Human protein S (PS), a cofactor of anticoagulant-activated protein C (APC), is a modular protein containing 4 epidermal growth factor (EGF)–like domains. EGF1 appears to mediate PS interaction with APC, but the roles of EGFs 2, 3, and 4 are less clear. We synthesized PS variants lacking single EGF domains (EGF2, 3, or 4) and assessed their APC cofactor activity in a factor Va inactivation assay. The variant lacking EGF2 (variant 134) showed the most dramatic loss of activity (∼10% of recombinant wild-type PS activity). Replacement of EGF2 by an additional EGF3 (variant 1334) resulted in a comparable loss of activity, suggesting that the loss of a specific rather than “spacer” function of EGF2 was responsible. We confirmed that the variant 134 had a functional γ-carboxyglutamic acid (Gla) domain and that EGF1 was correctly folded. This is the first clear evidence that EGF2 is required for the expression of PS activity.
Introduction
Protein S (PS) is a vitamin K–dependent plasma glycoprotein1 that acts as a nonenzymatic cofactor to activated protein C (APC) in the degradation of factors Va and VIIIa.2 PS is a modular protein comprising a γ-carboxyglutamic acid (Gla) domain, a thrombin-sensitive region (TSR), 4 epidermal growth factor (EGF)–like modules, and a large globular domain homologous to sex hormone–binding globulin (SHBG).3,4 The functions and interdependence of these modules are only partly understood. In plasma, APC cofactor activity is lost either by binding of the SHBG-like domain to C4b-binding protein (C4bBP)5 or on proteolytic cleavage of the TSR.6,7 The integrity of the TSR is necessary both for direct interaction with APC and to maintain the Gla domain in the correct configuration for phospholipid binding.8 9 The SHBG domain appears to contain the sites for interaction with C4bBP and factor V.
In contrast, the roles of the 4 EGF domains remain unresolved. The smallest PS molecule shown to express APC cofactor function is a “microprotein S” comprising the Gla domain, TSR, and EGF1, which has 30% cofactor activity compared with plasma-derived PS.10 The role of EGF1 in binding of APC was demonstrated by Hackeng et al10 using a chemically synthesized EGF1 to inhibit APC cofactor activity. Furthermore, the species specificity of PS APC cofactor activity is conferred by TSR-EGF1.11 The interaction between EGF1 and APC seems to involve the region around residue 103 where the naturally occurring qualitative (type II) mutation Thr103Asn was identified.12 In this study we have used serial deletion of EGF domains to demonstrate an important role of EGF2 in PS APC cofactor activity.
Study design
Construction of the expression vector for stable PS expression
The complete human PS cDNA (gift of Dr P. H. Reitsma, Leiden University Hospital, The Netherlands) was amplified by using oligonucleotides 5′-ATAAGATAGCGGCCGCAGACCGAGGCGCACAGGCT-3′ and 5′-ATAAGATAGCGGCCGCAATCCCAGGAAAGGACCAC-3′, introducing flankingNotI sites, to allow insertion into the vector pNeo1G572 (gift of Dr J. McVey, Imperial College, London, United Kingdom).13 pNeo1G572 was generated from pNeoIG561 using the oligonucleotides 5′-AGCTGACGCGGCCGCTA-3′ and 5′-AGCTTAGCGGCCGCGTC-3′ (Applied Biosystems, Foster City, CA) to introduce a NotI-containing linker into the multiple cloning site.
In vitro mutagenesis and PS expression
The oligonucleotide 5′-CTTGTGAAGATATCGATGAATGC-3′ was used to create an EcoRV site between EGF3 and EGF4, after subcloning the SacII fragment containing EGF1-4 into pBluescript II SK(+) (Stratagene, Cambridge, United Kingdom). The point mutation introduced is silent. The resulting vector was used to perform further mutagenesis after which the EGF domains were returned to the full-length cDNA for expression studies.
PS EGF2, EGF3, and EGF4 were each deleted by a loop-out technique using, respectively, the following oligonucleotides: 5′-GAAAAGTGTGAATTTGACGTGGATGAATGCTCTTTG-3′, 5′-CAAATAAGAAAGATTGTAAAGATATCGATGAATGCTCTGAGAAC-3′, and 5′-CAAAGTCTTGTGAAGATGTTGTTTCAGTGTGCCTT-3′. This generated the mutants designated 134, 124, and 123 with the following deletions: (a) PS EGF124: deletion of EGF3 (Val161-Asp202 inclusive); (b) PS EGF134: deletion of EGF2 (Ile117-Asp160 inclusive); and (c) PS EGF123: deletion of EGF4 (Ile203-Glu242 inclusive).
An additional EGF3 module was synthesized by annealing 2 overlapping oligonucleotides 5′-ATCTTCACAAGACTTTGATTTGAGAT- TATATCTGTAGCC-TTCGGGGCATTCACATTCAAAATCTCCTG-3′ and 5′-GTGGAGAATGCTCTTTGAAGCCAAGCATTTGTGGCACAGCT- GTGTGCAAGAACATCCCAGGAGATTT-3′ and making double stranded with T4 polymerase. This was cloned into theEcoRV site between EGF3 and EGF4, generating the variant 1334 with the insertion of EGF3 (Val161-Asp202 of wild-type PS [wtPS]) between Asp202 and Ile203 of wtPS.
The sequence and orientation of the mutated PS cDNA was confirmed after each mutagenic step. Transient and stable expression with methotrexate amplification of wtPS and variant PS were performed as previously described in COS and CHO cells, respectively.13-15 For each construct, transient transfections were performed in triplicate on a minimum of 5 separate occasions. Pulse-chase experiments were performed as previously described.14
Purification and characterization of rPS
The method described by He et al16 was adapted to a Biocad sprint reverse-phase automated chromatography system (Perceptive Biosystems, Framingham, MA). The concentration of PS; Western blotting; affinity for proteins S, C, and E (PS/PC/PE) vesicles; and APC cofactor activity were all assessed as previously described, except that sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) was performed under nonreducing conditions.14,15 The integrity of recombinant PS (rPS) EGF1 was assessed by the binding of the conformation and Ca2+-dependent monoclonal antibody HPS5417 (a kind gift of B. Dahlback, Malmo, Sweden) as previously described.15
Results and discussion
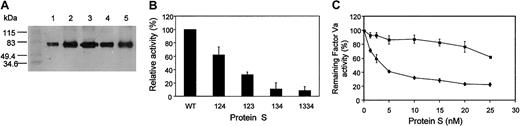
We have stably expressed PS deletion variants 123, 134, 124, and 1334 (where the numbers refer to the EGF domains present in an otherwise wtPS molecule, while retaining the normal EGF boundaries) in Chinese hamster ovary (CHO) cells augmented by methotrexate selection (Figure 1A). We assessed the APC cofactor function of the variants and wtPS in the inactivation of factor Va at 10 nM PS (Figure 1B). The modest effect on function resulting from deletion of EGF3 (variant 124) is in agreement with a previous study in which EGF3 was approximately deleted (deletion from Asp160 to Asp202), resulting in a functionally active variant.18 We detected a more substantial effect of EGF4 deletion on PS cofactor function, with variant 123 showing 32.2% ± 4.0% (SEM) of wtPS activity. This is also consistent with previously published data showing that EGF4 contains the highest affinity calcium-binding site and that modules 1 to 4 had greater inhibitory activity on APC-PS interaction than 1 to 3.19 20 However, removal of EGF2 (variant 134) was associated with the most dramatic reduction of APC cofactor activity (11% ± 9.16% of wtPS) and this was not corrected by replacement of EGF2 with an additional EGF3 (variant 1334). In additional experiments the greatly reduced activity of the 134 variant was confirmed over a wide range of PS concentrations (0-25 nM; Figure 1C).
Expression of PS variants with EGF domains deleted or replaced and their ability to enhance APC-catalyzed factor Va inactivation.
(A) Western blotting of wtPS and variant PS expressed in CHO cells. SDS-PAGE of about 50 ng rPS under nonreducing conditions and immmunoblotting with an anti-PS polyclonal antibody was performed as described in Rezende et al.15 Molecular weight markers are shown in kilodaltons on the left. Lane 1, wild-type; lane 2, variant 123; lane 3, variant 124; lane 4, variant 134; lane 5, variant 1334. The assay described in panels B and C uses the reduction in thrombin generation measured using a chromogenic substrate to assess the enhancement by PS of APC-catalyzed factor Va inactivation.14,25 (B) The results of the initial screening of the PS variants for their ability to enhance APC-catalyzed factor Va inactivation are shown. Although each variant and the wtPS were tested at several different concentrations, the results are presented for 10 nM PS. Results (means of 3 experiments ± SEM) are expressed as a percentage of wtPS (100%). We previously showed15 that recombinant wtPS has a similar specific activity to a commercial purified human PS preparation and for both, half-maximal inhibition of thrombin generation was reached at a PS concentration of about 1 nM. Identical results were obtained for purified, semipurified, and unpurified wtPS-containing medium. (C) The ability of the variant 134 (▪) to enhance APC-catalyzed inactivation of factor Va in comparison to wtPS (♦). The results are presented for the full range of tested PS concentrations (0, 1.25, 2.5, 5, 10, 15, 20, and 25 nM) and expressed relative to the remaining activity of factor Va measured in the absence of PS (100%) as a mean of 3 experiments ± SEM for each PS concentration. In some cases the SEM cannot be seen due to its small size. There were insufficient amounts of the other variants to perform this analysis at all concentrations.
Expression of PS variants with EGF domains deleted or replaced and their ability to enhance APC-catalyzed factor Va inactivation.
(A) Western blotting of wtPS and variant PS expressed in CHO cells. SDS-PAGE of about 50 ng rPS under nonreducing conditions and immmunoblotting with an anti-PS polyclonal antibody was performed as described in Rezende et al.15 Molecular weight markers are shown in kilodaltons on the left. Lane 1, wild-type; lane 2, variant 123; lane 3, variant 124; lane 4, variant 134; lane 5, variant 1334. The assay described in panels B and C uses the reduction in thrombin generation measured using a chromogenic substrate to assess the enhancement by PS of APC-catalyzed factor Va inactivation.14,25 (B) The results of the initial screening of the PS variants for their ability to enhance APC-catalyzed factor Va inactivation are shown. Although each variant and the wtPS were tested at several different concentrations, the results are presented for 10 nM PS. Results (means of 3 experiments ± SEM) are expressed as a percentage of wtPS (100%). We previously showed15 that recombinant wtPS has a similar specific activity to a commercial purified human PS preparation and for both, half-maximal inhibition of thrombin generation was reached at a PS concentration of about 1 nM. Identical results were obtained for purified, semipurified, and unpurified wtPS-containing medium. (C) The ability of the variant 134 (▪) to enhance APC-catalyzed inactivation of factor Va in comparison to wtPS (♦). The results are presented for the full range of tested PS concentrations (0, 1.25, 2.5, 5, 10, 15, 20, and 25 nM) and expressed relative to the remaining activity of factor Va measured in the absence of PS (100%) as a mean of 3 experiments ± SEM for each PS concentration. In some cases the SEM cannot be seen due to its small size. There were insufficient amounts of the other variants to perform this analysis at all concentrations.
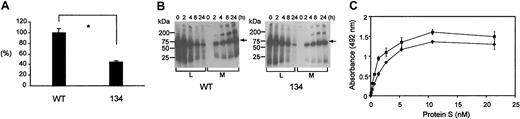
Transient expression of wtPS and variant 134 in COS-1 cells showed that the level of variant 134 in conditioned medium was approximately 2-fold reduced compared to wtPS (Figure 2A). This was confirmed by pulse-chase experiments, which showed that at 24 hours, the proportion of the initially labeled PS secreted was also reduced about 2-fold (Figure 2B). This indicates that loss of EGF2 is associated with a modest defect of secretion and suggests correct overall folding.
Secretion and phospholipid binding of the variant 134 compared to wtPS.
(A) Relative transient expression levels of recombinant wtPS and variant 134 in COS-1 cells. The concentrations of PS in cell supernatants were normalized according to reporter control (secreted alkaline phosphatase) values and expressed as a percentage of the wtPS. Each experiment was performed 3 times in duplicate. The error bars represent ± 2 SD. Unpaired t test was used to compare the levels between wild-type and variant 134 (*P = .0007). (B) Pulse-chase analysis of wild-type (WT) and variant 134 PS. Transfected cells were metabolically radiolabeled for 30 minutes and cell lysate (L) and conditioned medium (M) were collected at 0, 2, 4, 8, and 24 hours, immunoprecipitated with a polyclonal anti-PS antibody, and separated by SDS-PAGE. Molecular weight markers were used to calibrate each gel and the arrows identify the PS bands. At 24 hours the amount of PS secreted, as a proportion of the initially labeled material, was 68% ± 1% for wtPS and 36% ± 9% for the 134 variant. (C) Binding of recombinant wtPS and variant 134 to phospholipid. The binding of a range of rPS concentrations (wild-type, ♦; variant 134, ▪) to plates coated with PC/PS/PE (20:30:50) vesicles was detected with a polyclonal anti-PS antibody in the presence of 3 mM CaCl2. We did not detect any nonspecific binding to control wells without immobilized phospholipid or in the presence of EDTA (ethylenediaminetetraacetic acid). A wide range of PS concentrations was tested (0-21.4 nM) showing that variant 134 and wtPS bound to the phospholipid vesicles in a saturable manner with a very similar half-maximal binding.
Secretion and phospholipid binding of the variant 134 compared to wtPS.
(A) Relative transient expression levels of recombinant wtPS and variant 134 in COS-1 cells. The concentrations of PS in cell supernatants were normalized according to reporter control (secreted alkaline phosphatase) values and expressed as a percentage of the wtPS. Each experiment was performed 3 times in duplicate. The error bars represent ± 2 SD. Unpaired t test was used to compare the levels between wild-type and variant 134 (*P = .0007). (B) Pulse-chase analysis of wild-type (WT) and variant 134 PS. Transfected cells were metabolically radiolabeled for 30 minutes and cell lysate (L) and conditioned medium (M) were collected at 0, 2, 4, 8, and 24 hours, immunoprecipitated with a polyclonal anti-PS antibody, and separated by SDS-PAGE. Molecular weight markers were used to calibrate each gel and the arrows identify the PS bands. At 24 hours the amount of PS secreted, as a proportion of the initially labeled material, was 68% ± 1% for wtPS and 36% ± 9% for the 134 variant. (C) Binding of recombinant wtPS and variant 134 to phospholipid. The binding of a range of rPS concentrations (wild-type, ♦; variant 134, ▪) to plates coated with PC/PS/PE (20:30:50) vesicles was detected with a polyclonal anti-PS antibody in the presence of 3 mM CaCl2. We did not detect any nonspecific binding to control wells without immobilized phospholipid or in the presence of EDTA (ethylenediaminetetraacetic acid). A wide range of PS concentrations was tested (0-21.4 nM) showing that variant 134 and wtPS bound to the phospholipid vesicles in a saturable manner with a very similar half-maximal binding.
The analyses of the mutant PS molecules presented here allow some insight into the importance of the individual EGF domains. The importance of EGF1 has already been established but the “microprotein S” containing Gla, TSR, and EGF1 domains does not have full APC cofactor activity.21 The differences in functional activity between 124, 123, and 134 (Figure 1) indicate that EGF2, 3, and 4 each make some functional contribution and that they do not solely serve “spacer” functions. Nor are they equivalent; the most striking finding in this study is the very low activity resulting from the deletion of EGF2. These conclusions are supported by the failure of the replacement EGF3 to restore function in the variant 1334.
The loss of function in variant 134 is not the result of any gross conformational change in the fraction successfully secreted. The variants showed the same mobility as wtPS by SDS-PAGE under nonreducing conditions and by Western blotting (Figure 1A; loss of a single EGF domain cannot be resolved by this technique). Under reducing conditions all the PS preparations appeared homogeneous as single bands without evidence of internal cleavage (data not shown). Because binding of PS to phospholipid is essential for its anticoagulant activity, we compared the ability of wtPS and the variant 134 to bind to phospholipid vesicles. The results shown in Figure 2C indicate that variant 134 and wtPS both have functional Gla domains and that the deletion of EGF2 does not appreciably alter the binding of PS to phospholipids. PS EGF1 is essential for APC cofactor activity10 and therefore the structural integrity of this domain in variant 134 was assessed using the Ca2+-dependent monoclonal antibody HPS54 directed against an EGF1 epitope.17 There was no significant difference in binding between rwtPS and variant 134 over a range of PS concentrations (0-2.14 nM), strongly suggesting that EGF1 is structurally intact (data not shown).
It is not clear whether the importance of EGF2 established here results from a contribution to macromolecular binding sites or from a more complex structural role. The failure of EGF2-3 and EGF2-4 to inhibit PS APC cofactor activity suggests that the latter is more likely.20 An important role of the EGF modules may be to constrain the PS molecule in an appropriate configuration. For example, the presence of EGF4 increases the calcium affinities of EGF2 and EGF322 and the fragment EGF1-4 is 10-fold more active than EGF1-3 in inhibiting of the interaction between PS and APC, suggesting either a significant interaction between EGF4 and APC or an effect of EGF4 on the conformation of EGF1. A natural variant within EGF2 (Lys155Glu) has variously been reported to be phenotypically neutral23 or to have no ability to enhance APC activity.24 Our results confirm that EGF2 has an important role in PS anticoagulant function.
Prepublished online as Blood First Edition Paper, October 10, 2002; DOI 10.1182/blood-2002-08-2353.
Supported by a project grant (PG/96051) from the British Heart Foundation and a studentship from the Brazilian government (CAPES).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Michael A. Laffan, Department of Haematology, Faculty of Medicine, Imperial College, Hammersmith Hospital, Ducane Rd, London W12 ONN, United Kingdom; e-mail:m.laffan@ic.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal