors CCR7 and CCR6 were increased in acutely infected macaques and decreased in macaques with AIDS, whereas the expression of CCL21/6Ckine was not affected early in infection but was lower during AIDS infection. Unexpectedly, the lymph node subcapsular sinus of macaques was identified as a previously unrecognized site of homeostatic CCL20/MIP-3α expression. In addition, DC-SIGN and DC lysosome-associated membrane glycoprotein (DC-LAMP) mRNA expression levels were significantly reduced in lymphoid tissues during AIDS compared with uninfected controls, though the coexpression of CD68 and major histocompatibility complex (MHC) class II were not changed. These findings suggest that during pathogenic SIV infection of rhesus macaques, changes in expression of the CCR7 ligands CCL19/MIP-3β and CCL21/6Ckine affect the pool of DCs present in secondary lymphoid tissues. These perturbations likely contribute to ongoing viral replication and loss of systemic immune function.
Materials and methods
Animals and viral infection
These studies were performed under the approval of the University of Pittsburgh Institutional Animal Care and Use Committee. They included 16 adult rhesus macaques (Macaca mulatta) negative for SIV, simian retrovirus (type D), and simian T-lymphotropic viruses -1, -2, and -3. Macaques were infected intravenously with a characterized stock of the SIV/DeltaB670 primary isolate32; details regarding their clinicovirologic states have been described.33 Eight animals were killed 2 weeks after infection, whereas 6 animals were killed at the terminal stage of AIDS. Two age-matched, uninfected control animals were also included in these studies.
Cloning of rhesus macaque cDNAs
Our generation of a DC-SIGN cDNA has been described.34 To generate rhesus macaque cDNAs encoding DC-LAMP, DC-associated C-type lectin-1 receptor (DECTIN-1), CCR6, and CCR7, total RNAs were prepared from snap-frozen tissues using TRIZOL (Life Technologies, Rockville, MA). Reverse transcription–polymerase chain reaction (RT-PCR) was performed using the single-tube Access RT-PCR System (Promega, Madison, WI) or a 2-step RT then PCR method35 using standard thermocycling parameters. Primer sequences were based on the corresponding human sequences36-40 and are listed in Table1, along with the corresponding GenBank accession numbers. Products were ligated to the pGEM-T vector (Promega) and were sequenced completely in both directions using manual and automated strategies. The generation of rhesus macaque CCL20/MIP-3α, CCL19/MIP-3β, and CCL21/6Ckine cDNAs has been described.35
Rhesus macaque cDNAs and ISH probes
| cDNA . | GenBank accession no. (reference) . | Forward Primer (5′ → 3′) . | Reverse Primer (5′ → 3′) . | cDNA length (nt) . | Homology vs human, % . | |
|---|---|---|---|---|---|---|
| Nucleic acid . | Amino acid . | |||||
| DC-LAMP | AF416334 (Fuller et al43) | ATGCCCCGGCAGCTCAGCGCGGCGG | TTAGATTCTCTGGTATCCAGATGA | 1251 | 95.5 | 92.5 |
| DECTIN-1 | AF508729 | ATGGAATATCATCCTGATTTAG | TTACATTGAAAACTTCTTCTC | 744 | 96.7 | 94.9 |
| DC-SIGN | AF369755 (Baribaud et al34) | ATGAGTGACTCCAAGGAACCAA | CTACGCAGGAGGGGGGTTTGGGGT | 1146 | 93.2 | 90.3 |
| CCR6 | AF508730 | ACAATGAGCGGGGAATCAATGA | CTATCACATAGTGAAGGACGA | 1125 | 95.6 | 94.1 |
| CCR7 | AF508731 | ATGGACCTGGGGAAACCAATGA | AAGAGTCGCCTATGGGGAGAA | 1137 | 98.2 | 99.1 |
| CCL19/MIP-3β | AF449273 (Basu et al35) | ATGGCCCTGCTACTGGCCC | TTAACTGCTGCGGCGCTTCATCTT | 297 | 97.6 | 97.0 |
| CCL20/MIP-3α | AF449274 (Basu et al35 | ACCATGTGCTGTACCAAGAGTTTG | CTAAACCCTCCATGATGTGCAAGTGA | 291 | 91.8 | 86.6 |
| CCL21/6Ckine | AF449275 (Basu et al35) | ATGGCTCAGTCACTGGCTCTGA | CTATGGCCCTTTAGGGGTCTG | 396 | 94.6 | 91.9 |
| cDNA . | GenBank accession no. (reference) . | Forward Primer (5′ → 3′) . | Reverse Primer (5′ → 3′) . | cDNA length (nt) . | Homology vs human, % . | |
|---|---|---|---|---|---|---|
| Nucleic acid . | Amino acid . | |||||
| DC-LAMP | AF416334 (Fuller et al43) | ATGCCCCGGCAGCTCAGCGCGGCGG | TTAGATTCTCTGGTATCCAGATGA | 1251 | 95.5 | 92.5 |
| DECTIN-1 | AF508729 | ATGGAATATCATCCTGATTTAG | TTACATTGAAAACTTCTTCTC | 744 | 96.7 | 94.9 |
| DC-SIGN | AF369755 (Baribaud et al34) | ATGAGTGACTCCAAGGAACCAA | CTACGCAGGAGGGGGGTTTGGGGT | 1146 | 93.2 | 90.3 |
| CCR6 | AF508730 | ACAATGAGCGGGGAATCAATGA | CTATCACATAGTGAAGGACGA | 1125 | 95.6 | 94.1 |
| CCR7 | AF508731 | ATGGACCTGGGGAAACCAATGA | AAGAGTCGCCTATGGGGAGAA | 1137 | 98.2 | 99.1 |
| CCL19/MIP-3β | AF449273 (Basu et al35) | ATGGCCCTGCTACTGGCCC | TTAACTGCTGCGGCGCTTCATCTT | 297 | 97.6 | 97.0 |
| CCL20/MIP-3α | AF449274 (Basu et al35 | ACCATGTGCTGTACCAAGAGTTTG | CTAAACCCTCCATGATGTGCAAGTGA | 291 | 91.8 | 86.6 |
| CCL21/6Ckine | AF449275 (Basu et al35) | ATGGCTCAGTCACTGGCTCTGA | CTATGGCCCTTTAGGGGTCTG | 396 | 94.6 | 91.9 |
In situ hybridization and immunohistochemical staining
In situ hybridization (ISH) with [35S]-labeled riboprobes, immunohistochemical staining (IHC), and combined ISH/IHC were performed as described.33 41 Autoradiographic exposure times were 7 days. The DC-LAMP, DC-SIGN, CCR6, and CCR7 riboprobes consisted of separately labeled riboprobes encompassing the 5′ and 3′ portions of each cDNA, which were combined before hybridization. Sense and antisense [35S]-labeled riboprobes of DECTIN-1, CCL20/MIP-3α, CCL19/MIP-3β, and CCL21/6Ckine were generated from plasmid templates containing the full open-reading frame.
Antibodies used for IHC were specific for CD68 (KP1, DAKO, Carpinteria, CA) or MHC class II (human leukocyte antigen [HLA]–DR; TAL.1B5, DAKO). The percentages of mRNA+ cells that expressed CD68 or HLA-DR antigens were determined by examining 100 DC-LAMP or DC-SIGN mRNA+ cells per tissue section at a magnification × 600 and categorizing each cell as CD68 or HLA-DR positive or negative. The determinations were repeated 3 separate times.
Quantitative image analysis
For quantitation of the signals generated after ISH with [35S]-labeled probes and emulsion autoradiography, we used a quantitative image capture/analysis system.33 For each gene-specific probe, all tissue sections were hybridized, washed, exposed, and developed as a group, using antisense and control sense probes. Low-power (× 4) fields representing 5.6 mm2 of tissue area from each tissue section were captured with bright-field illumination (9-V halogen lamp) using an RT Slider Spot camera (Diagnostics Instruments, Sterling Heights, MI) and were analyzed using the MetaView software package (Version 4.5r4; Universal Imaging, West Chester, PA). Because the level of expression of DECTIN-1 mRNA was low, we captured 5 random fields from each tissue section hybridized with this probe using bright-field illumination and an objective magnification × 20. The captured fields were considered random for spleen tissue sections and, therefore, encompassed red pulp and white pulp. In lymph node sections, however, the captured fields were restricted to either cortical/paracortical regions (DC-LAMP, DECTIN-1, CCL19/MIP-3β, CCL21/6Ckine, CCR6, and CCR7) or medullary regions (DC-SIGN and DECTIN-1). Silver grains were differentiated using the color separation and pseudocolor features of MetaView, and the surface area (pixels) was measured with the threshold and measure object tools. Background-subtracted ISH signals were calculated for each of the microscopic fields and have been presented as the percentage of image surface area covered by silver grains. To compare the ISH signals for each mRNA among groups, we used the Student t test by using the Minitab software package (Minitab, State College, PA).P ≤ .05 was considered significant.
Results
Homeostatic chemokine expression is disrupted in macaque lymphoid tissues during SIV infection in vivo
Homeostatic chemokines are crucial regulators of lymphoid tissue composition11-13 and DC trafficking.3,42 Disruption of expression of these immunomodulators could contribute to the virologic and immunologic events that cause AIDS. We have addressed this issue using the SIV/macaque model to measure changes in the patterns and levels of expression of the CCR7 ligands CCL19/MIP-3β and CCL21/6Ckine in lymph node and spleen during early and late times after pathogenic SIV infection. Adult rhesus macaques included in these studies were infected intravenously with the SIV/DeltaB670 isolate and were killed during the acute phase of infection, when systemic viral replication is generally very high, or on the development of AIDS.33 43
Cells producing homeostatic chemokines were identified by in situ hybridization (ISH) and emulsion autoradiography using macaque-derived, gene-specific riboprobes (Table 1). This strategy was used because it reveals changes in mRNA expression that might not be discernible by population analyses of homogenized tissue and because it can provide insight into the types of cells expressing the mRNAs due to the microanatomic localization of signal. To provide a measure of mRNA expression levels, we used a quantitative image capture/analysis system33 to determine the surface area covered by autoradiographic silver grains per unit surface area of tissue in individual microscopic fields. We have previously demonstrated that this microscope slide-based strategy provides data that are concordant with population analyses of mRNA expression, such as real-time RT-PCR.33 For purposes of quantitative comparison, for each ISH probe, all lymph node and spleen tissue sections were hybridized at the same time and, concomitantly, with a sense control probe and were exposed for the same duration. Universally, the sense control probes provided no ISH signal.
In macaque lymph nodes, CCL19/MIP-3β and CCL21/6Ckine mRNAs were expressed in the T-lymphocyte–rich paracortical region (Figure1). In uninfected macaque lymph nodes, CCL19/MIP-3β was present but not abundant (Figure 1B), whereas CCL21/6Ckine was constitutively expressed to high levels (Figure 1C). During acute SIV infection, the level of expression of CCL19/MIP-3β increased dramatically (Figure 1E) and then decreased during AIDS to levels that nevertheless remained higher than in uninfected lymph nodes (Figure 1H). Quantitative image capture/analysis (Figure2A) revealed that CCL19/MIP-3β mRNA expression levels, measured in the cortex on a per surface area basis in lymph node, were significantly higher in acutely infected macaques than in uninfected macaques (P < .001; 20.8-fold) and in macaques that acquired AIDS (P < .05; 7.6-fold). Interestingly, though it is also a CCR7 ligand, CCL21/6Ckine mRNA did not increase in expression during acute infection but instead progressively decreased throughout the course of infection (Figure1C,F,I). During AIDS, CCL21/6Ckine expression was significantly lower (P < .01; 2.0-fold; Figure 2A) relative to uninfected and acutely infected macaques.
In situ hybridization detection of CCR7, CCL19/MIP-3β, and CCL21/6Ckine mRNAs in macaque lymph node during SIV infection.
Macaque lymph node tissue sections were hybridized in situ with [35S]-labeled riboprobes specific for CCR7, CCL19/MIP-3β, or CCL21/6Ckine, as indicated. A-C, D-F, and G-I are serial tissue sections. UI indicates uninfected; AC, acute infection. Autoradiographic exposure times were 7 days. Bar indicates 500 μm (A-I).
In situ hybridization detection of CCR7, CCL19/MIP-3β, and CCL21/6Ckine mRNAs in macaque lymph node during SIV infection.
Macaque lymph node tissue sections were hybridized in situ with [35S]-labeled riboprobes specific for CCR7, CCL19/MIP-3β, or CCL21/6Ckine, as indicated. A-C, D-F, and G-I are serial tissue sections. UI indicates uninfected; AC, acute infection. Autoradiographic exposure times were 7 days. Bar indicates 500 μm (A-I).
Quantitative image capture/analysis of ISH signals in lymphoid tissues from uninfected and SIV-infected macaques.
Quantitative image analysis was used to determine the autoradiographic signal intensities following ISH for CCL19/MIP-3β, CCL21/6Ckine, CCR6, CCR7, DC-LAMP, DC-SIGN, and DECTIN-1 in lymph node (A, C, E) and spleen (B, D, F) in the absence of infection or during acute infection or AIDS. The percentage of surface area in individual microscopic fields that was covered by silver grains was determined, and the values for the background-subtracted ISH signals are shown. Statistical significance was examined by the Student t test.P < .05 has been noted with the following symbols: acute infection versus uninfected (*), AIDS versus uninfected (†), AIDS versus acute infection (#). Mean (geometric) plasma viral loads in these groups were: uninfected, less than 100 copies/mL; acute, 12.0 × 107 copies/mL; and AIDS, 1.8 × 106copies/mL.
Quantitative image capture/analysis of ISH signals in lymphoid tissues from uninfected and SIV-infected macaques.
Quantitative image analysis was used to determine the autoradiographic signal intensities following ISH for CCL19/MIP-3β, CCL21/6Ckine, CCR6, CCR7, DC-LAMP, DC-SIGN, and DECTIN-1 in lymph node (A, C, E) and spleen (B, D, F) in the absence of infection or during acute infection or AIDS. The percentage of surface area in individual microscopic fields that was covered by silver grains was determined, and the values for the background-subtracted ISH signals are shown. Statistical significance was examined by the Student t test.P < .05 has been noted with the following symbols: acute infection versus uninfected (*), AIDS versus uninfected (†), AIDS versus acute infection (#). Mean (geometric) plasma viral loads in these groups were: uninfected, less than 100 copies/mL; acute, 12.0 × 107 copies/mL; and AIDS, 1.8 × 106copies/mL.
In spleen, CCL19/MIP-3β and CCL21/6Ckine mRNAs were expressed almost exclusively in the periarteriolar lymphoid sheath (PALS) and marginal zone of the white pulp (Figure 3). Similar to lymph node, in the absence of SIV infection, CCL19/MIP-3β mRNA was present but not abundant (Figure 3B), whereas CCL21/6Ckine was constitutively expressed to high levels (Figure 3C). Moreover, the microanatomic localization of these mRNAs changed markedly during the course of infection such that during AIDS, expression in PALS was minimal and most of the signal was localized to the marginal zones (Figures 3C,F,I; and Table 2). Although the changes in expression of these CCR7 ligands were clearly evident in microenvironments within the DC- and T-lymphocyte–rich microenvironments (Figure 3), quantitative image analysis of all splenic microenvironments combined did not reveal significant differences (Figure 2B).
In situ hybridization detection of CCR7, CCL19/MIP-3β, and CCL21/6Ckine mRNAs in macaque spleen during SIV infection.
Macaque spleen tissue sections were hybridized in situ with [35S]-labeled riboprobes specific for CCR7, CCL19/MIP-3β, or CCL21/6Ckine, as indicated and as described in the legend to Figure 1. A-C, D-F, and G-I are serial tissue sections. Bar indicates 500 μm (A-I).
In situ hybridization detection of CCR7, CCL19/MIP-3β, and CCL21/6Ckine mRNAs in macaque spleen during SIV infection.
Macaque spleen tissue sections were hybridized in situ with [35S]-labeled riboprobes specific for CCR7, CCL19/MIP-3β, or CCL21/6Ckine, as indicated and as described in the legend to Figure 1. A-C, D-F, and G-I are serial tissue sections. Bar indicates 500 μm (A-I).
Microanatomic locations of cells expressing mRNAs encoding DC-associated markers, chemokine receptors, or chemokines in macaque spleen
| Animal . | Disease status . | CCL19/MIP-3β . | CCL21/6Ckine . | CCR6 . | CCR7 . | DC-LAMP . | DC-SIGN . | DECTIN-1 . | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PALS . | MA . | RP . | PALS . | PWP . | RP . | PALS . | MA . | RP . | PALS . | MA . | RP . | PALS . | MA . | RP . | PALS . | MA . | RP . | PALS . | MA . | RP . | ||
| M5600 | Uninfected | +++ | − | − | +++ | + | − | − | ++ | − | +++ | − | − | ++ | − | − | − | + | + | − | − | + |
| M6600 | Uninfected | +++ | − | − | +++ | + | − | − | ++ | − | +++ | − | − | + | − | − | − | + | + | − | − | + |
| M0999 | Acute | +++ | − | − | +++ | + | − | − | ++ | − | +++ | − | − | ++ | − | − | − | + | + | + | − | + |
| M5299 | Acute | +++ | − | − | +++ | + | − | − | ++ | − | ++ | − | − | + | − | − | − | + | + | + | − | + |
| M5499 | Acute | +++ | − | − | +++ | + | − | − | + | − | +++ | − | − | + | − | − | − | + | + | + | − | + |
| M5599 | Acute | +++ | − | − | +++ | + | − | − | + | − | +++ | − | − | + | − | − | − | + | + | − | − | + |
| M5699 | Acute | +++ | + | − | +++ | + | − | − | ++ | − | +++ | − | − | ++ | + | − | − | + | + | + | − | + |
| M5899 | Acute | +++ | + | − | +++ | + | − | − | ++ | − | +++ | + | − | ++ | + | − | − | + | + | + | − | + |
| M5999 | Acute | +++ | + | − | +++ | + | − | − | ++ | − | +++ | − | − | ++ | + | − | − | + | ++ | + | − | + |
| M6299 | Acute | +++ | − | − | +++ | + | − | − | + | − | +++ | − | − | ++ | + | − | − | + | − | + | − | + |
| M1799 | AIDS | + | ++ | − | + | ++ | − | − | + | − | + | + | − | − | + | − | − | + | − | + | − | + |
| M5199 | AIDS | + | + | − | + | ++ | − | − | − | − | + | + | − | − | + | − | − | − | − | − | − | + |
| M5799 | AIDS | + | + | − | + | + | − | − | − | − | − | − | − | − | − | − | − | + | − | − | − | + |
| M6199 | AIDS | ++ | ++ | − | + | ++ | − | − | + | − | + | ++ | − | − | ++ | − | − | − | − | + | − | + |
| M8397 | AIDS | ++ | − | − | + | ++ | − | − | + | − | + | +++ | − | − | + | − | − | + | − | + | − | + |
| M1198 | AIDS | + | ++ | − | + | +++ | − | − | + | − | + | ++ | − | − | + | − | − | + | − | + | − | + |
| Animal . | Disease status . | CCL19/MIP-3β . | CCL21/6Ckine . | CCR6 . | CCR7 . | DC-LAMP . | DC-SIGN . | DECTIN-1 . | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PALS . | MA . | RP . | PALS . | PWP . | RP . | PALS . | MA . | RP . | PALS . | MA . | RP . | PALS . | MA . | RP . | PALS . | MA . | RP . | PALS . | MA . | RP . | ||
| M5600 | Uninfected | +++ | − | − | +++ | + | − | − | ++ | − | +++ | − | − | ++ | − | − | − | + | + | − | − | + |
| M6600 | Uninfected | +++ | − | − | +++ | + | − | − | ++ | − | +++ | − | − | + | − | − | − | + | + | − | − | + |
| M0999 | Acute | +++ | − | − | +++ | + | − | − | ++ | − | +++ | − | − | ++ | − | − | − | + | + | + | − | + |
| M5299 | Acute | +++ | − | − | +++ | + | − | − | ++ | − | ++ | − | − | + | − | − | − | + | + | + | − | + |
| M5499 | Acute | +++ | − | − | +++ | + | − | − | + | − | +++ | − | − | + | − | − | − | + | + | + | − | + |
| M5599 | Acute | +++ | − | − | +++ | + | − | − | + | − | +++ | − | − | + | − | − | − | + | + | − | − | + |
| M5699 | Acute | +++ | + | − | +++ | + | − | − | ++ | − | +++ | − | − | ++ | + | − | − | + | + | + | − | + |
| M5899 | Acute | +++ | + | − | +++ | + | − | − | ++ | − | +++ | + | − | ++ | + | − | − | + | + | + | − | + |
| M5999 | Acute | +++ | + | − | +++ | + | − | − | ++ | − | +++ | − | − | ++ | + | − | − | + | ++ | + | − | + |
| M6299 | Acute | +++ | − | − | +++ | + | − | − | + | − | +++ | − | − | ++ | + | − | − | + | − | + | − | + |
| M1799 | AIDS | + | ++ | − | + | ++ | − | − | + | − | + | + | − | − | + | − | − | + | − | + | − | + |
| M5199 | AIDS | + | + | − | + | ++ | − | − | − | − | + | + | − | − | + | − | − | − | − | − | − | + |
| M5799 | AIDS | + | + | − | + | + | − | − | − | − | − | − | − | − | − | − | − | + | − | − | − | + |
| M6199 | AIDS | ++ | ++ | − | + | ++ | − | − | + | − | + | ++ | − | − | ++ | − | − | − | − | + | − | + |
| M8397 | AIDS | ++ | − | − | + | ++ | − | − | + | − | + | +++ | − | − | + | − | − | + | − | + | − | + |
| M1198 | AIDS | + | ++ | − | + | +++ | − | − | + | − | + | ++ | − | − | + | − | − | + | − | + | − | + |
PALS indicates periarteriolar lymphoid sheath; MA, marginal zone or follicular mantle zone of white pulp; PWP, proximal to white pulp; RP, red pulp.
Scoring was based on the percentage of surface area covered by autoradiographic silver grains in microscopic images captured through a × 4 objective, except for DECTIN-1 ISHs, which were captured through a × 20 objective: 0% to 1% (−); 1% to 5% (+); 5% to 10% (++); 10% and greater (+++).
To determine whether the early increases in CCL19/MIP-3β and late decreases in CCL21/6Ckine expression during SIV infection were associated with changes in expression of their receptor, CCR7, we examined its expression levels and patterns. In lymph node, CCR7 was expressed in the paracortex in the same diffuse pattern as CCL21/6Ckine (Figure 1). In uninfected lymph node, CCR7 was present but not abundant (Figure 1A), and it increased dramatically during acute infection, in parallel with CCL19/MIP-3β (Figure 1D). By quantitative image analysis, CCR7 expression levels in lymph node were significantly (P < .01; 4.9-fold) higher in acutely infected macaques than in uninfected macaques and macaques that acquired AIDS (Figure2C). In spleen, CCR7 was expressed almost exclusively in the T-lymphocyte–rich PALS of the white pulp (Figure 3), with measurable, but not abundant, expression in uninfected animals. Following SIV infection, the patterns and levels of expression of CCR7 closely paralleled those of CCL19/MIP-3β and, to a lesser extent, CCL21/6Ckine (Figure 3D,G).
We next determined the patterns and levels of expression of chemokine CCL20/MIP-3α and its receptor, CCR6, which are involved in the trafficking of DC precursors and iDCs.18 19 Unexpectedly, CCL20/MIP-3α mRNA was abundantly expressed in the subcapsular microanatomic compartment of lymph nodes in all uninfected and SIV-infected macaques (Figure 4A,D,G). Hybridization with a sense control probe invariably provided no ISH signal (Figure 4D, inset). In contrast, CCL20/MIP-3α mRNA was not detected in spleen in any macaques (data not shown). Consistent with the pattern of CCL20/MIP-3α expression in lymph node, CCR6 was expressed in subcapsular regions and in perifollicular regions, including the follicular mantle zones (Figure 4B,E,H), with levels that increased significantly (P < .05) by 7.8-fold during acute infection and then decreased during AIDS (Figure 2C). In spleen, CCR6 mRNA was also detected in the follicular mantle zones (Figure4C,F,I), but there was a progressive decrease in expression throughout infection to the point at which CCR6 mRNA levels were significantly (P < .02) lower during AIDS (Figure 2D). These data demonstrate that in nonhuman primate lymph nodes, CCL20/MIP-3α is expressed homeostatically at the interface between the afferent lymphatic conduit and the lymph node and that the expression levels of this chemokine and its receptor CCR6 are altered by SIV infection.
In situ hybridization detection of CCL20/MIP-3α and CCR6 mRNAs in macaque lymph node and spleen during SIV infection.
Tissue sections from lymph node (A-B, D-E, G-H) and spleen (C, F, I) were hybridized in situ with [35S]-labeled riboprobes specific for CCL20/MIP-3α or CCR6 as indicated and as described in the legend to Figure 1. The inset in panel D is a representative ISH with the control sense riboprobes. Bar indicates 200 μm (A-B, D-E, G-H) and 500 μm (C, F, I).
In situ hybridization detection of CCL20/MIP-3α and CCR6 mRNAs in macaque lymph node and spleen during SIV infection.
Tissue sections from lymph node (A-B, D-E, G-H) and spleen (C, F, I) were hybridized in situ with [35S]-labeled riboprobes specific for CCL20/MIP-3α or CCR6 as indicated and as described in the legend to Figure 1. The inset in panel D is a representative ISH with the control sense riboprobes. Bar indicates 200 μm (A-B, D-E, G-H) and 500 μm (C, F, I).
SIV infection alters the patterns and levels of expression of DC-associated mRNAs in macaque lymphoid tissues
The disruption of homeostatic chemokine expression we observed during SIV infection could lead to perturbation of the DC populations in secondary lymphoid tissues. Accordingly, we examined the patterns and levels of expression of 3 DC-associated mRNAs—DC-LAMP, DC-SIGN, and DECTIN-1. Although no single marker thus far uniquely identifies DCs, these markers were chosen because it has been demonstrated that they are highly expressed by DCs. Among these markers, DC-SIGN is thought to play a significant role in the pathogenesis of HIV-1 in that it can bind to HIV-121-24 and SIV22 23virions and can efficiently transmit the virus to T-lymphocytes.
In lymph node, DC-LAMP+ cells were localized predominantly in the T-lymphocyte–rich paracortical regions in all animals, and their microanatomic localization did not change during the course of SIV infection (Figure 5A,E,I). As measured by quantitative image analysis, DC-LAMP expression in the cortical/paracortical regions of lymph nodes increased early during infection (9.5% ± 3.6% of imaged tissue surface area covered by silver grains) and then decreased significantly during AIDS (P < .001; 1.5% ± 1.0%), relative to uninfected macaques (7.2% ± 2.7%; Figure 2E). These data indicate that there is an early increase in the pool of mature or activated DCs and a dramatic loss associated with AIDS. Unexpectedly, DC-SIGN was expressed in a reciprocal pattern, with nearly all mRNA+ cells residing in medullary sinuses (Figure 5B,F,J). DC-SIGN ISH signals in lymph node did not change significantly during the course of infection (Figure 2E), indicating that the local pool of DC-SIGN+cells available for local transmission of virus was maintained throughout infection. DECTIN-1 mRNA+ cells were observed in the paracortical and medullary regions of lymph node in all animals (data not shown), though the ISH signals were low (Figure 2E). Nevertheless, the DECTIN-1 mRNA ISH signals were significantly higher during acute SIV infection (0.9% ± 0.6%) than in uninfected macaques (0.2% ± 0.1%; P < .05) and in macaques developing AIDS (0.4% ± 0.3%, P < .05; Figure 2E).
In situ hybridization detection of DC-SIGN, DC-LAMP, and DECTIN-1 mRNAs in macaque lymph nodes and spleen during SIV infection.
Tissue sections from lymph node were hybridized in situ with [35S]-labeled riboprobes specific for DC-LAMP or DC-SIGN as indicated. Insets in panels D and E are ISHs with control sense riboprobes. co/pa indicates cortex/paracortex; me, medullary region; g, germinal center; p, PALS; rp, red pulp. Bar indicates 125 μm (A-B,E-F,I-J) and 200 μm (C-D,G-H,K-L).
In situ hybridization detection of DC-SIGN, DC-LAMP, and DECTIN-1 mRNAs in macaque lymph nodes and spleen during SIV infection.
Tissue sections from lymph node were hybridized in situ with [35S]-labeled riboprobes specific for DC-LAMP or DC-SIGN as indicated. Insets in panels D and E are ISHs with control sense riboprobes. co/pa indicates cortex/paracortex; me, medullary region; g, germinal center; p, PALS; rp, red pulp. Bar indicates 125 μm (A-B,E-F,I-J) and 200 μm (C-D,G-H,K-L).
In spleen, the microanatomic locations of DC-LAMP+ and DC-SIGN+ cells did not appreciably overlap, and their localization and expression levels were altered during SIV infection. In uninfected and acutely infected macaques, DC-LAMP+ cells were present primarily within the T-lymphocyte–rich PALS and marginal zones (Figure 5C,G; Table 2). However, during AIDS the rare DC-LAMP+ cells that remained in the spleen were restricted mainly to the marginal zones (Figure 5K). DC-LAMP ISH signals measured over the entire tissue in spleens progressively decreased during the course of SIV infection such that macaques with AIDS had significantly lower levels of expression than uninfected macaques (P < .05; Figure 2F). In uninfected and acutely infected macaques, DC-SIGN+ cells were present in red pulp and proximal to white pulp, whereas in macaques with AIDS, they were observed nearly entirely proximal to white pulp, just beyond the marginal zone (Figure 5D,H,L; Table 2). Similar to DC-LAMP, though more dramatic, DC-SIGN ISH signals progressively decreased during disease, with higher ISH signals in uninfected and acutely infected macaques than in macaques developing AIDS (P < .05; Figure 2F), in which there was a striking absence of DC-SIGN+ cells (Figure 5L). Finally, DECTIN-1 mRNA+ cells were present in red pulp and white pulp (Table 2), and the patterns of localization and the levels of expression did not change significantly during the course of infection (Figure 2F).
To determine whether the phenotypes of DCs expressing DC-LAMP or DC-SIGN mRNAs in macaque spleen sections were altered during the course of SIV infection, we simultaneously performed ISH for these markers and IHC for CD68 or HLA-DR. The percentages of mRNA+ cells that were also CD68+ or HLA-DR+ did not change significantly during the course of SIV infection. Nearly all DC-SIGN mRNA+ cells (98.2% ± 0.7%), but only a fraction of DC-LAMP mRNA+ cells (7.5% ± 0.7%), were positive for CD68 protein. In contrast, approximately half the DC-SIGN mRNA+ cells (53.2% ± 0.8%) and most DC-LAMP mRNA+ cells (89.9% ± 1.4%) were positive for HLA-DR protein. In summary, these data demonstrate that SIV infection leads to significant alterations in the expression of multiple DC-associated mRNAs in lymph node and spleen, culminating in dramatic decreases during AIDS.
Discussion
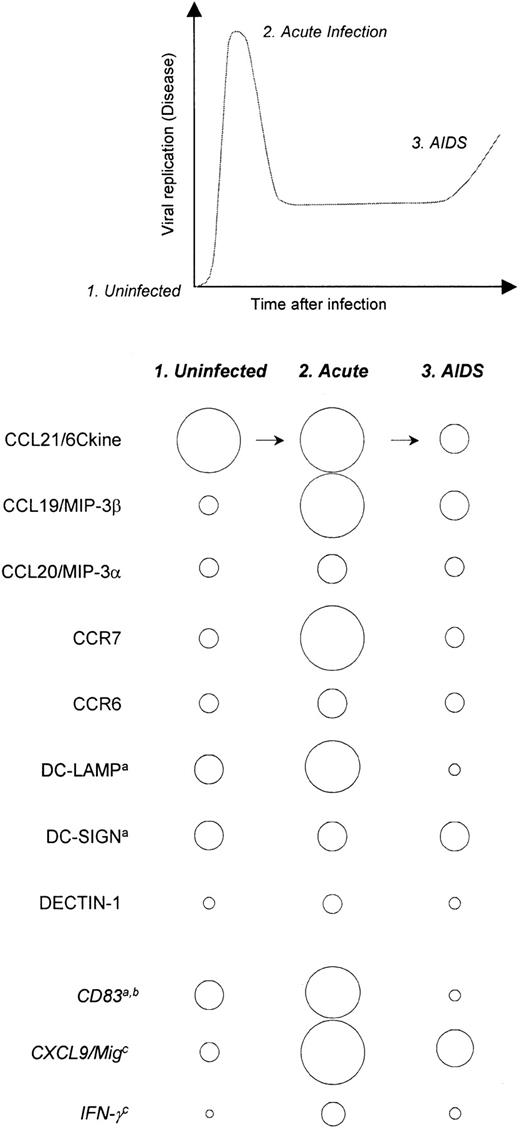
In this report we have demonstrated that SIV infection disrupts the expression of homeostatic chemokines in macaque secondary lymphoid tissues. These findings provide new insight into fundamental aspects of DC biology in lymph nodes and the effects of pathogenic SIV infection on DC-recruiting chemokines and DC markers in lymph nodes. The changes we identified in homeostatic chemokine expression were associated with local changes in the expression of DC-associated chemokine receptors and DC-associated markers (summarized in Figure6). DC networks in macaque lymphoid tissues were dramatically altered during SIV infection and AIDS, as demonstrated by significantly decreased expression levels of DC-LAMP in lymph node and spleen and of DC-SIGN in spleen, and they were associated with changes in expression of the chemokines CCL19/MIP-3β and CCL21/6Ckine. These perturbations caused by SIV infection likely contribute to the early replicative burst that occurs during in vivo infection and the establishment of immunodeficiency.
Summary of HIV/SIV-induced alterations in chemokine, chemokine receptor, and DC marker expression.
Relative levels of expression of the genes listed to the left, during health and states of high viral replication, are schematically represented by the sizes of the circles. Also indicated are changes in lymphoid tissue gene expression supported by previously published reports: (a) Lore et al,31 (b) Zimmer et al,30 (c) Reinhart et al.33
Summary of HIV/SIV-induced alterations in chemokine, chemokine receptor, and DC marker expression.
Relative levels of expression of the genes listed to the left, during health and states of high viral replication, are schematically represented by the sizes of the circles. Also indicated are changes in lymphoid tissue gene expression supported by previously published reports: (a) Lore et al,31 (b) Zimmer et al,30 (c) Reinhart et al.33
Regarding fundamental aspects of DC biology in lymph nodes, we have shown that CCL20/MIP-3α and its receptor CCR6 are constitutively expressed in lymph nodes during health and throughout the course of SIV infection. The expression of CCL20/MIP-3α and CCR6 in lymph node cortex and subcapsular sinus, respectively, has not been previously described, though CCL20/MIP-3α expression has been demonstrated in inflamed epithelial crypts of human tonsils7 and Peyer patches.44 These findings suggest that CCL20/MIP-3α could play a role in the constitutive trafficking of peripheral DCs along the lymphatic conduit to the outer surfaces of lymph nodes, in addition to its role in recruiting DC precursors to peripheral sites of antigen acquisition.45 Based on these findings, it is conceivable that the transition from iDC to mDC does not involve a full switch in chemokine receptor expression from CCR6 to CCR7, as is implied from in vitro studies of cord blood–derived DCs.7Rather, CCR6 could maintain an active role in getting DCs to the outer edge of the lymph node, where signaling through CCR7 by CCL21/6Ckine becomes dominant and moves DCs to the paracortex. Additional studies of DC phenotypes in vivo are required to determine whether the simultaneous expression of CCR6 and CCR7 is involved in the trafficking of DCs to lymph nodes and to determine what role additional chemokine receptors, such as CCR5 and CXCR4, and their respective ligands play in the net migration patterns of DCs during health and SIV-associated disease. DC-based vaccination therapies could potentially be augmented if strategies were designed to exploit the homeostatic expression of CCL20/MIP-3α at the outer surfaces of lymph nodes.
In macaque spleen, though CCR6 mRNA was abundant in follicular mantle zone microenvironments in the absence of infection and decreased significantly during AIDS, we were unable to detect any appreciable CCL20/MIP-3α mRNA in the spleen, consistent with findings in murine spleen.44 We note that though the cells that are expressing CCR6 and are lost during SIV infection in macaque spleen might be splenic lymphoid DCs, which express CCR6 to higher levels than myeloid DCs,46 other cell types are also known to express CCR6, including subsets of B-lymphocytes46,47 and T-lymphocytes.46 48 Therefore, the CCR6+population of cells in spleen and lymph node could be composed of multiple cell types, not just DCs.
Another aspect of fundamental DC biology in lymph nodes we identified was that the levels of expression of the mDC recruiting chemokines, CCL19/MIP-3β and CCL21/6Ckine, were different in health and during SIV-associated disease. These CCR7 ligands are not redundant, given that CCL21/6Ckine is expressed constitutively to high levels and thus functions as a homeostatic chemokine, whereas CCL19/MIP-3β is expressed to high levels only after SIV infection and thus appears to function as an inflammatory chemokine. Consistent with our data in macaques, murine CCL21/6Ckine is constitutively expressed to higher levels than CCL19/MIP-3β.49
The data presented here are, to our knowledge, the first examination of homeostatic chemokine expression levels and patterns in vivo during HIV- or SIV-induced immunodeficiency. During the course of SIV infection, we found that CCL19/MIP-3β expression levels dramatically increased in lymph node and splenic white pulp during acute infection, in contrast to CCL21/6Ckine expression levels, which in these same compartments were abundant in the absence of SIV infection and then progressively decreased. These changes in local expression of homeostatic chemokines are likely a major force driving increased expression of DC-associated markers in lymphoid tissues early during SIV infection and decreased expression late during SIV infection. Given that CCL19/MIP-3β is one of the most differentially expressed genes in mDCs relative to iDCs or monocytes,50-52 it is likely that CCL19/MIP-3β is not induced exclusively by inflammatory signals but, rather, is also expressed by the increased recruitment of mDCs to lymph nodes during acute infection. Evidence for increased recruitment of mDCs to lymph nodes during acute infection is provided by our demonstration of increased DC-LAMP (Figures 1, 2) and CCR7 (Figure 4) ISH signals and the previous demonstration of increased CD83 ISH signals in these very same animals.30 Moreover, CD83, DC-LAMP, and other DC markers have been shown to increase in tonsillar tissue early during HIV-1 infection.31 Increased expression of CCL19/MIP-3β early in infection likely establishes a positive feedback loop whereby increased CCL19/MIP-3β recruits CCR7+ mDCs expressing additional CCL19/MIP-3β. Increased expression of CCR7 in lymphoid tissues likely results from the recruitment of CCR7+ naive and central memory T-lymphocytes,42,53 in addition to mDCs. It is important to note that though our data suggest that the changes in chemokine expression we have identified lead to altered homing of DCs, T-lymphocytes, and potentially other cell types, detailed in vivo trafficking studies will be required to directly measure overall effects on DC and lymphocyte homing. Consistent with the prediction from our data that T-lymphocytes are more likely to home to lymphoid tissues during HIV-1/SIV infection, recent data indicate that the rate of trafficking of CD4+ T-lymphocytes to lymph nodes is higher in HIV-1–infected patients than uninfected control patients or in hepatitis B-virus–infected control patients.54
It is reasonable to expect that there are severe pathologic consequences to these changes in local gene expression and cellular composition within lymphoid tissues during SIV infection. One consequence would be the dramatic fueling of virus propagation early during the course of infection, when virus-specific immune responses are just developing and expanding. Increased expression levels of CCL19/MIP-3β would recruit both mDCs, which are potent activators of T lymphocytes,1-3 and naive T lymphocytes,53which in vivo are highly susceptible to SIV and HIV-1 infection.55,56 Recruitment of these cells, and their multiple interactions, would fuel virus propagation because the pool of locally replicating viruses is extensive,57 virus is deposited and maintained in an infectious form on follicular dendritic cells58 and on cells expressing DC-SIGN21-23and SIGNR,22 DC/T-lymphocyte interactions create a permissive environment for viral replication,17 and abundant CCL21/6Ckine expression locally (Figures 4-5) increases viral gene expression.59 Inappropriate homing of T-lymphocytes has been proposed as a critical mechanism by which T-lymphocytes are recruited to secondary lymphoid tissues and rendered unavailable for trafficking to peripheral sites, where their effector activities are needed.33,60 The early increases in CCL19/MIP-3β (Figure2) and CXCL9/Mig,33 late decreases in CCL20/6Ckine (Figure2), and sustained increase in CXCL9/Mig33 could be expected to lead to inappropriate trafficking of multiple cell types with myriad virologic and immunologic consequences that over time would be detrimental to the host.
We interpret the altered levels of expression of DC-associated markers to reflect changes in the types and the numbers of DCs within the lymphoid tissues. In support of this, Zimmer et al30have demonstrated, in tissues from the same animals examined here, that the numbers of CD83+ DCs and the CD83+expression levels on those DCs in macaque lymph nodes increase during acute SIV infection and then decrease during AIDS. However, our observation that nearly all (98.2%) DC-SIGN mRNA+ cells were also positive for CD68 protein and that most (89.9%) DC-LAMP mRNA+ cells were positive for HLA-DR protein, regardless of disease state, suggests that SIV infection does not entirely alter the phenotypes of DCs in lymphoid tissues. Further analyses are required to more fully define the phenotypic and functional changes occurring in lymphoid tissue DCs during the different stages of SIV infection.
One limitation of our study is that the markers detected by our ISH probes for DC-associated markers are not absolutely specific for DCs and might also be expressed by some populations of monocytes or macrophages. This is an issue that cannot be resolved without the identification of a pan-DC–specific marker; currently, there are none. Nevertheless, the changes we observed in the expression of DC-LAMP, DC-SIGN, and DECTIN-1 represent disruption of the networks of antigen-presenting cells, even if a fraction of the cells expressing them are monocytes/macrophages. Our findings that DC-SIGN expression levels in lymph node do not change significantly could be the result of simultaneous detection of the related DC-SIGNR,22 which is expressed in lymph node but not spleen. Nevertheless, the maintenance of high combined levels of expression of DC-SIGN and DC-SIGNR in lymph nodes is an important finding, given that both molecules can bind HIV-1 and SIV and can facilitate the transfer of virus to susceptible cells,21 22 such as trafficking T-lymphocytes. The maintenance of expression of these SIGN molecules in lymph node could, therefore, contribute to ongoing viral replication throughout the entire course of disease.
In summary, these findings indicate there are significant alterations in the networks of DCs in microenvironments within macaque lymphoid tissues and in the chemokine networks involved in establishing and maintaining the DC networks. Collectively, these alterations likely result in a reduced capability to mount primary and memory immune responses and contribute to the development of systemic immunodeficiency. Therapeutic strategies designed to reduce or reverse this process might aid in reducing the systemic immunopathologic consequences of viral infection.
We thank Dawn McClemens-McBride, Saverio Capuano III, Melanie O'Malley, and Shane Ritchey for assistance with project coordination and animal care, and Craig Fuller for assistance with statistical analyses.
Prepublished online as Blood First Edition Paper, October 24, 2002; DOI 10.1182/blood-2002-08-2653.
Supported by National Institutes of Health grants HL62056 and MH61205 (T.A.R.). Y.K.C. was supported, in part, by the Postdoctoral Fellowship Program of The Korean Science and Engineering Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Todd A. Reinhart, Department of Infectious Diseases and Microbiology, Graduate School of Public Health, University of Pittsburgh, 606 Parran Hall, 130 DeSoto St, Pittsburgh, PA; e-mail:reinhar@pitt.edu.

![Fig. 1. In situ hybridization detection of CCR7, CCL19/MIP-3β, and CCL21/6Ckine mRNAs in macaque lymph node during SIV infection. / Macaque lymph node tissue sections were hybridized in situ with [35S]-labeled riboprobes specific for CCR7, CCL19/MIP-3β, or CCL21/6Ckine, as indicated. A-C, D-F, and G-I are serial tissue sections. UI indicates uninfected; AC, acute infection. Autoradiographic exposure times were 7 days. Bar indicates 500 μm (A-I).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/5/10.1182_blood-2002-08-2653/3/m_h80533908001.jpeg?Expires=1767765307&Signature=F2FAShvtx1B4fACFMe2KlZyxl3vD8TrOb-zzRUctOxWoODG~ZQTEattac~aFSiTTS~br~cb6QPpXEmSKa9y9a1cynKIfkL2znwnnqni3bpmQ0uK9O~E4H9Fc~XniDfWL08BM92zKLMuEHyO-JJwIsaBCTTJmlIcdmCQEYoZyf9i4Gd0VYc21C5nY-O6uKz6cLQ76KxXYUJKWdQgpUntMX1ZC3xt7oES5L4tCC62wWyee2yBHzYBW1OkoepgyPNwF5LUj5ZeDydSUGBffSyg43MK8oPYysGGjAICaKCYAkchPcv7lspHBzgqbhnd1WrVk8OJ6FdtABZKxtDajJXf0Qg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 3. In situ hybridization detection of CCR7, CCL19/MIP-3β, and CCL21/6Ckine mRNAs in macaque spleen during SIV infection. / Macaque spleen tissue sections were hybridized in situ with [35S]-labeled riboprobes specific for CCR7, CCL19/MIP-3β, or CCL21/6Ckine, as indicated and as described in the legend to Figure 1. A-C, D-F, and G-I are serial tissue sections. Bar indicates 500 μm (A-I).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/5/10.1182_blood-2002-08-2653/3/m_h80533908003.jpeg?Expires=1767765307&Signature=Joim1Z-xdzTTxOpnjnM4E6lqCwvb4hNq6CkQo-XAXgvZuJdLVtzWlaFQG9b9---akczXEYd8NQVSQTSslWTdAk7Dy17ZZev1MNI6LPX3ewzmMykiX6brou7E2oj-udniracreOAAQzssAXzT-giKTRMdZ3F7pWU7ecBuJciGt9-G4OfqzhDIH4i4~8eaSfyZ5ObyWMGlnlG29a4wlffAJhKiF3FBdqBMOgb493zJipGv7~9Uctj53E4-gdU2eN2V0pBUn-UH5bfpLkPj~hFyCFNQTRFMWuugucHHHWEXJIIkMP73xj6oE10265CcF0jG71HK3-IqSNmho6avgo3bJA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. In situ hybridization detection of CCL20/MIP-3α and CCR6 mRNAs in macaque lymph node and spleen during SIV infection. / Tissue sections from lymph node (A-B, D-E, G-H) and spleen (C, F, I) were hybridized in situ with [35S]-labeled riboprobes specific for CCL20/MIP-3α or CCR6 as indicated and as described in the legend to Figure 1. The inset in panel D is a representative ISH with the control sense riboprobes. Bar indicates 200 μm (A-B, D-E, G-H) and 500 μm (C, F, I).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/5/10.1182_blood-2002-08-2653/3/m_h80533908004.jpeg?Expires=1767765307&Signature=SLYyRFOaW5fL5wnEYtUmbFzusndxGFlx5PXq3azoZrU3O8net44D29L34pAdXNFnPydq8uA5vLlA5-4AxpvmF-S9IDI8mwUMdz-ro1HEVzd0AmhzlGhyd2Te39S29Ne0e~Ykj785YoABqJx57i88PCs95omk0HMirUw5061~FuaGGeu-tz~e67siodNFn5ktBmSVuqWAFQxFUpYFEeueu8fdH~j~XBzBJmrnLObkN7qX0qeer-Ior9t726o1g~tqYeOontdIn5GHub6gXRZWcjq59YrroyQsSCdIccVcP3Y3pBR5KbltKJA1q4r4H2ts15MydHETUoz-ywAHGRv3Yw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. In situ hybridization detection of DC-SIGN, DC-LAMP, and DECTIN-1 mRNAs in macaque lymph nodes and spleen during SIV infection. / Tissue sections from lymph node were hybridized in situ with [35S]-labeled riboprobes specific for DC-LAMP or DC-SIGN as indicated. Insets in panels D and E are ISHs with control sense riboprobes. co/pa indicates cortex/paracortex; me, medullary region; g, germinal center; p, PALS; rp, red pulp. Bar indicates 125 μm (A-B,E-F,I-J) and 200 μm (C-D,G-H,K-L).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/5/10.1182_blood-2002-08-2653/3/m_h80533908005.jpeg?Expires=1767765307&Signature=xGG4gLACbXB5zqFZ0eWe3gLLOO7YA1V-atCfw2SCTmsLX0cvUCCgx3fexYp6DNyF2Oij0755-ZapjKxClAeN6VLVGh6cs8Rh4wwc6v~Tgn4Q0r1gEm4MAy4nxOHyEJvaJ8U5sPyqooxI1BV~8k940DBvI7dQnd5TYjGoCIUEPPL~Vj8KsBTyAPX7Khflbn7yyzs1ue135N8Kff2j3Oolzv9fwoUOuf~ck1lv-J722~acga3e-QAZoNiV5u0Agx5F8-5FzcDESckoc0nEYBsDkms4NVN94Z7CMNh-2IQ1zQYPjBnWjyzYxk3froiMzVSfu1FmFG~p9k-QwTBCDfKqDQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal