A frequent outcome of allogeneic stem cell transplantation (alloSCT) in the treatment of leukemia is the destruction of the host hematolymphoid compartment and, thus, the malignancy, through the combined action of high-dose chemoradiotherapy and a T-cell–mediated graft-versus-host effect. Unfortunately, alloSCT is frequently limited by toxicity, including graft-versus-host disease (GVHD), and has not been successful in the treatment of tumors derived from solid organs. Here we report a novel cooperation between host and donor T cells in the response to a tumor cell vaccine given after a nonmyeloablative allogeneic stem cell transplantation (NST) protocol that achieves stable mixed bone marrow chimerism. Treatment of animals with NST, posttransplantation donor lymphocyte infusions (DLIs), and a vaccine, comprising irradiated autologous tumor cells mixed with a granulocyte-macrophage colony-stimulating factor (GM-CSF)–producing bystander line, results in potent and specific antitumor immunity. This combined modality immunotherapy, administered after surgical removal of the primary tumor, cured metastatic mammary cancer in most animals without inducing GVHD. Cured animals contained tumor-specific T cells of both host and donor origin, but immunodeficient hosts could not be cured by NST, DLI, and vaccine administration. Thus, transfer of allogeneic donor T cells may help break functional tolerance of a host immune system to a solid tumor, thereby providing a rationale for the generation of mixed hematopoietic chimerism by NST prior to tumor cell vaccination.
Introduction
Most patients with early-stage cancers of solid organs, including lung, breast, and colon, can be cured by surgical removal of the primary tumor. Unfortunately, many patients present or relapse with hematogenous metastases which, with rare exceptions, cannot be cured by currently available modalities, including surgery, radiation therapy, chemotherapy, or allogeneic stem cell transplantation (alloSCT). Likewise, although newer engineered cancer vaccines show significant potency in animal models of recently established disease, once the tumor has been established for more than 5 days or metastases have occurred, vaccines are generally ineffective as single agents.1 This is in part because tumor establishment is typically associated with induction of tolerance to tumor antigens, which must be broken to achieve successful therapy.2,3 Vaccination after myeloablative alloSCT has produced incremental improvements but is still unable to affect tumors established for more than 3 days.4 We report here that vaccination after a nonmyeloablative allogeneic stem cell transplantation (NST) protocol that achieves stable mixed bone marrow chimerism generates significantly enhanced tumor-specific immune responses capable of eliminating metastases 2 weeks after establishment of the primary tumor without inducing graft-versus-host disease (GVHD). The significantly enhanced efficacy of this strategy relative to vaccination alone or vaccination after either autologous SCT or full alloSCT depends on the action of both host and donor immune systems, which interact in the setting of mixed chimerism.
Materials and methods
Mice
DBA/2 (H-2d), BALB/cAnNCr (H-2d, Ly9.1+), and C.B17-scid (severe combined immunodeficiency disease) mice were obtained from the National Cancer Institute, Frederick, MD. B10.D2 (B10.D2-H2d H2-T18cHc0/oSnJ; H-2d, Ly9.1−) mice and CD40-deficient mice on the BALB/c genetic background (CNCr.129P2-Tnfrsf5tm1Kik) were obtained from Jackson Laboratories, Bar Harbor, ME. Mice were maintained in microisolator cages and were fed ad libitum with autoclaved laboratory chow and acidified water. All mice were approximately 8 to 12 weeks of age at the time of transplantation.
Tumor cell lines
4T1, a 6-thioguanine–resistant cell line derived from a spontaneous BALB/c mammary carcinoma, was obtained from Dr Fred R. Miller (Barbara Ann Karmanos Cancer Institute, Detroit, MI). CT26 is a colon epithelial tumor induced by intrarectal injections of BALB/c mice with N-nitroso-N-methylurethane.5DA-3, a mammary carcinoma of BALB/c origin, was kindly provided by Dr Eduardo Sotomayor (H. Lee Moffitt Cancer Center, Tampa, FL). NT2 was derived from a spontaneous mammary carcinoma of neu-N transgenic mice.6 Cells were cultured in vitro in Eagle Hanks amino acid (EHAA) medium (Biofluids, Rockville, MD), 10% fetal calf serum (FCS; GIBCO BRL, Gaithersburg, MD), 5 × 10−5 M 2-mercaptoethanol (2-ME), glutamine, and antibiotics (complete medium; CM). B78H1/GM-CSF, a major histocompatibility complex (MHC)–negative, granulocyte-macrophage colony-stimulating factor (GM-CSF)–producing melanoma cell line of C57BL/6 (H-2b) origin,7was maintained in CM supplemented with 1200 μg/mL hygromycin B (Boehringer-Mannheim, Indianapolis, IN).
Cell preparations
Donor spleens were removed aseptically and pressed through a nylon mesh to obtain single cell suspensions. Bone marrow cell suspensions were prepared by flushing sterile medium through the femoral and tibial marrow canals. Cell suspensions were vigorously pipetted, counted, and washed in sterile phosphate buffered saline (PBS) prior to injection.
Hematopoietic cell transplantation and GVHD assay
Animals were irradiated by a dual source 137Cs irradiator (Gammacell 40; Atomic Energy of Canada, Ottawa, ON) at an exposure rate of approximately 72 cGy/minute. Conditioning for NST consisted of 200-cGy total body irradiation (TBI) on the day before, and cyclophosphamide (Bristol-Myers, Evansville, IN) 200 mg/kg intraperitoneally 3 days after marrow transplantation. Donor cells were always injected into a tail vein in a final volume of 0.5 mL PBS. In some experiments, T cells were depleted from donor splenocytes by incubation with antibodies to CD4 (RL172.4; gift of Dr Albert Bendelac, Princeton University, Princeton, NJ), CD8 (3.155; American Type Culture Collection, Manassas, VA), and guinea pig complement (GIBCO BRL), as previously described.8 Primed lymphocytes were obtained from DBA/2 or B10.D2 mice that had received an intraperitoneal injection of 10 to 20 million BALB/c splenocytes 2 to 3 weeks prior to killing them. To quantify GVHD, 15 μg lipopolysaccharide (LPS; Escherichia coli O55:B5, Calbiochem-Novabiochem, San Diego, CA) was administered 2 weeks after DLI, and death within 24 hours was scored.
Ld-IgG, antibodies, cell staining, and flow cytometric analysis
Splenocytes from unimmunized or vaccinated animals were obtained, and red blood cells were removed by using an ammonium chloride lysis buffer. The single cell suspensions were labeled with an antibody against CD8 and were passed through a CD8+ T-cell isolation column (Miltenyi Biotec, Auburn, CA). At least 4 million purified CD8+ T cells, obtained from the column eluate, were stimulated with 10 μg/mL AH1 (SPSYVYHQF) peptide in 2 mL medium containing 10 U/mL interleukin 2 (IL-2). One week later, 1 million CD8+ T cells were incubated on ice with approximately 100 ng Ld-immunoglobulin G (IgG1) loaded with AH1 or control β-gal peptide (TPHPARIGL), and stained for flow cytometry by using anti-CD8–CyChrome (BD PharMingen, San Diego, CA), biotinylated antihuman IgG1(BD PharMingen), phycoerythrin (PE)–streptavidin (Caltag, Burlingame, CA), and anti-Ly9.1–fluorescein isothiocyanate (FITC; BD PharMingen), as described.9
The AH1423-431/Ld tetramer (AH1 tetramer), conjugated with PE, was obtained through the National Institute of Allergy and Infectious Diseases (NIAID) Tetramer Facility and the National Institutes of Health (NIH) AIDS Research and Reference Reagent program. Splenocytes were collected, and erythrocytes were lysed by using standard procedures followed by washing with flow cytometry buffer. After washing, splenocytes were resuspended at 2 × 107 cells/mL in flow cytometry buffer containing “Fc block”(anti-CD16/CD32; BD PharMingen) for 10 minutes at room temperature. After washing, cells were triple-stained using PE-conjugated AH1 tetramer, FITC-conjugated anti-Ly9.1, and CyChrome-conjugated anti-CD8 Cy antibody and incubated for 45 minutes at room temperature. After 2 washes cells were immediately analyzed by flow cytometry.
Mixed lymphocyte cultures and immunologic cytotoxicity assays
Four million responder cells were cultured with 2 × 106 irradiated (30 Gy) stimulator spleen cells or 2 × 105 irradiated (100 Gy) 4T1 cells in individual wells of a 24-well plate, each well containing 2 mL CM. For depletion experiments spleens were collected from vaccinated and control mice, single cell suspensions were prepared and washed in PBS, and red blood cells were removed by using an ammonium chloride lysis buffer. The single cell suspensions were labeled with a biotinylated antibody against Ly 9.1 and avidin-conjugated beads and then were passed through a Miltenyi column (Miltenyi Biotec). Ly9.1+ cells of BALB/c origin were eluted from the column and stimulated as outlined earlier except for the addition of 10 U/mL recombinant mouse IL-2. After 5 days of culture in a 37°C, 5% CO2 incubator, responder cells were harvested, washed, and plated for 4 hours, in a standard chromium release assay, at various responder-to-target (R/T) ratios in triplicate wells of 96-well U-bottom plates, each well containing 0.2 mL CM and 10451Cr-labeled target cells (spleen cells cultured for 48 hours in CM containing 2 μg/mL Concanavalin A or tumor cells). Each data point was performed in triplicate and then averaged. Responder-to-target ratios indicated in the figure are based on the initial number of responder cells plated in mixed lymphocyte culture.
In vivo tumor growth and surgery
Primary tumors were measured every 3 to 4 days by calipers. The mean tumor diameter was calculated as the square root of the product of 2 perpendicular diameters. At designated time points, mice were anesthetized with metophane (Pittman-Moore, Mundelein, IN), and, once the animals were unconscious, tumors were removed surgically using sterile technique. Following surgical removal of tumors with minimal removal of surrounding tissue, wound sites were closed with clips, which were removed 8 to 10 days after surgery. In all experiments, animals that appeared severely ill or moribund were humanely killed to prevent suffering and were examined for the presence of visible metastatic disease.
Statistical analyses
All survival data were analyzed by the nonparametric rank sum test of Wilcoxon. Mean tumor sizes were compared by using the analysis of variance (ANOVA) test. P < .05 was considered statistically significant.
Results
Vaccination after an NST + DLI protocol achieving mixed chimerism cures animals with established metastatic cancer
To model the common but extremely difficult clinical scenario of established metastatic cancer, we chose to study 4T1, a poorly immunogenic mammary cancer of BALB/c origin that metastasizes to lung, liver, and brain within 13 days of subcutaneous injection. By day 13, the disease cannot be cured by surgery, chemotherapy, or lethal TBI followed by alloSCT. Consistent with previous observations, we found that allogeneic T cells could retard the development and/or growth of systemic metastases10,11(supplemental Figure S1a on the Blood website; see the Supplemental Figure link at the top of the online article), but only if the T cells had been taken from donors previously primed with cells that are syngeneic to the tumor. However, primed cells caused significant GVHD when administered shortly after lethal conditioning and alloSCT,12,13 and they ultimately failed to control the growth of the primary tumor.14 These results prompted 3 changes in the therapeutic approach, depicted in Figure1A.
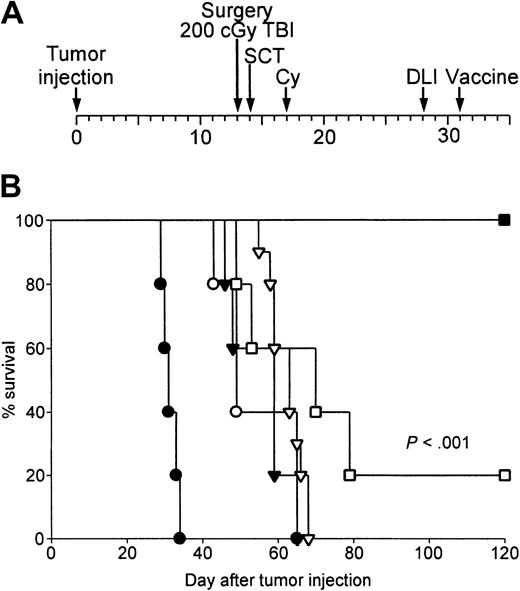
Successful therapy of metastatic breast cancer with surgery, NST, DLI, and autologous tumor cell vaccines.
(A) Experimental scheme. (B) BALB/c mice received 104 4T1 cells subcutaneously on day 0. The subcutaneous tumor was resected on day 13, prior to NST from syngeneic BALB/c or MHC-compatible B10.D2 donors. NST consisted of 200 cGy TBI on day 13, 10 million donor marrow cells intravenously on day 14, and cyclophosphamide 200 mg/kg intraperitoneally on day 17. Mice receiving B10.D2 marrow then received nothing (○), 20 million B10.D2 splenocytes on day 28 (▾), autologous tumor vaccine on day 31 (▿; 106 irradiated autologous tumor cells mixed with 5 × 105 B78H1/GM-CSF, a GM-CSF–secreting, MHC-negative bystander cell line), or 20 million B10.D2 splenocytes plus vaccine (▪). Mice receiving BALB/c marrow also received 20 million BALB/c splenocytes plus vaccine (■). An additional group of BALB/c mice received 4T1 subcutaneously on day 0 and surgery on day 13 without further therapy (●). Tumor-free survival is plotted as a function of time after subcutaneous tumor inoculation. This experiment has been repeated twice, but without syngeneic NST controls, with similar results.
Successful therapy of metastatic breast cancer with surgery, NST, DLI, and autologous tumor cell vaccines.
(A) Experimental scheme. (B) BALB/c mice received 104 4T1 cells subcutaneously on day 0. The subcutaneous tumor was resected on day 13, prior to NST from syngeneic BALB/c or MHC-compatible B10.D2 donors. NST consisted of 200 cGy TBI on day 13, 10 million donor marrow cells intravenously on day 14, and cyclophosphamide 200 mg/kg intraperitoneally on day 17. Mice receiving B10.D2 marrow then received nothing (○), 20 million B10.D2 splenocytes on day 28 (▾), autologous tumor vaccine on day 31 (▿; 106 irradiated autologous tumor cells mixed with 5 × 105 B78H1/GM-CSF, a GM-CSF–secreting, MHC-negative bystander cell line), or 20 million B10.D2 splenocytes plus vaccine (▪). Mice receiving BALB/c marrow also received 20 million BALB/c splenocytes plus vaccine (■). An additional group of BALB/c mice received 4T1 subcutaneously on day 0 and surgery on day 13 without further therapy (●). Tumor-free survival is plotted as a function of time after subcutaneous tumor inoculation. This experiment has been repeated twice, but without syngeneic NST controls, with similar results.
First, to reduce the risk of local complications, the subcutaneous primary tumor was removed surgically prior to alloSCT. Second, to reduce the risk of GVHD,15 we used nonmyeloablative conditioning, consisting of 200-cGy TBI 1 day before and cyclophosphamide (Cy) 200 mg/kg intraperitoneally 3 days after the transplantation of B10.D2 (H-2d) marrow cells. This regimen consistently results in the induction of mixed hematopoietic chimerism, the stable coexistence of donor and host blood cells, without GVHD.16 Importantly, previous work has shown that alloreactive lymphocytes within mixed chimeras, even those transfused from donors primed against host antigens, can protect against17 or even mediate the regression of established solid tumors without causing lethal GVHD.18 Third, to augment the activity of DLIs against metastases, we administered a tumor vaccine, comprising 106 irradiated 4T1 cells mixed with 5 × 105 irradiated B78H1–GM-CSF, a GM-CSF–secreting, MHC-negative “bystander” melanoma cell line.7 In mouse models, vaccines containing a paracrine source of GM-CSF elicit protective antitumor immunity when administered after syngeneic1 or T-cell–depleted myeloablative allogeneic SCT.19 However, tumor vaccines administered after myeloablative SCT are typically ineffective in the treatment of tumors that have been established in vivo for longer than 3 days.4,20 21
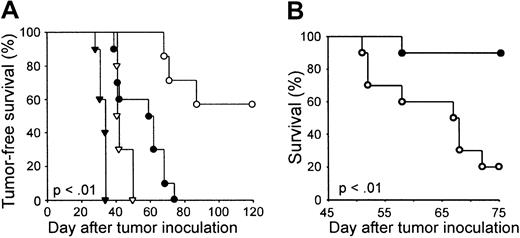
Remarkably, all 5 BALB/c mice that were treated with surgery on day 13 after tumor injection, NST on day 14, DLI (20 million unprimed B10.D2 spleen cells intravenously) on day 28, and tumor vaccine on day 31, survived tumor free for more than 120 days (Figure 1B). The survival of these animals was significantly longer (P < .001) than the survival of animals that received all components of the therapy except for DLI or tumor vaccine, and of animals that received syngeneic marrow, syngeneic DLI, and vaccine. This latter result suggests that an allogeneic graft-versus-host reaction augmented the antitumor immune response rather than harmed it. Four of 5 cured animals, but none of 5 control animals not receiving transplants, also survived tumor free for an additional 120 days after intravenous rechallenge with 104 4T1 cells, indicating the presence of long-term immunity to the tumor (data not shown).
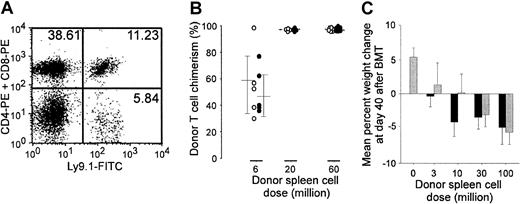
Tumor vaccines do not augment DLI-induced GVHD
In humans, the establishment of complete donor hematopoietic chimerism accompanies DLI-induced graft-versus-tumor reactions. However, the 4 animals that resisted 4T1 rechallenge following curative therapy were stable, long-term mixed hematopoietic chimeras (Figure2A), containing a mean (± SD) of 64.3% (± 8.81%) donor T cells. None of these animals, nor any control animals receiving NST, DLI, and tumor vaccine without tumor challenge, demonstrated any histologic or clinical signs of GVHD, such as dermatitis, diarrhea, weight loss, hunched posture, or ruffled fur (data not shown). However, because the vaccine contains autologous tumor cells, a source of host histocompatibility antigens, and GM-CSF, a potent growth factor for dendritic cells, we tested whether the vaccine exacerbates GVHD by using 3 sensitive assays: LPS-induced mortality, augmentation of donor T-cell chimerism, and weight loss.22 Fourteen days after receiving NST from unprimed B10.D2 donors, groups of 9 to 11 BALB/c mice received graded doses of BALB/c-sensitized B10.D2 spleen cells, with or without 4T1/B78H1–GM-CSF vaccine. Two weeks later, 5 mice per group received an intravenous injection of LPS, which kills mice with subclinical GVHD.23 Regardless of whether vaccine was administered, all mice receiving 20 million or more primed spleen cells died following LPS injection, whereas all mice receiving 6 million or fewer primed spleen cells lived (Table 1). Donor T-cell chimerism, which was measured at 12 weeks after DLI with or without vaccine in the remaining mice, was also not significantly augmented by vaccine administration (Figure 2B). Because establishment of full donor T-cell chimerism precedes and is associated with a higher risk of clinical GVHD, this result suggests that vaccine administration does not increase the risk of GVHD following DLI. Finally, infusion of unprimed B10.D2 spleen cells induced a dose-dependent weight loss in sublethally irradiated, immunodeficient C.B17-scid mice, which are unusually sensitive to GVHD (Figure2C). However, mice receiving the GM-CSF–based autologous tumor cell vaccine 3 days after DLI did not lose more weight than comparable unvaccinated mice. Thus, by 3 sensitive measures, posttransplantation administration of the autologous tumor cell vaccine did not augment GVHD after NST.
Autologous tumor cell vaccines do not exacerbate DLI-induced GVHD after NST.
(A) Cured animals are mixed hematopoietic chimeras. Peripheral blood from animals cured of 4T1 by surgery, NST, DLI, and vaccine was obtained 6 months after DLI and vaccine administration and stained with FITC-conjugated antibody against Ly9.1, which is specific for host BALB/c lymphocytes, and PE-conjugated antibodies against CD4 and CD8. Dual-color flow cytometry from a representative animal is shown. (B-C) Effects on graft-versus-host disease (Table 1). (B) Graded doses of spleen cells (from 0 to 60 million) from BALB/c-sensitized B10.D2 donors were administered, with or without 4T1/B78H1–GM-CSF vaccine, to groups of 9 to 11 BALB/c mice that had received NST (as described in the legend to Figure 1) 2 weeks earlier. (B) Twelve weeks after spleen cell infusion, donor T-cell chimerism was measured by staining splenocytes of recipients (n = 4-6 per group) with FITC-conjugated antibody against Ly9.1 (host) and PE-conjugated antibodies against CD4 and CD8. ○ indicates individual vaccinated animals; ●, individual nonvaccinated animals. (C) C.B17-scid mice (n = 5 per group) received 200 cGy TBI on day −1 and graded doses (from 0 to 100 million) of unprimed B10.D2 spleen cells on day 0, with or without 4T1/B78H1–GM-CSF vaccine on day 3. Mice were weighed on day −1 and day 40, and percentage of weight change from baseline is plotted as a function of spleen cell dose. ░ indicates vaccinated animals; ▪, nonvaccinated animals.
Autologous tumor cell vaccines do not exacerbate DLI-induced GVHD after NST.
(A) Cured animals are mixed hematopoietic chimeras. Peripheral blood from animals cured of 4T1 by surgery, NST, DLI, and vaccine was obtained 6 months after DLI and vaccine administration and stained with FITC-conjugated antibody against Ly9.1, which is specific for host BALB/c lymphocytes, and PE-conjugated antibodies against CD4 and CD8. Dual-color flow cytometry from a representative animal is shown. (B-C) Effects on graft-versus-host disease (Table 1). (B) Graded doses of spleen cells (from 0 to 60 million) from BALB/c-sensitized B10.D2 donors were administered, with or without 4T1/B78H1–GM-CSF vaccine, to groups of 9 to 11 BALB/c mice that had received NST (as described in the legend to Figure 1) 2 weeks earlier. (B) Twelve weeks after spleen cell infusion, donor T-cell chimerism was measured by staining splenocytes of recipients (n = 4-6 per group) with FITC-conjugated antibody against Ly9.1 (host) and PE-conjugated antibodies against CD4 and CD8. ○ indicates individual vaccinated animals; ●, individual nonvaccinated animals. (C) C.B17-scid mice (n = 5 per group) received 200 cGy TBI on day −1 and graded doses (from 0 to 100 million) of unprimed B10.D2 spleen cells on day 0, with or without 4T1/B78H1–GM-CSF vaccine on day 3. Mice were weighed on day −1 and day 40, and percentage of weight change from baseline is plotted as a function of spleen cell dose. ░ indicates vaccinated animals; ▪, nonvaccinated animals.
Effects of vaccine on graft-versus-host disease
| Sensitized donor cell dose, million . | Dead/total . | |
|---|---|---|
| Unvaccinated . | Vaccinated . | |
| 0 | ND | 0/5 |
| 6 | 0/5 | 0/5 |
| 20 | 5/5 | 5/5 |
| 60 | 5/5 | 5/5 |
| Sensitized donor cell dose, million . | Dead/total . | |
|---|---|---|
| Unvaccinated . | Vaccinated . | |
| 0 | ND | 0/5 |
| 6 | 0/5 | 0/5 |
| 20 | 5/5 | 5/5 |
| 60 | 5/5 | 5/5 |
Two weeks after spleen cell infusion, 5 mice per group received 15 μg E coli LPS intravenously, and mortality was scored 24 hours later.
ND indicates not done.
Requirements for protective or therapeutic antitumor immunity
The bone marrow transplantation (BMT) procedure and posttransplantation vaccination were both required for the induction of long-term protection against tumor challenge. Four of 5 BALB/c mice not receiving transplants died after a tumor challenge administered 2 weeks after vaccination, as did 5 of 5 BALB/c mice that received NST and DLI without vaccine 4 months earlier (Figure3B).
Protective and therapeutic antitumor immunity requires allogeneic cell transplantation, and CD4+ and CD8+ T cells in DLI and autologous tumor cell vaccination.
(A) Scheme of experiment in panel B. (B) Protective antitumor immunity requires NST and posttransplantation vaccine. BALB/c mice underwent NST from B10.D2 donors. Beginning 2 weeks after transplantation, chimeras then received nothing (▾), 20 million B10.D2 splenocytes intravenously (▿), or 20 million B10.D2 splenocytes and 4T1 tumor vaccine (▪; N = 5 per group), as shown in panel A. Four months later, all mice that received transplants, and groups of 5 untreated (●) or vaccinated BALB/c controls (○), were challenged with 104 4T1 cells intravenously. Tumor-free survival is plotted as a function of time after intravenous tumor challenge. (C) Scheme of experiment in panel D. (D) Therapeutic antitumor immunity requires CD4+ and CD8+ T cells in the DLI. BALB/c mice bearing 13-day-old 4T1 tumors received surgery and NST from B10.D2 donors as illustrated in Figure 2A. On day 28, mice received 2 × 107 B10.D2 splenocytes, either unmodified (●, ○) or depleted of CD4+ (▾) or CD8+ (▿) T cells. Three days later, mice received 4T1 vaccine (●, ▾, ▿) or nothing (○). Tumor-free survival was then monitored. (E) Therapeutic antitumor immunity requires autologous cells in the vaccine. Tumor-bearing BALB/c mice received surgery, NST, DLI, and vaccine consisting of the bystander line mixed with nothing (▾), irradiated 4T1 cells (●), irradiated DA-3 cells (○; a BALB/c breast cancer), or irradiated NT-2 cells (▿; a mammary cancer of FVB/N [H-2q] mice). Tumor-free survival is plotted as a function of time after tumor injection.
Protective and therapeutic antitumor immunity requires allogeneic cell transplantation, and CD4+ and CD8+ T cells in DLI and autologous tumor cell vaccination.
(A) Scheme of experiment in panel B. (B) Protective antitumor immunity requires NST and posttransplantation vaccine. BALB/c mice underwent NST from B10.D2 donors. Beginning 2 weeks after transplantation, chimeras then received nothing (▾), 20 million B10.D2 splenocytes intravenously (▿), or 20 million B10.D2 splenocytes and 4T1 tumor vaccine (▪; N = 5 per group), as shown in panel A. Four months later, all mice that received transplants, and groups of 5 untreated (●) or vaccinated BALB/c controls (○), were challenged with 104 4T1 cells intravenously. Tumor-free survival is plotted as a function of time after intravenous tumor challenge. (C) Scheme of experiment in panel D. (D) Therapeutic antitumor immunity requires CD4+ and CD8+ T cells in the DLI. BALB/c mice bearing 13-day-old 4T1 tumors received surgery and NST from B10.D2 donors as illustrated in Figure 2A. On day 28, mice received 2 × 107 B10.D2 splenocytes, either unmodified (●, ○) or depleted of CD4+ (▾) or CD8+ (▿) T cells. Three days later, mice received 4T1 vaccine (●, ▾, ▿) or nothing (○). Tumor-free survival was then monitored. (E) Therapeutic antitumor immunity requires autologous cells in the vaccine. Tumor-bearing BALB/c mice received surgery, NST, DLI, and vaccine consisting of the bystander line mixed with nothing (▾), irradiated 4T1 cells (●), irradiated DA-3 cells (○; a BALB/c breast cancer), or irradiated NT-2 cells (▿; a mammary cancer of FVB/N [H-2q] mice). Tumor-free survival is plotted as a function of time after tumor injection.
In contrast, all 5 animals that received NST, DLI, and vaccine resisted tumor challenge 4 months later (P < .001 versus NST ± DLI). Likewise, the optimal therapeutic effect required both CD4+ and CD8+ T cells in the DLI, as depletion of either T-cell subset from the transfused spleen cells diminished the tumor-free survival of tumor-bearing mice undergoing surgery, NST, DLI, and tumor vaccine (P < .001; Figure 3D). In addition, tumor-free survival of 4T1 tumor-bearing mice was also diminished if irradiated 4T1 cells were omitted from the vaccine, or if they were replaced by irradiated DA-3 cells, a breast cancer of BALB/c origin, or by irradiated NT-2 cells, a breast cancer of FVB/N (H-2q) origin (P < .001; Figure 3E). This result indicates the use of autologous tumor cells in the vaccine is required for the cure of most of the tumor-bearing animals.
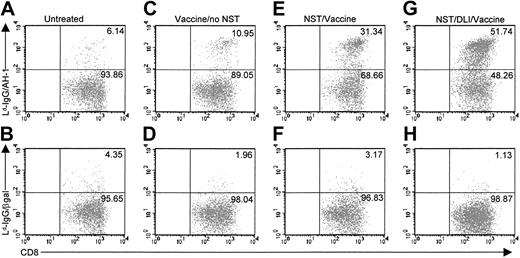
Vaccination after NST induces T cells specific for tumor-associated antigens
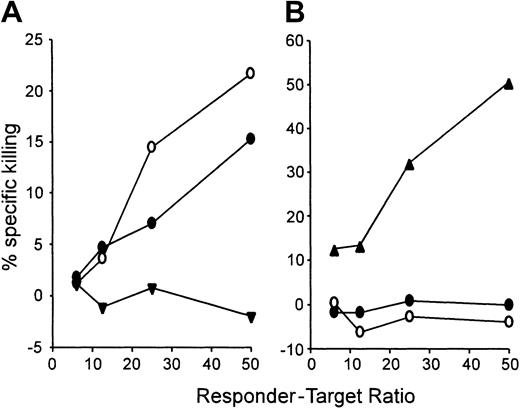
The long-lived resistance of cured animals to tumor challenge in the absence of ongoing GVHD suggests that the tumor vaccine induced an immune response against tumor-associated antigens (TAAs) with restricted tissue distribution. Consistent with this hypothesis, killer cells against 4T1 targets were generated by culturing spleen cells from the cured animals with irradiated 4T1 cells in vitro (Figure4A), but culture of the same spleen cells with irradiated BALB/c cells failed to generate killers against BALB/c spleen targets (Figure 4B). This latter finding correlates with the maintenance of mixed hematopoietic chimerism in mice receiving the combination of NST, DLI, and vaccine. To assess whether posttransplantation immunization induced antigen-specific T cells, we exploited 4T1's expression of AH1, an MHC class I (H-2Ld)-restricted, TAA derived from GP70, the envelope glycoprotein of an endogenous ecotropic murine leukemia virus.24,25 AH1 is expressed by other tumors of BALB/c origin but not by normal adult tissue; thus, it is a tumor-associated rather than a tumor-specific antigen.24GP70gene expression in 4T1 cells was confirmed by reverse transcription polymerase chain reaction (RT-PCR), and presentation of AH1 by H-2Ld was verified by sensitivity to killing by an AH1-specific, H-2Ld-restricted T-cell clone (data not shown). To directly quantitate AH1-specific CD8+ cells, we have used AH1 peptide-loaded complexes of Ld-IgG dimers.9 Purified CD8+ cells from immunized or unimmunized mice were stimulated for 7 days in vitro with AH1 peptide and IL-2 and then analyzed with Ld-IgG dimers using flow cytometry. Compared with the percentage of AH1-specific T cells in unvaccinated BALB/c mice (Figure 5A), administration of a single vaccine, consisting of 4T1 cells mixed with a GM-CSF–producing bystander cell line, led to a small but detectable increase in the percentage of AH1-specific T cells (Figure 5C). In contrast, AH1-specific T cells were readily detected in the spleens of BALB/c mice that received a single vaccine after NST from B10.D2 donors (Figure 5E). Chimeras that received DLI prior to vaccine administration also had a high percentage of AH1-specific T cells at 6 weeks (Figure5G) or 4 months (data not shown) after vaccination. Regardless of whether or not mice received transplants, tumor vaccination did not induce H-2Ld-restricted T cells against an irrelevant peptide from β-galactosidase (Figure 5D,F,H). The detection of AH1-specific T cells 4 months after immunization of a chimera indicates that posttransplantation vaccination induced long-lived antigen-specific T cells. Taken together with the absence of increased GVHD, our results suggest that the combination of NST, DLI, and vaccine selectively activates tumor-specific T cells relative to T cells specific for widely expressed host minor histocompatibility antigens.
Chimeras that have been cured of metastatic breast cancer exhibit CTL activity against tumor cells but tolerance of normal host antigens.
Splenocytes from representative cured animals (●, ○), from unprimed BALB/c mice (▾), or from B10.D2 mice primed previously with BALB/c cells (▴) were cultured for 5 days with irradiated 4T1 cells or BALB/c splenocytes and then tested for killing of 4T1 (A) or BALB/c (B) targets in a 4-hour chromium release assay.
Chimeras that have been cured of metastatic breast cancer exhibit CTL activity against tumor cells but tolerance of normal host antigens.
Splenocytes from representative cured animals (●, ○), from unprimed BALB/c mice (▾), or from B10.D2 mice primed previously with BALB/c cells (▴) were cultured for 5 days with irradiated 4T1 cells or BALB/c splenocytes and then tested for killing of 4T1 (A) or BALB/c (B) targets in a 4-hour chromium release assay.
Vaccine-induced expansion of tumor-specific T cells is enhanced by NST and administration of DLI.
BALB/c mice (A-D) or B10.D2→BALB/c chimeras generated on day 0 by NST (E-H, see legend to Figure 1) received no treatment (A-B), autologous tumor cell vaccine (C-F), or 20 million B10.D2 spleen cells followed by autologous tumor cell vaccine (G-H). Six weeks after vaccine administration, recipients' spleens were harvested, and CD8+ T cells were purified and incubated for 7 days with AH1 peptide in complete medium supplemented with 10 U/mL IL-2. AH1-specific T cells in culture were identified by 2-color flow cytometry after staining with CyChrome-conjugated antibody to mouse CD8 and Ld-Ig dimers loaded with AH1 peptide, followed by biotinylated goat antibody to mouse IgG1 and phycoerythrin-conjugated avidin (A,C,E,G). As a negative control, T cells were also stained with Ld-Ig dimers loaded with an irrelevant peptide from mouse β-galactosidase (Ld-Ig/β-gal; B,D,F,H).
Vaccine-induced expansion of tumor-specific T cells is enhanced by NST and administration of DLI.
BALB/c mice (A-D) or B10.D2→BALB/c chimeras generated on day 0 by NST (E-H, see legend to Figure 1) received no treatment (A-B), autologous tumor cell vaccine (C-F), or 20 million B10.D2 spleen cells followed by autologous tumor cell vaccine (G-H). Six weeks after vaccine administration, recipients' spleens were harvested, and CD8+ T cells were purified and incubated for 7 days with AH1 peptide in complete medium supplemented with 10 U/mL IL-2. AH1-specific T cells in culture were identified by 2-color flow cytometry after staining with CyChrome-conjugated antibody to mouse CD8 and Ld-Ig dimers loaded with AH1 peptide, followed by biotinylated goat antibody to mouse IgG1 and phycoerythrin-conjugated avidin (A,C,E,G). As a negative control, T cells were also stained with Ld-Ig dimers loaded with an irrelevant peptide from mouse β-galactosidase (Ld-Ig/β-gal; B,D,F,H).
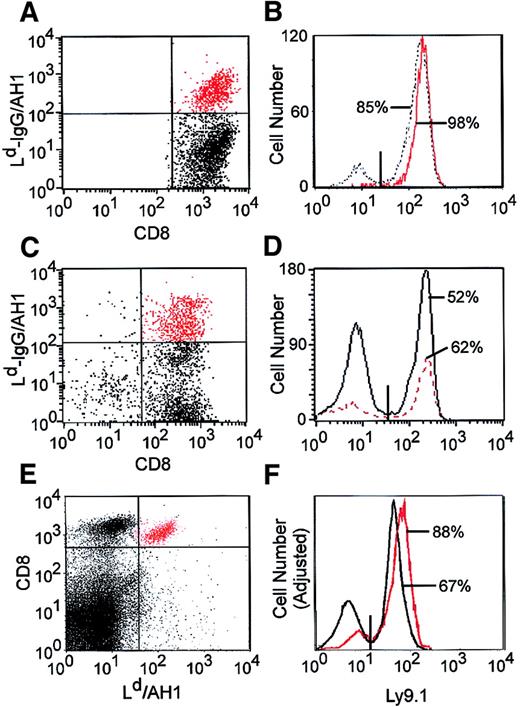
After NST/DLI, most of vaccine-induced, tumor-specific T cells are derived from the host
Because the NST protocol used here results in stable mixed hematopoietic chimerism (Figure 2A), we characterized the origin (donor versus host) of the tumor-specific T cells elicited by vaccination. BALB/c mice received NST, DLI, and 4T1 vaccine as in Figure 5G, spleen cells from these animals were stimulated for 7 days with AH1 peptide and IL-2, and total versus AH1-specific CD8+ T cells were characterized for the expression of the Ly9.1 allele of host BALB/c origin. Surprisingly, 85% of total CD8+ T cells and 98% of AH1-specific CD8+ T cells were of host origin (Figure 6A-B). In a second experiment, BALB/c mice were treated as in Figure 5G but vaccinated with CT26, a BALB/c colon cancer that expresses high levels of AH124 and induces a robust AH1-specific T-cell response. Following in vitro stimulation with AH1 peptide, 52% of CD8+ T cells and 62% of AH1-specific CD8+ T cells were host derived (Figure 6C-D).
The Ld-IgG dimers that were used to identify AH1-specific T cells could not detect such cells directly from the spleen or peripheral blood of vaccinated animals; hence, the requirement for a period of in vitro stimulation with AH1 peptide and IL-2 to expand these cells above the threshold level of detection. To avoid the potential for skewing of the relative percentages of donor versus host T cells by in vitro stimulation, H-2Ld tetramers were synthesized and used to detect AH1-specific T cells directly from the spleen. AH1-specific CD8+ T cells could not be detected in the spleens of untreated BALB/c mice (data not shown) but were readily detectable in the spleens (or peripheral blood; data not shown) of BALB/c mice treated with NST, DLI, and a CT26 vaccine (Figure 6E); of these cells, 88% were derived from the donor and 12% were derived from the host (Figure 6F). Thus, a significant percentage of host T cells participates in the immune response to tumor cell vaccination after NST.
Vaccine-induced, AH1-specific T cells arise from donor and host.
(A) BALB/c mice received NST, DLI, and 4T1 tumor cell vaccine as described in Figure 3A. Four months after vaccine administration, splenic CD8+ T cells were purified and cultured for 1 week with AH1 peptide and IL-2, and viable cells were stained with Ld-IgG/AH1 dimers, biotinylated antihuman IgG, PE-streptavidin, CyChrome-conjugated anti-CD8, and FITC-conjugated anti-Ly9.1. (B) The host (Ly9.1+) versus donor origin of total CD8+ (black line) and AH1-specific CD8+ T cells (red line) is shown. (C-D) Same as in A and B, except that the vaccine consisted of 106 CT26 cells and 5 × 105 B78H1–GM-CSF cells, which were mixed together and irradiated (5000 cGy). (E) Spleen cells were taken from BALB/c mice treated with NST, DLI, and CT26 vaccine (as in C-D) and were stained directly ex vivo with FITC-conjugated anti-Ly9.1, CyChrome-conjugated anti-CD8, and PE-conjugated Ld/AH1 tetramers. (F) The host (Ly9.1+) versus donor origin of total CD8+(black line) and AH1-specific CD8+ T cells (red line) is shown.
Vaccine-induced, AH1-specific T cells arise from donor and host.
(A) BALB/c mice received NST, DLI, and 4T1 tumor cell vaccine as described in Figure 3A. Four months after vaccine administration, splenic CD8+ T cells were purified and cultured for 1 week with AH1 peptide and IL-2, and viable cells were stained with Ld-IgG/AH1 dimers, biotinylated antihuman IgG, PE-streptavidin, CyChrome-conjugated anti-CD8, and FITC-conjugated anti-Ly9.1. (B) The host (Ly9.1+) versus donor origin of total CD8+ (black line) and AH1-specific CD8+ T cells (red line) is shown. (C-D) Same as in A and B, except that the vaccine consisted of 106 CT26 cells and 5 × 105 B78H1–GM-CSF cells, which were mixed together and irradiated (5000 cGy). (E) Spleen cells were taken from BALB/c mice treated with NST, DLI, and CT26 vaccine (as in C-D) and were stained directly ex vivo with FITC-conjugated anti-Ly9.1, CyChrome-conjugated anti-CD8, and PE-conjugated Ld/AH1 tetramers. (F) The host (Ly9.1+) versus donor origin of total CD8+(black line) and AH1-specific CD8+ T cells (red line) is shown.
The host immune system participates in antitumor immunity
Studies have shown that cell-based tumor vaccines, administered after high-dose TBI and syngeneic or allogeneic SCT, can induce protective antitumor immunity4,19-21 but fail to eradicate even recently established disease.4 20 Lethal conditioning and alloSCT generally result in the establishment of complete donor myeloid and lymphoid chimerism and, consequently, ablation of host immunity. On the basis of the host origin of tumor-specific T cells shown earlier, we hypothesized that the ability of tumor vaccines to eradicate disseminated cancer after NST has induced mixed chimerism is due, at least in part, to the preservation of a host antitumor response. To characterize the role of host immunity in vivo, the antitumor effect of surgery, NST, DLI, and tumor vaccine was compared in BALB/c versus immunodeficient C.B17-scid recipients. Tumor-free survival following surgery alone was slightly prolonged in BALB/c mice compared with scid recipients, although no animal was cured (Figure 7A). The superior survival of BALB/c mice demonstrates the existence of a weak endogenous antitumor immune response that fails to eradicate the malignancy in the absence of active or adoptive immunotherapy. With addition of NST, DLI, and tumor vaccine, cure of metastatic mammary cancer was achieved in most of the BALB/c mice but in none of the scid recipients, indicating that a host immune system is indeed required for cure of 4T1. We are currently conducting experiments in which host T cells are eliminated from mixed chimeras just prior to vaccination, to define more precisely their contribution to the vaccine-induced antitumor immune response.
Host T cells participate in the therapeutic response to NST, DLI, and autologous tumor cell vaccines.
(A) Groups of 10 C.B17-scid (▾, ●) or BALB/c mice (▿, ○) received 104 4T1 cells subcutaneously on day 0 followed by surgery on day 13 (▾, ▿), or surgery on day 13 plus NST, DLI, and autologous tumor cell vaccine (●, ○), as schematized in Figure 1A. (B) Comparison of NST to myeloablative alloSCT. Two groups of 10 BALB/c mice each received 104 4T1 cells subcutaneously on day 0, surgery on day 13, 107 B10.D2 bone marrow cells intravenously on day 14, 2 × 107 B10.D2 spleen cells intravenously on day 28, and autologous tumor cell vaccine on day 31. Conditioning for alloSCT consisted of 200 cGy TBI (●) or 850 cGy TBI (○) on day 13 and Cy 200 mg/kg intraperitoneally on day 17. Tumor-free survival is plotted as a function of day after tumor inoculation.
Host T cells participate in the therapeutic response to NST, DLI, and autologous tumor cell vaccines.
(A) Groups of 10 C.B17-scid (▾, ●) or BALB/c mice (▿, ○) received 104 4T1 cells subcutaneously on day 0 followed by surgery on day 13 (▾, ▿), or surgery on day 13 plus NST, DLI, and autologous tumor cell vaccine (●, ○), as schematized in Figure 1A. (B) Comparison of NST to myeloablative alloSCT. Two groups of 10 BALB/c mice each received 104 4T1 cells subcutaneously on day 0, surgery on day 13, 107 B10.D2 bone marrow cells intravenously on day 14, 2 × 107 B10.D2 spleen cells intravenously on day 28, and autologous tumor cell vaccine on day 31. Conditioning for alloSCT consisted of 200 cGy TBI (●) or 850 cGy TBI (○) on day 13 and Cy 200 mg/kg intraperitoneally on day 17. Tumor-free survival is plotted as a function of day after tumor inoculation.
Finally, because the death of host T cells and antigen-presenting cells (APCs) is proportional to the dose of TBI used for transplantation conditioning, we predicted that increasing the dose of TBI would paradoxically increase the risk of relapse. Indeed, tumor-free survival was significantly longer in mice treated with DLI and vaccine after 200 cGy TBI and NST than in mice receiving the same treatment after 850 cGy TBI, a standard myeloablative conditioning regimen, and alloSCT (Figure 7B). Metastatic lung nodules were found in all of the recipients of myeloablative conditioning that were humanely killed (data not shown), indicating that posttransplantation vaccination of these animals was not sufficient to achieve tumor eradication. These results suggest that preservation of host immunity by induction of mixed hematopoietic chimerism optimizes the therapeutic outcome of posttransplantation tumor cell vaccination.
Discussion
AlloSCT was originally developed as a method of rescuing patients with leukemia from fatal aplasia after attempts were made to destroy all malignant cells with high-dose TBI with or without cytotoxic chemotherapy. Protocols using reduced intensity conditioning prior to alloSCT were undertaken only after it was demonstrated that adoptive immunotherapy alone is sufficient to induce sustained remissions in patients with relapsed leukemia after alloSCT.26 Because animal and human studies showed that graft-versus-leukemia reactions are associated with and/or follow the establishment of complete donor hematopoietic chimerism,15,27 initial clinical trials of NST were designed to achieve exclusively donor-derived hematopoiesis, for example by scheduling DLIs for patients with mixed hematopoietic chimerism after the initial procedure.27,28 In light of the substantial evidence for specific2,3,29 and generalized30 immune response defects in patients with cancer, the loss of host immunity following alloSCT/DLI may not have been considered to be detrimental to the patient's long-term prognosis. However, the value of establishing complete donor chimerism after alloSCT has not been assessed rigorously in the treatment of tumors of solid organs.
The studies undertaken here demonstrate cooperation between host and donor T cells in the eradication of metastatic cancer in the setting of mixed chimerism following NST. Indeed, the finding of host-derived cytotoxic T lymphocytes (CTLs) against tumor cells and an improved therapeutic outcome in immunocompetent recipients suggest that endogenous T cells are not rendered irreversibly tolerant of tumor-associated antigens by the presence of growing tumor cells. In this regard, data suggest that, in mice with growing tumors, tumor-specific CD8+ T cells exist in a state of partial activation that is insufficient for tumor rejection31 but can be prodded to mediate tumor regression by a combination of tumor vaccination and administration of an agonistic antibody against CD40.32-34 It is therefore possible that tolerance in tumor-specific CD4+ T cells is the major cause of failure of the immune system to reject growing tumors3,35 and that tumor regression may be stimulated by enhanced presentation of tumor antigen to CD8+ T cells combined with replacement of helper signals, either through agonistic CD40 antibodies or, in our system, alloreactive donor CD4+ T cells. This explanation would also account for the requirement for histocompatibility differences between the infused lymphocytes and the host for the optimal vaccine effect. Interestingly, even when CD8+ T cells are “seeing” antigen in tumor-draining lymph nodes, replacement of CD4+ T cells help with agonistic anti-CD40 antibodies is insufficient for induction of tumor regression, and concomitant provision of antigen is required.32,33 This need for exogenously supplied antigen may explain why DLI without concomitant tumor vaccine administration had no effect in our model and why clinical responses to DLI alone do not occur in patients who maintain the mixed chimeric state after DLI.36 Taken together, these and our results suggest the existence of 2 distinct categories of antitumor immune responses after alloSCT.37 The first category, a nonspecific graft-versus-host reaction, is mediated exclusively by donor T cells reactive to host histocompatibility antigens, is associated with GVHD and full donor hematopoietic chimerism, and cures only hematolymphoid malignancies. The second category, tumor-specific immunity, may be mediated by host and/or donor T cells, does not augment donor chimerism or the risk of GVHD, requires both enhanced antigen-presentation and APC activation, and may be effective in the treatment of solid tumors.
In light of the significant incidence of GVHD following NST in humans, the most appealing feature of our therapeutic approach is that specific antitumor immunity can be induced without exacerbating GVHD by immunizing donor T cells that are infused into mixed hematopoietic chimeras. Indeed, it is not overtly obvious why vaccination of an allogeneic donor with autologous tumor cells leads to GVHD after alloSCT,12,13 whereas vaccination of freshly infused donor lymphocytes within the NST recipient can induce specific antitumor immunity without GVHD. Perhaps this latter phenomenon relates to the differential ratios of immunogenic to tolerogenic APCs presenting tumor-specific versus minor histocompatibility antigens in a mixed hematopoietic chimera following vaccine administration. The efficacy of GM-CSF–based tumor cell vaccines is attributable to the capacity of GM-CSF to recruit immunogenic APCs to the vaccine site. These APCs would be expected to capture both tumor-specific and minor histocompatibility antigens from the injected, nonviable tumor cells and transport these antigens to the draining lymph nodes, thereby promoting tumor-specific immunity and GVHD, respectively. Away from the vaccine site, resting cells presenting histocompatibility antigens, including host tissue cells and residual host APCs, might be expected to induce tolerance in or “exhaust” alloreactive donor T cells.38 Indeed, a common outcome of DLI, especially from unprimed donors, is the absence of any detectable antihost response,39 which suggests that histocompatibility differences between donor and host are not a sufficient condition for the continuous activation of alloreactive donor T cells. Thus, alloreactive T cells that have been activated at the vaccine site may subsequently be inactivated following encounter with resting host APCs, whereas tumor-specific T cells, after eliminating residual tumor cells, would be expected to persist in a long-lived memory state. Regardless of the mechanism of action, the administration of GM-CSF–based tumor vaccines in conjunction with DLI after NST represents a potential strategy for the treatment of patients with metastatic solid tumors in the clinic.
Prepublished online as Blood First Edition Paper, October 24, 2002; DOI 10.1182/blood-2002-07-2233.
Supported by a Career Development Award (DAMD17-99-1-9235) from the United States Department of Defense (E.J.F.) and a Clinical Investigator Award from the Cancer Research Institute (E.J.F.). L.L. is a recipient of an American Association for Cancer Research-Amgen Translational Award and a research award from the Amy Strelzer Manasevit Scholars Program funded by the Marrow Foundation in cooperation with the National Marrow Donor Program.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Ephraim J. Fuchs, 488 Bunting-Blaustein Cancer Research Bldg, 1650 Orleans St, Baltimore, MD 21231; e-mail:fuchsep@jhmi.edu.

![Fig. 3. Protective and therapeutic antitumor immunity requires allogeneic cell transplantation, and CD4+ and CD8+ T cells in DLI and autologous tumor cell vaccination. / (A) Scheme of experiment in panel B. (B) Protective antitumor immunity requires NST and posttransplantation vaccine. BALB/c mice underwent NST from B10.D2 donors. Beginning 2 weeks after transplantation, chimeras then received nothing (▾), 20 million B10.D2 splenocytes intravenously (▿), or 20 million B10.D2 splenocytes and 4T1 tumor vaccine (▪; N = 5 per group), as shown in panel A. Four months later, all mice that received transplants, and groups of 5 untreated (●) or vaccinated BALB/c controls (○), were challenged with 104 4T1 cells intravenously. Tumor-free survival is plotted as a function of time after intravenous tumor challenge. (C) Scheme of experiment in panel D. (D) Therapeutic antitumor immunity requires CD4+ and CD8+ T cells in the DLI. BALB/c mice bearing 13-day-old 4T1 tumors received surgery and NST from B10.D2 donors as illustrated in Figure 2A. On day 28, mice received 2 × 107 B10.D2 splenocytes, either unmodified (●, ○) or depleted of CD4+ (▾) or CD8+ (▿) T cells. Three days later, mice received 4T1 vaccine (●, ▾, ▿) or nothing (○). Tumor-free survival was then monitored. (E) Therapeutic antitumor immunity requires autologous cells in the vaccine. Tumor-bearing BALB/c mice received surgery, NST, DLI, and vaccine consisting of the bystander line mixed with nothing (▾), irradiated 4T1 cells (●), irradiated DA-3 cells (○; a BALB/c breast cancer), or irradiated NT-2 cells (▿; a mammary cancer of FVB/N [H-2q] mice). Tumor-free survival is plotted as a function of time after tumor injection.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/4/10.1182_blood-2002-07-2233/3/m_h80433832003.jpeg?Expires=1769082592&Signature=5GQ1eFjJz6FLNy767dEznJsiMpcIGK8KHB9W746kZoqj48uU21X3Vd3GWD1F7hb7U~nzWyTZ6kRLIfPrSvc~JnHvnO4N-C7eeXKeRw-wikyDIx5D5ycZFtie4S5Wxg90be~94DQBz5g8Ul4fCcH7uNESyaTog-Vfz04CzDh16OmsDU-rtqWjz02mKgWPQE4R5Bqoate4fGkODkCf1dBdBrUQL7cUBL~eMnuWNDfzpyc4htIhPTJN-rxmij0v0WxzM3~IcmiFCLv3XWJtCtaKZicXrxfkNcml9gffxbyGSv-RsjZHWpxc364e1VekEtroCirqcarwoD-QV3B1YhLURA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal