Some cases of heparin-induced thrombocytopenia (HIT) have been reported to be associated with antibodies against interleukin-8 (IL-8), a chemokine related to platelet factor 4. We found that sera from 5 HIT patients containing immunoglobulin G (IgG) or IgM antibodies to IL-8, as evidenced using surface plasmon resonance spectroscopy, were able to trigger IL-8–dependent activation of washed platelets, leading to procoagulant activity. This activation occurred at IL-8 concentrations achievable in vivo and was facilitated by heparin (0.1 U/mL). Activation was also induced by affinity-purified anti–IL-8 IgG and involved FcγRIIa. In the 2 patients who could be followed up, antibodies were no longer detectable 4 months after heparin withdrawal. One additional patient with paraneoplastic recurrent thrombosis without thrombocytopenia was found to have platelet-activating anti–IL-8 IgM, but in this case heparin was inhibitory. This is another example of potentially pathogenic platelet activation by antibodies.
Introduction
In most patients with heparin-induced thrombocytopenia (HIT), antibodies bind to complexes of heparin and platelet factor 4 (PF4). If these antibodies are immunoglobulin G (IgG), they interact with FcγRIIa receptors and trigger platelet activation.1,2 However, previous investigators using enzyme-linked immunosorbent assay (ELISA) have reported that a few HIT patients have antibodies specific to another, PF4-related α-chemokine—interleukin-8 (IL-8), alone3,4 or in combination with antibodies to heparin-PF4 complexes.5These antibodies were infrequently detected in patients with heparin exposure but no thrombocytopenia and in patients with thrombocytopenia from another etiology.3
The main known property of IL-8 is to induce neutrophil activation, and anti–IL-8 autoantibodies have been shown to inhibit IL-8 interaction with its specific receptors on neutrophils.6 IL-8 also binds to heparin,7 and there may be IL-8 receptors on platelets.8-10
This study was undertaken to investigate whether antibodies to IL-8 are able to induce platelet activation in vitro and whether they are dependent on heparin.
Study design
Sera were obtained from 5 selected patients (P1-P5) who fulfilled the diagnostic criteria for HIT1,11 (suggestive temporal pattern of platelet counts in relation to heparin therapy and evidence for heparin-dependent platelet-activating antibodies) but without IgG to heparin-PF4 complexes.3 Patient 6 had typical HIT with IgG to heparin-PF4 only. One additional patient (P7), who had experienced fatal recurrent paraneoplastic thromboses while on heparin therapy without thrombocytopenia,12 was also studied. Small volumes of blood were collected from samples required for routine patient management and were withdrawn after heparin therapy was stopped; heparin was no longer detectable with anti-Xa assay. ELISA (Asserachrom HPIA; Diagnostica Stago, Asnières, France) was used to detect antibodies to heparin-PF4.13
IgG was purified by affinity chromatography on protein G–Sepharose (Pharmacia Biotech, Uppsala, Sweden). IgM was isolated as previously described,14 and IgG contamination was 1.5% or less. IgG and IgM were similarly purified from the serum of one healthy donor, and concentrations were determined for all preparations using an in-house–designed ELISA.
Detection and purification of antibodies to IL-8
Anti–IL-8 antibodies were detected using surface plasmon resonance spectroscopy (SPR). Recombinant human IL-8 without carrier protein (Innogenetics; Valbiotech, Paris, France) was covalently coupled to a carboxylated dextran negatively charged matrix (sensor chip CM5; Biacore, Uppsala, Sweden). Binding experiments were performed as previously described15 using serum and purified IgG. After the injection of diluted serum, bound antibodies were revealed by the injection of goat anti-human IgG (Sigma-Aldrich, St Quentin Fallavier, France) or rabbit anti-human IgM (DAKO, Glostrup, Denmark). Nonspecific binding was avoided by adding free carboxymethyl-dextran to the samples before injection.
Anti–IL-8 IgG was affinity purified using SPR by injecting purified total IgG over the IL–8-coated biosensor. After washing, bound antibodies were eluted with 100 mM HCl and were neutralized with 1 M Tris-HCl, pH 8.0.
Platelet activation assay
All sera were heat-inactivated for 30 minutes at 56°C and were centrifuged at 13 000g for 45 minutes. The platelet procoagulant assay was performed according to Warner et al16 in combination with the measurement of annexin V–fluorescein isothiocyanate (FITC; Bioproduct, Bagneux, France) binding to platelets by flow cytometry.
Results and discussion
We demonstrated for the first time in this study that antibodies to IL-8 are able to induce in vitro platelet activation. We first used SPR technology to visualize directly the binding of antibodies to IL-8. Four patients had antibodies to IL-8 alone, either IgG (P1 and P2) or IgM (P3 and P4). Patient 5 had IgG and IgM antibodies to IL-8, in combination with IgM antibodies to heparin-PF4. Patient 7 had only anti–IL-8 IgM.
The platelet procoagulant assay was chosen as an activation endpoint because washed platelets are sensitive to activation by antibodies,1 and this microassay allows simultaneous testing of numerous samples. In addition, the generation of procoagulant activity indicates strong platelet activation related to the pathogenesis of the HIT syndrome.16-18 IL-8–dependent platelet activation was induced by the 6 sera containing anti–IL-8 antibodies. Neither binding of antibodies to IL-8 (Figure1A) nor serum-induced IL-8–dependent platelet activation required exogenous heparin. Activation was maximal in the range of 0.125 to 1.25 pmol/L IL-8, and similar levels have been reported in human serum.19 Moreover, Arepally et al20 also recently showed that antibodies from HIT patients induced IL-8 synthesis and secretion by monocytes. A decrease in platelet response was observed at higher concentrations of IL-8 (Figures 1B and 2A), supporting the hypothesis of formation of immune complexes in the fluid phase that may prevent the binding of antibodies to surface-bound IL-8, as previously reported.12 Another explanation is that the potency of immune complexes to activate platelets might be weaker when the antibody-to-antigen ratio is lower.
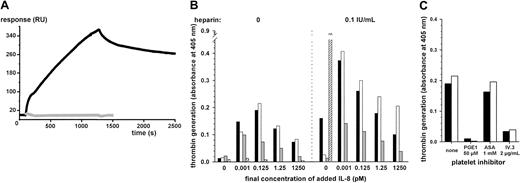
IgG binding to immobilized IL-8 and IL-8–dependent platelet procoagulant activity induced by IgG.
(A) Purified total IgG from patient P1 (black line) was injected over the IL-8–coated biosensor for 1200 seconds, followed by running buffer (spontaneous dissociation) for an additional 1200 seconds. Control sensorgram obtained with purified total IgG from a healthy donor is displayed as the gray line. (B) Washed human platelets from a healthy donor were incubated with serum (black bars; 5 mg IgG/mL), purified total IgG (white bars; 2.4 mg/mL), or affinity-purified anti–IL-8 IgG (gray bars; 7.7 μg/mL) from patient 1. Varying concentrations of IL-8 were added to the mixture, without or with unfractionated heparin (0.1 U/mL). With P6 control serum containing only IgG against heparin-PF4 (hatched bars, 2.35 mg IgG/mL), significant activation was recorded only in the presence of heparin alone. (C) Platelets were pretreated with buffer alone, acetylsalicylic acid (ASA), PGE1, or FcγRIIa receptor–blocking antibody IV.3 before incubation with P1 serum (black bars) or purified total IgG (white bars).
IgG binding to immobilized IL-8 and IL-8–dependent platelet procoagulant activity induced by IgG.
(A) Purified total IgG from patient P1 (black line) was injected over the IL-8–coated biosensor for 1200 seconds, followed by running buffer (spontaneous dissociation) for an additional 1200 seconds. Control sensorgram obtained with purified total IgG from a healthy donor is displayed as the gray line. (B) Washed human platelets from a healthy donor were incubated with serum (black bars; 5 mg IgG/mL), purified total IgG (white bars; 2.4 mg/mL), or affinity-purified anti–IL-8 IgG (gray bars; 7.7 μg/mL) from patient 1. Varying concentrations of IL-8 were added to the mixture, without or with unfractionated heparin (0.1 U/mL). With P6 control serum containing only IgG against heparin-PF4 (hatched bars, 2.35 mg IgG/mL), significant activation was recorded only in the presence of heparin alone. (C) Platelets were pretreated with buffer alone, acetylsalicylic acid (ASA), PGE1, or FcγRIIa receptor–blocking antibody IV.3 before incubation with P1 serum (black bars) or purified total IgG (white bars).
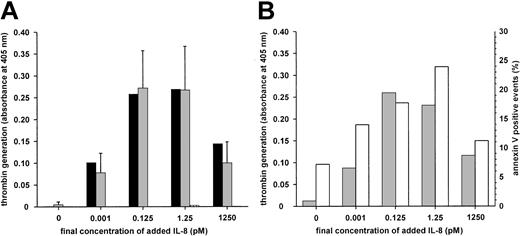
IgM-mediated IL-8–dependent platelet activation.
(A) Washed human platelets from a healthy donor were incubated with 1:10 diluted serum (black bars, 205 μg IgM/mL), isolated total IgM (gridded bars, 35 μg/mL) from P7, or isolated total IgM (gray bars, 35 μg/mL) from the healthy donor as control. Similar results regarding isolated IgM were obtained from 5 experiments with different platelet preparations from the healthy donor (results shown as mean ± SD) or washed platelets from another donor (not shown). (B) Platelets from the healthy donor were incubated with isolated total IgM from P7. Procoagulant activity (gray bars) and annexin V–FITC binding (white bars) were taken as the assay endpoints.
IgM-mediated IL-8–dependent platelet activation.
(A) Washed human platelets from a healthy donor were incubated with 1:10 diluted serum (black bars, 205 μg IgM/mL), isolated total IgM (gridded bars, 35 μg/mL) from P7, or isolated total IgM (gray bars, 35 μg/mL) from the healthy donor as control. Similar results regarding isolated IgM were obtained from 5 experiments with different platelet preparations from the healthy donor (results shown as mean ± SD) or washed platelets from another donor (not shown). (B) Platelets from the healthy donor were incubated with isolated total IgM from P7. Procoagulant activity (gray bars) and annexin V–FITC binding (white bars) were taken as the assay endpoints.
Similar results were obtained with serum, total purified IgG, and specific anti–IL-8 IgG from patient 1, indicating that activation was specifically caused by anti–IL-8 IgG (Figure 1A). In the presence of heparin, significant thrombin generation was detected with the patient's serum without exogenous IL-8. The patient's serum might have provided sufficient amounts of the chemokine to trigger antibody-mediated platelet activation. In contrast, no signal was obtained in the presence of IL-8 with either normal serum or normal IgG or with P6 serum containing only anti–heparin-PF4 IgG (Figure 1B). Activation of platelets by anti–heparin-PF4 IgG required the presence of heparin, whereas heparin was not necessary but substantially increased the platelet response to anti–IL-8 IgG (Figure 1B). Platelet activation by anti–IL-8 IgG was inhibited by the blocking antibody IV.3 and, like anti–heparin-PF4 IgG, probably involved binding to platelet FcγRIIa through the Fc portion. The procoagulant response was abolished by prostaglandin E1 (PGE1), whereas it was only slightly affected by acetylsalicylic acid, suggesting that anti–IL-8 antibodies are potent platelet activators (Figure 1C).
Serum and isolated IgM from patient 7 consistently activated platelets in an IL–8-dependent manner (Figure 2A). Exposure of procoagulant phospholipids was also evidenced by the binding of annexin V (Figure2B). There was no increase in lactate dehydrogenase, ruling out platelet lysis; moreover, PGE1 was inhibitory. This activation occurred with isolated IgM and washed platelets, suggesting that complement was not required. As expected, antibody IV.3 did not affect the response. The P5 serum containing a mixture of antibodies also induced similar IL–8-dependent platelet activation, which was inhibited by antibody IV.3 and thus probably was attributed only to anti–IL-8 IgG. In the 2 patients who could be followed up, antibodies to IL-8 were no longer detectable 4 months after heparin withdrawal.
The different effects of heparin in vitro suggest at least 2 categories of anti–IL-8 antibodies related to different epitopes. On the one hand, heparin (0.1 U/mL) facilitated IL–8-dependent platelet activation induced by antibodies from HIT patients P1 to P5, as illustrated in Figure 1B. Therefore, epitopes recognized by such antibodies remained accessible in the presence of heparin, which could have increased the density of IL-8 on the platelet surface.7 On the other hand, the same concentration of heparin (ie, 0.1 U/mL) completely inhibited the platelet response to P7 antibodies (data not shown). Thus, heparin might have hindered the epitopes for anti–IL-8 antibodies from this particular patient with paraneoplastic thromboses and no HIT.12
Our study provides evidence that antibodies to IL-8 can trigger platelet activation that is strong enough to promote procoagulant activity. As for anti–heparin-PF4 IgG,21 anti–IL-8 IgG antibodies could first bind to the antigen on the platelet surface, and subsequent cell activation could occur through FcγRIIa. The mechanism of activation induced by IgM remains to be elucidated, but it could be antigen cross-linking.22
We thank Pascale Crapsky for her skillful technical assistance, Agnès Mulot for help with clinical data management, and Doreen Raine for editing this paper.
Prepublished online as Blood First Edition Paper, October 10, 2002; DOI 10.1182/blood-2002-02-0620.
Supported in part by grants from the district of Nancy, the Region of Lorraine, and the University Henri Poincaré–Nancy 1.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Thomas Lecompte, Hématologie, EA 3452, Faculté de Médecine, Université Henri Poincaré, Boite Postale 184, 54505 Vandoeuvre-lès-Nancy, France; e-mail:thomas.lecompte@chu-nancy.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal