Fanconi anemia (FA) is a chromosomal instability disorder characterized by a progressive bone marrow (BM) failure and an increased incidence of myeloid leukemias. Children with FA are currently being enrolled in clinical trials to evaluate the safety of retroviral-mediated gene transfer. Previously, we usedFancc−/− mice to show thatFancc−/− hematopoietic stem cells (HSCs) have a profound defect in repopulating ability. Here, we examined whether retroviral-mediated gene transfer of recombinantFancc (rFancc) would restore the repopulating ability of Fancc−/− HSC to wild-type levels. Fancc−/− HSCs transduced with a retrovirus encoding rFancc exhibited a repopulating ability that approached wild-type levels. Interestingly, ∼30% of primary recipients (7 of 22) transplanted with uncorrectedFancc−/− cells developed a range of hematopoietic abnormalities including pancytopenia and BM hypoplasia similar to individuals with FA. Hematopoietic abnormalities were detected in only 1 of 22 mice transplanted withFancc−/− cells transduced with a retrovirus encoding rFancc. Moreover, several mice with hematopoietic defects had progenitors that displayed a marked resistance to IFN-γ, TNF-α, and MIP-1α compared to both Fancc−/−progenitors, which are uniquely hypersensitive to these cytokines, and wild-type progenitors. These data are analogous to studies using progenitors from patients with myelodysplasia and provide functional support for clonal evolution in these mice. Collectively, these data show that gene transfer can enhance HSC repopulating ability and suppresses the tendency for clonal evolution. These studies also reveal potential detrimental effects of ex vivo manipulation for untransducedFancc−/− HSCs.
Introduction
Fanconi anemia (FA) is a heterogeneous autosomal recessive disorder manifested by congenital malformations, bone marrow (BM) failure, clonal hematopoietic disorders including myelodysplasia, and malignancies, especially acute myelogenous leukemia.1-5 Although multiple organ systems are affected by the loss of FA protein function, the major causes of morbidity and mortality of FA relate to the hematopoietic disease, with 80% of patients dying from BM failure. In addition, 10% of FA patients develop myeloid leukemias, with a mean age of death being 19 years.3 Currently, the only definitive treatment for the hematologic manifestations of FA is HLA-identical hematopoietic stem cell (HSC) transplantation. There are 7 cDNAs corresponding to distinct FA complementation types that have been cloned (FANCA,FANCC, FANCD2, FANCE, FANCF, FANCG, andBRCA2),6-13 raising the potential of using gene transfer technology to introduce a functional cDNA into autologous stem cells.
An initial phase 1 trial designed to evaluate the safety of gene transfer for FA patients did not result in long-term hematopoietic reconstitution with genetically corrected stem cells in 4 FA complementation type C (FA-C) patients.14 Although it is difficult to assess why long-term reconstitution of transduced cells was not observed, potential explanations include decreased survival of HSCs during ex vivo culture, low HSC transduction efficiency, inability of transduced HSCs to engraft and outcompete endogenous HSCs, and/or insufficient retroviral-mediated FANCC expression to correct long-term repopulating ability. In other systems, murine models have provided significant advancements in answering basic questions related to transduction and expression of retroviral-mediated transgenes in HSCs as well as the impact of gene transfer on HSC proliferation and self-renewal potential. The availability of murine models deficient in FA protein expression are especially valuable to evaluate HSC function before and after gene replacement therapy, since in vivo reconstitution represents the only universally accepted functional assay for HSCs.
Two recent studies examined whether retroviral-mediated gene transfer of Fancc restored normal sensitivity to genotoxins 4 to 6 months after transplantation.15 16 These data showed that transduced repopulating cells had increased resistance to genotoxins, including mitomycin C (MMC). However, no data are available examining the potential of recombinant Fancc (rFancc) to restore HSC repopulating ability, a key physiologic function critical for successful treatment of BM failure in FA-C patients. In addition, no data are available evaluating the potential of gene transfer technology to prevent clonal hematopoietic disorders in FA.
Previously, we and others established that HSCs fromFancc−/− mice have profound defects in repopulating ability17 and self-renewal potential.18 In the studies reported here, we usedFancc−/− mice to determine whether introduction of rFancc into Fancc−/− HSCs would restore normal in vivo proliferation and self-renewal potentials to wild-type (WT) levels. Competitive repopulation studies demonstrated that Fancc−/− HSC repopulating ability was enhanced significantly compared to the repopulating potential ofFancc−/− HSCs lacking the recombinant protein. An unexpected finding was that ex vivo culture of uncorrectedFancc−/− cells resulted in an increased incidence of hematologic abnormalities including BM failure, myelofibrosis, and disrupted splenic histology in primary and secondary recipients transplanted with these cells. In addition, uncorrectedFancc−/− progenitors from several mice with hematologic alterations displayed a marked resistance to multiple inhibitory cytokines known to preferentially induce apoptosis inFancc−/− progenitors compared to WT progenitors. These data are the first demonstration of the profound hematologic phenotypes characteristic of the human disease in a murine model of FA without genotoxin treatment. Thus, data presented here enhance the understanding of both the potential benefits and limitations of retroviral-mediated gene transfer strategies to treat the hematologic manifestations of FA and provide an animal model to study the pathogenesis of clonal hematopoietic disorders in FA.
Materials and methods
Mouse colonies
Fancc+/− mice were intercrossed with C57Bl/6J mice for more than 10 generations to develop an inbred strain. Since Fancc−/− mice are infertile,Fancc+/− mice (CD45.2+) were bred to generate Fancc−/− and WT mice. Congenic C57Bl/6J (CD45.2+) and B6.SJL-PtrcaPep3b/BoyJ (B6.BoyJ, CD45.1+) mice were purchased from Jackson Laboratories (Bar Harbor, ME) for transplantation experiments. Indiana University's laboratory animal research committee approved all animal studies conducted.
Retroviral constructs
Two retroviruses containing either human (MFG-FAC) or murineFancc (MSCV-Fancc) were used for these studies, as well as 2 independent control vectors (MSCVpac and MSCV-EGFP). The cloning strategy for MFG-FAC was previously described.19 The retroviral backbone MSCVpac was obtained from Dr R. Hawley (Holland Laboratory, American Red Cross),20 and the enhanced green fluorescent protein (EGFP) cDNA was obtained from Clontech (Palo Alto, CA) in the pEGFPN1 plasmid. All molecular biology reagents were purchased from New England Biolabs (Beverly, MA) unless otherwise stated. To construct a retroviral vector containing EGFP, the puromycin resistance cDNA in MSCVpac (3′ of phosphoglycerate kinase promotor) was replaced by the EGFP cDNA (MSCV-EGFP) using the following method. MSCVpac was digested with ClaI, blunt ended using Klenow and deoxynucleoside triphosphates (dNTPs), and then digested withHindIII. To isolate the EGFP cDNA, pEGFPN1 was digested withNotI, blunt ended using Klenow and dNTPs, and then digested with HindIII. EGFP was ligated into the retroviral backbone using T4 DNA ligase (MSCV-EGFP). To clone the murine FancccDNA into the multiple cloning site of MSCV-EGFP (5′of phosphoglycerate kinase promotor), this vector was digested with BglII andHpaI. The murine Fancc cDNA was amplified from pmfac2 (a generous gift from Manuel Buchwald) using Pfu and primers containing a 5′ BglII restriction site and a 3′HpaI restriction site. The polymerase chain reaction (PCR) product was digested with BglII andHpaI. Vector and insert were ligated using T4 DNA ligase (MSCV-Fancc). Fidelity of amplified Fancc was verified by sequencing (Davis Sequencing Facility, Davis, CA).
High-titer stable ecotropic packaging lines for MSCVpac, MSCV-EGFP, and MSCV-Fancc were established as previously described for MFG-FAC,19 except selection was conducted using either puromycin resistance or EGFP expression. Retroviral supernatants were collected from packaging cells once 80% confluent and stored at −80°C. Supernatants were titered on NIH-3T3 cells and were determined to be ∼1 × 106 viral particles/mL for all retroviruses.
Transduction protocol
To obtain sufficient numbers of cells for competitive repopulation experiments, BM cells were harvested from 20 to 30Fancc−/− mice and 6-10 WT mice for each of the 3 experiments. The transduction protocol was as previously described with minor modifications.19,21,22 Low-density cells (Ficoll-Hypaque density 1.119; Sigma, St Louis, MO) were prestimulated with 200 units/mL human (h)IL-6 and 100 ng/mL murine stem cell factor (mSCF) (Peprotech, Rocky Hill, NJ) for 48 hours. After prestimulation, cells were transduced on recombinant human fibronectin fragment CH-296, RetroNectin (a generous gift from TAKARA BIO, Otsu, Japan) as previously described.19,21,22Fancc−/− cells were transduced with either a control retrovirus (MSCVpac or MSCV-EGFP) or a retrovirus encoding rFancc (MFG-FAC or MSCV-Fancc), and WT cells were transduced with a control retrovirus only. An aliquot of cells from each transduction group was plated in progenitor assays with MMC as previously described,23 while the majority of transduced cells were either directly transplanted into lethally irradiated recipients or used in competitive repopulation assays.
Competitive repopulation assays/secondary transplants
Four independent competitive repopulation experiments were conducted similar to previous methods.17 Experiments 1 to 3 used transduced cell populations as donor cells: (1) WT cells transduced with control virus; (2) Fancc−/−cells transduced with control virus, and (3)Fancc−/− cells transduced with a retrovirus containing either the human or (4) murine Fancc cDNA. One to two million cells from each of the 3 test cell populations were mixed with a common pool of B6.BoyJ low-density competitors (3-7 × 105 cells) that had been prestimulated with hIL-6 and mSCF for 48 to 72 hours. Experiment 4 usedFancc−/− and WT cells that had either been freshly isolated or cultured for 4 days with hIL-6 and mSCF. In order to obtain approximately equivalent levels of donor chimerism in mice transplanted with freshly isolated cells, a 6:1 test-to-competitor cell ratio was used for recipients transplanted withFancc−/− test cells and a 3:1 test-to-competitor cell ratio for mice transplanted with WT test cells.
Each cell mixture was resuspended in 0.5 mL Iscove modified Dulbecco medium (IMDM; GIBCO BRL, Gaithersburg, MD), 20% fetal calf serum (FCS) (Hyclone Laboratories, Logan, UT), and injected into the tail vein of 5 to 8 lethally irradiated recipients. Six mice were transplanted with only competitor cells (CD45.1+) to ensure that donor chimerism measurements (CD45.2+) were not contaminated by endogenous hematopoiesis from lethally irradiated C57Bl/6J recipients (CD45.2+). CD45.1 and CD45.2 chimerism were analyzed as previously described.17 Mean donor chimerism were analyzed using a Mann-Whitney nonparametric test to evaluate for significant differences between transduction groups. A Fisher exact test was used to determine whether the number of mice that exhibited high chimerism was different between transduction groups.
Secondary transplants were conducted using unfractionated BM cells harvested from 2 to 3 primary recipients in each of the 4 experimental groups from experiment 3: control Fancc−/−donor cells with high chimerism, controlFancc−/− donor cells with low chimerism,Fancc−/− donor cells transduced with MSCV-Fancc, and control Fancc+/+ donor cells. Five million cells were transplanted into lethally irradiated recipients, and chimerism analyzed as described above.
Progenitor assays using BM from mice receiving transplants
Low-density cells (density, 1.119 g/mL) were harvested from selected primary recipients in experiment 2 to examine MMC and inhibitory cytokine sensitivity in progenitors. Chimeric BM cells were stained with CD45.2-fluorescein isothiocyanate (PharMingen, San Diego, CA). Cells were washed, resuspended in cold phosphate-buffered saline (PBS) 0.1% bovine serum albumin (BSA), and sorted for CD45.2+ cells using a Becton Dickinson FACSTAR sorter (San Jose, CA) to purify donor cells and exclude competitor cells from progenitor assays. Sorted CD45.2+ cells were plated in the absence or presence of either MMC (10-200 nM), IFN-γ (10 ng/mL), TNF-α (10 ng/mL), or MIP-1α (50 ng/mL) as previously described.23 In addition, the proportion of CD45.2+ progenitors in S phase was estimated using3H-thymidine suicide assays as previously described.23
Histologic analysis of femur/spleen
The spleen and one long bone from selected mice were collected and fixed in 1% formaldehyde at room temperature for 6 hours and then embedded in paraffin. Hematoxylin and eosin (H&E) staining was performed using routine methods. All antibodies and immunohistochemical reagents were purchased from DAKO (Carpinteria, CA) unless otherwise specified. Immunohistochemical staining of spleen sections was performed with antibodies that identify myeloid cells (myeloperoxidase and lysozyme), T lymphocytes (CD3), and B lymphocytes (B220; PharMingen). Spleen slides were treated with 3% H2O2 for 15 minutes and then incubated with primary antibodies to either myeloperoxidase (5 minutes), lysozyme (10 minutes), CD3 (30 minutes), or B220 (30 minutes). Slides were then stained with secondary rabbit anti–human antibody for 5 minutes, incubated with streptavidin conjugated to horseradish peroxidase (10 minutes), followed by diaminobenzidine (DAB) treatment (5 minutes) and counterstaining with diluted hematoxylin. All slides were evaluated by an experienced hematopathologist (Dr Attilio Orazi), who was blinded to experimental treatment groups.
Polyclonal antibody production
Polyclonal antibodies were developed in female New Zealand rabbits against a synthetic peptide RSEKLARELLKELRTQV corresponding to carboxy terminal residues of human FANCC (amino acids 541-558) containing a cysteine residue on both the N- and C-termini for coupling. The peptide was coupled to keyhole limpet hemocyanin (KLH) as described by the manufacturer (Pierce Chemical, Rockford, IL). The rabbits were given a primary injection of 0.25 mg peptide-KLH emulsified in Freund complete adjuvant and subsequently injected with 0.1 mg peptide-KLH emulsified in Freund incomplete adjuvant at 2-week intervals. Rabbit serum was analyzed for reactive antibody against both human and murine Fancc by Western blotting. The antibody was purified by column chromatography using the peptide as the antigen.
Western blotting
Whole cell lysates were obtained from 5 × 106 BM cells and quantitated using a bicinchoninic (BCA) assay (Pierce Chemical). Whole cell lysates from Fancc−/−murine embryonic fibroblasts transduced with MSCV-Fancc were used as a positive control. Equivalent amounts of protein (100 μg) from BM samples were loaded on a 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gel and transferred onto a nitrocellulose membrane. Membranes were blocked with Tris-buffered saline (TBS) Tween containing 5% nonfat milk for 1 hour. For immunodetection of Fancc protein, rabbit anti–Fancc antibody was used at a 1:100 dilution for one hour, and the secondary antibody, anti-rabbit–horseradish peroxidase (HRP) (Amersham Biosciences, Piscataway, NJ) was used at a 1:1000 dilution for 1 hour before visualizing by chemiluminescence (Amersham Biosciences). Equal protein loading was documented by using β-actin as an internal control.
Semiquantitative PCR
HEL cells were transduced with a limiting dilution of retroviral supernatant to obtain a single integration per transduced cell. EGFP-positive cells were sorted by fluorescence cytometry and expanded to use as a standard for dilutional PCR. Genomic DNA was isolated from HEL and “test” peripheral blood cells using a Puregene DNA isolation kit (Gentra Systems, Minneapolis, MN). Ten nanograms of DNA from the “test” sample were amplified using EGFP-specific primers (forward primer 5′GAAGTTCATCTGCACCACCGGCAA3′ and reverse primer 5′TAGTGGTTGTCGGGCAGCAGCACG3′). In addition, the single copy standard DNA was diluted 1:5, 1:10, 1:50, 1:100, and 1:500 before amplification to compare “test” DNA signal. Each sample was amplified for 30 cycles (94°C for 60 seconds, 64°C for 60 seconds, and 72°C for 60 seconds). Amplified PCR products were run on a 1% agarose gel and assessed for the intensity of the 468–base pair (bp) band similar to previously described methods.24-26
Results
Expression of rFancc in Fancc−/−HSCs maintained in long-term reconstituted mice
To determine whether functional rFancc expression could be detected in vivo following long-term hematopoietic cell reconstitution,Fancc−/− cells were transduced with one of 2 recombinant retroviruses encoding the Fancc transgene and transplanted into lethally irradiated, WT mice. Six months following transplantation, BM cells were evaluated for Fancc expression by Western blotting, and colony assays were conducted to examine whether introduction of the Fancc transgene into BM progenitors from reconstituted mice corrected MMC hypersensitivity. One of 4 representative Western blots showed that BM harvested from a mouse transplanted with Fancc−/− cells transduced with MSCV-Fancc clearly expressed rFancc (Figure1A, lane 2), while BM from a mouse transplanted with Fancc−/− cells transduced with a control virus did not (lane 3). Furthermore, BM progenitors from all mice transplanted with Fancc−/− cells transduced with MSCV-Fancc displayed a normal sensitivity to MMC equivalent to that observed in progenitors from mice transplanted with WT cells transduced with the control virus (Figure 1B). Similar results were obtained when Fancc−/− cells were transduced with MFG-FAC (data not shown). Consistent with previous studies using other constructs,15 16 these data suggest that the Fancc transgene was introduced into long-term reconstituting cells and was sufficiently expressed in progeny cells to correct the characteristic hypersensitivity to bifunctional alkylating agents such as MMC.
Fancc expression and mitomycin C sensitivity of BM cells from long-term reconstituted mice.
(A) Fancc Western. Whole cell lysates were extracted from the BM of long-term reconstituted mice transplanted withFancc−/− cells transduced with either MSCV-Fancc (lane 2) or MSCV-EGFP (lane 3).Fancc−/− murine embryonic fibroblasts transduced with MSCV-Fancc were used as a positive (+) control (lane 1). One of 4 representative blots is shown. (B) MMC progenitor assay. Six months following transplantation, BM cells were harvested from mice transplanted with either Fancc−/− cells transduced with MSCV-Fancc (▪),Fancc−/− cells transduced with a reporter gene only (●), or wild-type cells transduced with a reporter gene only (▴). BM cells were cultured in methylcellulose progenitor assays at 5 × 104 cells/mL with increasing concentrations of MMC. Each condition was plated in triplicate and scored on day 7 of culture. The percent maximal colony formation was determined by dividing the number of colonies scored at each MMC concentration by untreated control cultures. Error bars represent standard error of the means (SEM), Student t test *P ≤ .001.
Fancc expression and mitomycin C sensitivity of BM cells from long-term reconstituted mice.
(A) Fancc Western. Whole cell lysates were extracted from the BM of long-term reconstituted mice transplanted withFancc−/− cells transduced with either MSCV-Fancc (lane 2) or MSCV-EGFP (lane 3).Fancc−/− murine embryonic fibroblasts transduced with MSCV-Fancc were used as a positive (+) control (lane 1). One of 4 representative blots is shown. (B) MMC progenitor assay. Six months following transplantation, BM cells were harvested from mice transplanted with either Fancc−/− cells transduced with MSCV-Fancc (▪),Fancc−/− cells transduced with a reporter gene only (●), or wild-type cells transduced with a reporter gene only (▴). BM cells were cultured in methylcellulose progenitor assays at 5 × 104 cells/mL with increasing concentrations of MMC. Each condition was plated in triplicate and scored on day 7 of culture. The percent maximal colony formation was determined by dividing the number of colonies scored at each MMC concentration by untreated control cultures. Error bars represent standard error of the means (SEM), Student t test *P ≤ .001.
Ex vivo culture and transplantation of controlFancc−/−cells results in an increased risk of developing abnormal reconstituting ability
Competitive repopulation assays assess relative differences in repopulating activity of distinct HSC sources.27-32Previously, we demonstrated that Fancc−/− HSCs have a marked reduction in repopulating ability compared to WT HSCs.17 To determine whether the repopulating defect could be corrected via expression of rFancc,Fancc−/− BM cells were transduced with a retrovirus encoding Fancc or, alternatively, a reporter gene and used as donor cells in competitive repopulation studies. Donor cells (CD45.2+) from each transduction group were cotransplanted into irradiated recipients with a common pool of syngeneic competitor cells (CD45.1+, Figure2). Peripheral blood chimerism of transplanted mice was evaluated by fluorescence cytometry. Three independent experiments were conducted with similar results. Table1 summarizes retroviral vectors that were used in each experiment, and the number of mice receiving transplants for each transduction group.
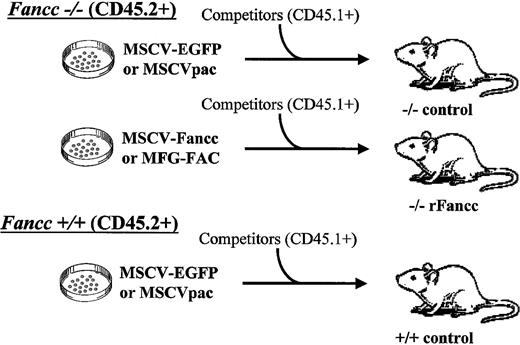
Schematic of gene therapy competitive repopulation experiments.
Donor BM cells from Fancc−/−mice were transduced with either a retrovirus containing a reporter gene (MSCV-EGFP or MSCVpac) or a retrovirus encoding rFancc (MSCV-Fancc or MFG-FAC). WT donor cells were transduced with a retrovirus containing a reporter gene only (MSCV-EGFP or MSCVpac). Each respective donor cell population was cotransplanted into 5-8 lethally irradiated recipient mice with competitor cells from B6.BoyJ mice that were genetically identical to C57Bl/6J mice with the exception of distinct CD45 isoantigen expression.
Schematic of gene therapy competitive repopulation experiments.
Donor BM cells from Fancc−/−mice were transduced with either a retrovirus containing a reporter gene (MSCV-EGFP or MSCVpac) or a retrovirus encoding rFancc (MSCV-Fancc or MFG-FAC). WT donor cells were transduced with a retrovirus containing a reporter gene only (MSCV-EGFP or MSCVpac). Each respective donor cell population was cotransplanted into 5-8 lethally irradiated recipient mice with competitor cells from B6.BoyJ mice that were genetically identical to C57Bl/6J mice with the exception of distinct CD45 isoantigen expression.
Number of mice transplanted in each donor cell transplant group
| Experiment . | Control virus . | Recombinant Fancc virus . |
|---|---|---|
| 1 | MSCVpac | MFG-FAC (n = 6) |
| Fancc−/−, n = 7 | ||
| Fancc+/+, n = 8 | ||
| 2 | MSCV-EGFP | MSCV-Fancc (n = 8) |
| Fancc−/−, n = 7 | ||
| Fancc+/+, n = 6 | ||
| 3 | MSCV-EGFP | MSCV-Fancc (n = 3) |
| Fancc−/−, n = 8 | MFG-FAC (n = 5) | |
| Fancc+/+, n = 6 |
| Experiment . | Control virus . | Recombinant Fancc virus . |
|---|---|---|
| 1 | MSCVpac | MFG-FAC (n = 6) |
| Fancc−/−, n = 7 | ||
| Fancc+/+, n = 8 | ||
| 2 | MSCV-EGFP | MSCV-Fancc (n = 8) |
| Fancc−/−, n = 7 | ||
| Fancc+/+, n = 6 | ||
| 3 | MSCV-EGFP | MSCV-Fancc (n = 3) |
| Fancc−/−, n = 8 | MFG-FAC (n = 5) | |
| Fancc+/+, n = 6 |
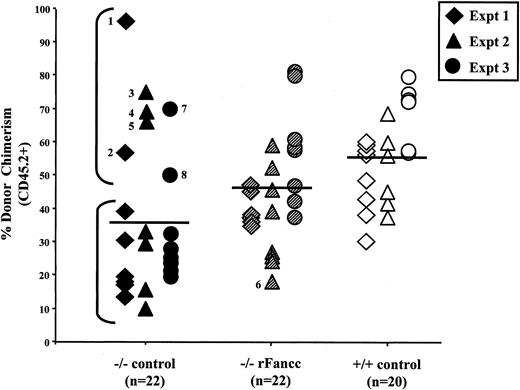
Donor chimerism of individual recipients from all experiments were analyzed 6 months following transplant (Figure3). As expected, mean donor chimerism of mice transplanted with Fancc−/− cells transduced with a retrovirus encoding a reporter gene only (−/− control) was significantly lower than recipients transplanted with WT donor cells (+/+ control, 36.7% ± 5.1% vs 56.4% ± 3.1%,P < .002), although −/− control chimerism was highly variable (range, 10%-97%). Furthermore,Fancc−/− donor cells transduced with a retrovirus encoding rFancc exhibited enhanced reconstituting ability compared to −/− control cells (45.0% ± 3.4% vs 36.7% ± 5.1%, P < .04), though not consistently to WT levels. Our prediction, based on previous experiments with freshly isolated cells,17 was that −/− control cells would exhibit a level of chimerism at least 2-fold lower than that supported by +/+ control HSCs. This prediction was generally observed in 15 of 22 −/− control mice in experiments 1 to 3 (Figure 3, lower bracket). Surprisingly, 7 mice had a markedly elevated chimerism compared to the other 15 mice in this transplant group (Figure 3, upper bracket). These data demonstrate a segregation of mice transplanted with −/− control donor cells into 2 distinct groups. This observation was not detected in mice transplanted with WT donor cells transduced with the same retroviral supernatants or in Fancc−/− cells transduced with rFancc. To ensure that the high chimerism observed in some −/− control mice was not due to preferential damage to Fancc−/− cells from EGFP expression, peripheral blood cells were examined for EGFP expression in experiments 2 and 3, where MSCV-EGFP was used as the control retrovirus. EGFP expression was low in all −/− control and +/+ control transduction group recipients (0% to 25% EGFP+ cells; and no differences in intensity of EGFP expression). Furthermore, no correlation was observed between the level of donor chimerism and EGFP expression in the −/− control transduction group (data not shown).
Donor chimerism of individual mice transplanted with transduced test cells.
Peripheral blood donor chimerism was determined by fluorescence cytometry 6 months following transplantation. Individual data points represent donor chimerism of single mice transplanted with eitherFancc−/− cells transduced with a reporter gene only (−/− control), Fancc−/− cells transduced with a retrovirus encoding rFancc (−/− rFancc), or WT cells transduced with a reporter only (+/+ control). All data from these 3 transduction groups in experiments 1-3 are shown. Mean donor chimerism of mice transplanted with −/− control cells was significantly lower than +/+ control (*P < .002). Transduction of Fancc−/− cells with rFancc (−/− rFancc) resulted in a significant increase in repopulating ability compared to −/− control test cells (**P ≤ .04). The lower bracket outlines expected chimerism of mice transplanted with −/− control cells, and the upper bracket outlines mice (mice 1-5, 7, and 8) with a marked increase in donor chimerism. Mouse 6 in the −/− rFancc transduction group exhibited low chimerism at this timepoint but had a marked increase in chimerism 16 months after transplant. A Fisher exact test confirmed that the incidence of aberrant chimerism in the −/− control (7 of 22) transduction group was significantly greater than in the −/− rFancc (1 of 22) transduction group (P < .05).
Donor chimerism of individual mice transplanted with transduced test cells.
Peripheral blood donor chimerism was determined by fluorescence cytometry 6 months following transplantation. Individual data points represent donor chimerism of single mice transplanted with eitherFancc−/− cells transduced with a reporter gene only (−/− control), Fancc−/− cells transduced with a retrovirus encoding rFancc (−/− rFancc), or WT cells transduced with a reporter only (+/+ control). All data from these 3 transduction groups in experiments 1-3 are shown. Mean donor chimerism of mice transplanted with −/− control cells was significantly lower than +/+ control (*P < .002). Transduction of Fancc−/− cells with rFancc (−/− rFancc) resulted in a significant increase in repopulating ability compared to −/− control test cells (**P ≤ .04). The lower bracket outlines expected chimerism of mice transplanted with −/− control cells, and the upper bracket outlines mice (mice 1-5, 7, and 8) with a marked increase in donor chimerism. Mouse 6 in the −/− rFancc transduction group exhibited low chimerism at this timepoint but had a marked increase in chimerism 16 months after transplant. A Fisher exact test confirmed that the incidence of aberrant chimerism in the −/− control (7 of 22) transduction group was significantly greater than in the −/− rFancc (1 of 22) transduction group (P < .05).
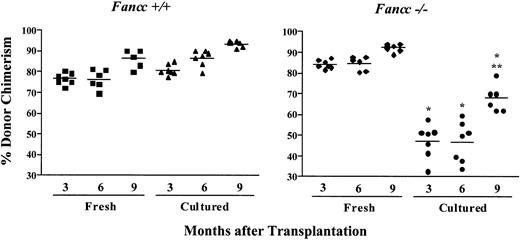
The elevated chimerism in the 7 animals in the −/− control transduction group suggested that ex vivo manipulation and/or retroviral transduction contributed to the development of aberrant chimerism. To examine whether ex vivo culture is sufficient to predispose Fancc−/− repopulating cells to develop aberrant reconstituting ability, competitive repopulation experiments were conducted using either freshly isolated cells or cells cultured ex vivo for 4 days as test populations (Figure4). To achieve similar donor chimerism in mice transplanted with freshly isolated cells, it was necessary to double the Fancc−/− test-to-competitor cell ratio 6:1 compared to WT test-to-competitor cell ratio 3:1 sinceFancc−/− HSCs exhibit a significant defect in repopulating ability.17 In addition, to further decrease the repopulating stress of transplantation, twice as many cells were transplanted per recipient compared to gene transfer experiments. Mice transplanted with WT donor cells displayed relatively stable levels of donor chimerism over time regardless of whether cells were freshly isolated or cultured. Chimerism of mice transplanted with freshly isolated Fancc−/− cells also was stable. Interestingly, cultured Fancc−/− donor cells exhibited a marked reduction in repopulating ability at all timepoints evaluated compared to freshly isolatedFancc−/− cells (Figure 4). However, in contrast to the chimerism of mice transplanted with freshly isolatedFancc−/− cells, chimerism of individual mice transplanted with cultured Fancc−/− cells at the 3- and 6-month timepoints was highly variable (33%-60%). Further, 9 months following transplantation mean donor chimerism increased significantly (from 47% at 6 months to 68% at 9 months), a time when long-term repopulating ability should be stable.31 32Collectively, these data suggest that ex vivo culture selectively compromised Fancc−/− but not WT HSC repopulating ability. Furthermore, the wide variability of donor chimerism and the unexpected rise in chimerism of mice transplanted with cultured Fancc−/− cells are consistent with data from experiments 1 to 3, where mice in the −/− control transduction group displayed highly variable chimerism (Figure 3).
Ex vivo culture of Fancc−/−cells compromises repopulating ability.
Peripheral blood donor chimerism was determined by fluorescence cytometry 3, 6, and 9 months following transplantation. Individual data points represent donor chimerism of single mice transplanted with either WT or Fancc−/− donor cells that were freshly isolated or cultured for 4 days in hIL-6 and mSCF, identical to the culture conditions used to maintain donor cells in the gene transfer competitive repopulation experiments. Repopulating ability ofFancc−/− cells after ex vivo culture was significantly lower than the repopulating ability of freshly isolatedFancc−/− cells at all timepoints evaluated (Student t test *P < .0006). Donor chimerism of mice transplanted with ex vivo culturedFancc−/− cells increased significantly from 6 to 9 months after transplantation (Student t test **P < .001).
Ex vivo culture of Fancc−/−cells compromises repopulating ability.
Peripheral blood donor chimerism was determined by fluorescence cytometry 3, 6, and 9 months following transplantation. Individual data points represent donor chimerism of single mice transplanted with either WT or Fancc−/− donor cells that were freshly isolated or cultured for 4 days in hIL-6 and mSCF, identical to the culture conditions used to maintain donor cells in the gene transfer competitive repopulation experiments. Repopulating ability ofFancc−/− cells after ex vivo culture was significantly lower than the repopulating ability of freshly isolatedFancc−/− cells at all timepoints evaluated (Student t test *P < .0006). Donor chimerism of mice transplanted with ex vivo culturedFancc−/− cells increased significantly from 6 to 9 months after transplantation (Student t test **P < .001).
Fancc expression protects primary and secondary recipients from developing aberrant hematopoiesis
To determine whether the observed abnormalities in reconstitution predisposed mice to develop BM failure or clonal hematopoietic disorders, mice from experiments 1 to 3 were analyzed for evidence of hematologic alterations in primary or secondary recipients. Mice in experiment 1 were analyzed as primary recipients 6 months following transplantation. These studies revealed that peripheral blood counts and BM cellularity of all mice were unremarkable between transduction groups. However, the mouse transplanted with −/− control cells and 97% chimerism (Figure 3, mouse 1; Table2) developed splenomegaly (198 mg vs normal transplanted mice 102 ± 11 mg), although the histology was normal (data not shown).
Hematologic alterations in primary recipients with aberrant donor chimerism from experiments 1 and 2
| Mouse no.* . | Detected abnormalities† (time of analysis in months) . |
|---|---|
| 1 | Splenomegaly (6) |
| 2 | None (6) |
| 3 | Pancytopenia, hypoplastic bone marrow, splenomegaly with abnormal architecture and increased proportion of immature myeloid cells (16) |
| 4 | Unexpected death (16) |
| 5 | Splenomegaly (16) |
| 6 | Bone marrow myeloid hyperplasia and myelofibrosis with immature appearing myeloid cells (16) |
| Mouse no.* . | Detected abnormalities† (time of analysis in months) . |
|---|---|
| 1 | Splenomegaly (6) |
| 2 | None (6) |
| 3 | Pancytopenia, hypoplastic bone marrow, splenomegaly with abnormal architecture and increased proportion of immature myeloid cells (16) |
| 4 | Unexpected death (16) |
| 5 | Splenomegaly (16) |
| 6 | Bone marrow myeloid hyperplasia and myelofibrosis with immature appearing myeloid cells (16) |
Chimerism data illustrated in Figure 3.
Analyzed peripheral blood counts, bone marrow and spleen cellularity, spleen weight, and bone marrow and spleen histology.
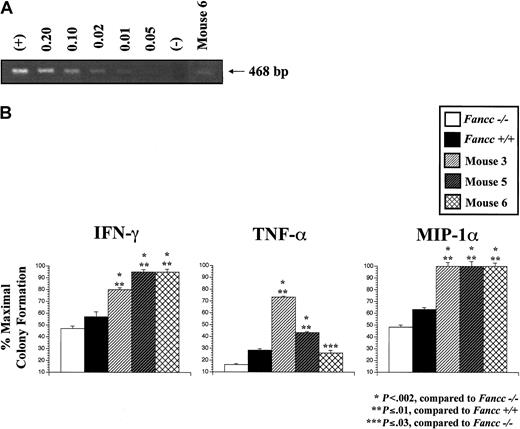
We hypothesized that 6 months may be insufficient time after transplantation for the evolution of BM failure or myelodysplasia/leukemia. To determine whether the high aberrant chimerism in control Fancc−/− donor cells predispose to the eventual development of aplastic anemia or clonal hematopoietic disorders, mice from experiment 2 were followed sequentially over time, while BM cells from mice in experiment 3 were transplanted into secondary recipients. Sixteen months after transplantation, chimerism in 3 of 7 mice transplanted with controlFancc−/− donor cells in experiment 2 remained elevated, similar to the 6-month timepoint (Figure 3, mice 3-5). In addition, one of the 8 mice in experiment 2 transplanted withFancc−/− donor cells transduced with a retrovirus containing rFancc developed progressively higher donor chimerism over time. This mouse (Figure 3, mouse 6) exhibited low chimerism (17%) at the 6-month timepoint, similar to mice transplanted with −/− control cells (Figure 3). However, by the 16-month timepoint donor chimerism increased significantly to 49%. To determine whether the increase in donor chimerism was due to expansion of untransduced or transduced cells, a semiquantitative PCR was conducted using primers specific to the proviral DNA. PCR of peripheral blood cells showed that only 1%-2% of cells contained the provirus (Figure5A), suggesting that the increase in chimerism was due to expansion of untransducedFancc−/− cells. These data suggest that mouse 6 did not exhibit sufficient HSC functional correction required for normal repopulating ability or defense from developing aberrant chimerism. The remaining 7 mice transplanted withFancc−/− cells transduced with rFancc maintained stable donor chimerism.
Mice transplanted with either −/− control cells or a low frequency of −/− rFancc cells had progenitors that were resistant to multiple inhibitory cytokines.
(A) Semiquantitative PCR. HEL cells with a single provirus copy were used as the standard DNA for dilution, and untransduced HEL cells were used as the negative control. DNA extracted from the peripheral blood of mouse 6 was amplified with primers specific for proviral DNA. Approximately 1%-2% of peripheral blood cells from mouse 6 contained proviral DNA. (B) Inhibitory cytokine progenitor assay. Sorted CD45.2+ cells from mice 3, 5, and 6 were cultured in methylcellulose progenitor assays at 5 × 104 cells/mL with either IFN-γ (10 ng/mL), TNF-α (10 ng/mL), or MIP-1α (50 ng/mL). Each condition was plated in triplicate and scored on day 7 of culture. The percent maximal colony formation was determined by dividing the number of colonies scored with each inhibitory cytokine by untreated control cultures. Low-density cells fromFancc−/− and WT mice were used as controls. Baseline progenitor numbers scored in the untreated groups were 84 forFancc−/− , 82 forFancc+/+, 195 for mouse 3, 106 for mouse 5, and 89 for mouse 6. Error bars represent SEM.
Mice transplanted with either −/− control cells or a low frequency of −/− rFancc cells had progenitors that were resistant to multiple inhibitory cytokines.
(A) Semiquantitative PCR. HEL cells with a single provirus copy were used as the standard DNA for dilution, and untransduced HEL cells were used as the negative control. DNA extracted from the peripheral blood of mouse 6 was amplified with primers specific for proviral DNA. Approximately 1%-2% of peripheral blood cells from mouse 6 contained proviral DNA. (B) Inhibitory cytokine progenitor assay. Sorted CD45.2+ cells from mice 3, 5, and 6 were cultured in methylcellulose progenitor assays at 5 × 104 cells/mL with either IFN-γ (10 ng/mL), TNF-α (10 ng/mL), or MIP-1α (50 ng/mL). Each condition was plated in triplicate and scored on day 7 of culture. The percent maximal colony formation was determined by dividing the number of colonies scored with each inhibitory cytokine by untreated control cultures. Low-density cells fromFancc−/− and WT mice were used as controls. Baseline progenitor numbers scored in the untreated groups were 84 forFancc−/− , 82 forFancc+/+, 195 for mouse 3, 106 for mouse 5, and 89 for mouse 6. Error bars represent SEM.
The increase in chimerism in mice 3 to 6 suggested thatFancc−/− donor cells may have developed adaptive mutations during ex vivo culture that allowed for a proliferation and/or survival advantage. To evaluate for functional evidence of an adaptive mutation, we examined BM progenitor sensitivity to IFN-γ, TNF-α, and MIP-1α, whichFancc−/− progenitors are normally hypersensitive.23 33 Mouse 4 died unexpectedly before detailed analyses could be conducted to determine the cause of death 16 months after transplantation. Low-density cells from the surviving 3 animals were purified by fluorescence cytometry to isolate donor cells only (CD45.2+) before conducting progenitor assays. Purified progenitors from mice 3, 5, and 6 remained hypersensitive to MMC (data not shown). As expected, progenitors from the BM of unmanipulated Fancc−/− mice were hypersensitive to all 3 inhibitory cytokines evaluated as compared to WT progenitors (P < .04, Figure 5B). In striking contrast, Fancc−/− progenitors from mice 3, 5, and 6 were more resistant to IFN-γ, TNF-α, and MIP-1α compared toFancc−/− control progenitors, and in most cases, compared to WT progenitors. The loss of cytokine responsiveness was not due to the lack of cycling as determined by3H-thymidine suicide assays (% suicide, 38%-40%). These functional data support the concept that transplanted mice with high chimerism developed an adaptive mutation during ex vivo culture providing an advantage for the expansion of a clonal cell population.
To examine whether the aberrant chimerism predisposed mice 3, 5, and 6 to develop hematologic defects, mice in experiment 2 were killed 16 months following transplantation, and peripheral blood counts (red blood cells [RBCs], white blood cells [WBCs] with differential, and platelets), BM cellularity, spleen weight, and BM and spleen histology were examined. Abnormal hematologic findings were detected only in mice with high chimerism (summarized in Table 2). Mouse 3 displayed pancytopenia with reduced BM cellularity (BM cellularity 4.6 × 106 cells compared to +/+ control 38.4 ± 0.8 × 106 cells/2 femurs) and splenomegaly (spleen weight, 217 mg). Histologic examination revealed a hypoplastic BM (Figure 6C-D) and a high proportion of immature myeloid precursors in the spleen with loss of normal splenic architecture (Figure 6E-F). Mouse 5 displayed profound splenomegaly (spleen weight, 228 mg) with normal BM and spleen histology. Histologic analysis of mouse 6 demonstrated a profound myeloid hyperplasia associated with discrete areas of myelofibrosis (demonstrated by increased reticulin staining), containing immature appearing myeloid cells (Figure 6G-J). Collectively, these data suggest that ex vivo culture provided an environment for the evolution of clones with adaptive mutations increasing the risk of transplanted mice to develop hematopoietic defects that are reminiscent of human FA hematologic manifestations.
Mice from experiment 3 were used for secondary transplant studies 8 months after ex vivo culture and transplantation to address 3 experimental questions. First, was the secondary repopulating defect of Fancc−/− reconstituting cells corrected by introduction of rFancc? Second, was the high chimerism observed in some mice reconstituted with −/− control cells a consequence of a defect in a self-renewing HSC? And finally, would secondary transplantation predispose mice transplanted with −/− control cells to develop hematologic defects? To answer these questions, the secondary repopulating ability of 4 experimental groups was analyzed: −/− control cells with low chimerism, −/− control cells with high chimerism, −/− cells transduced with rFancc, and +/+ control cells (Figure 3). Secondary recipient chimerism was analyzed 8 months following transplantation (Figure7). Secondary recipients transplanted with either +/+ control or −/− rFancc donor cells exhibited similar donor chimerism as primary recipients, suggesting that introduction of rFancc into Fancc−/− HSCs enhanced both primary and secondary repopulating ability in vivo. In addition, the high chimerism observed in primary recipient mice 7 and 8 (Figure 3) originally transplanted with −/− control cells also was maintained in secondary recipients, indicating that the molecular event resulting in high chimerism occurred at the level of a self-renewing HSC.
Histologic evidence of BM hypoplasia and myelofibrosis.
(A) H&E staining of the BM from a mouse transplanted withFancc+/+ cells (magnification × 100). (B) H&E staining of the spleen from a mouse transplanted withFancc+/+ cells (magnification × 100). (C) H&E staining of the BM from mouse 3 (magnification × 100). (D) Magnification × 200 revealed a hypoplastic marrow. (E) H&E staining of the spleen from mouse 3 (magnification × 200). (F) Magnification × 400 revealed abnormal splenic architecture with an increased number of immature myeloid cells. (G) H&E staining of the BM from mouse 6 (magnification × 200). (H-I) Magnification × 400 revealed a hypercellular BM with a marked degree of myeloid hyperplasia and significant myelofibrosis. (J) Reticulin staining of the BM from mouse 6 (magnification × 400) to identify collagen deposition, which confirms myelofibrosis.
Histologic evidence of BM hypoplasia and myelofibrosis.
(A) H&E staining of the BM from a mouse transplanted withFancc+/+ cells (magnification × 100). (B) H&E staining of the spleen from a mouse transplanted withFancc+/+ cells (magnification × 100). (C) H&E staining of the BM from mouse 3 (magnification × 100). (D) Magnification × 200 revealed a hypoplastic marrow. (E) H&E staining of the spleen from mouse 3 (magnification × 200). (F) Magnification × 400 revealed abnormal splenic architecture with an increased number of immature myeloid cells. (G) H&E staining of the BM from mouse 6 (magnification × 200). (H-I) Magnification × 400 revealed a hypercellular BM with a marked degree of myeloid hyperplasia and significant myelofibrosis. (J) Reticulin staining of the BM from mouse 6 (magnification × 400) to identify collagen deposition, which confirms myelofibrosis.
Donor chimerism of secondary recipients transplanted with BM cells from one of the 4 primary recipient experimental groups.
Mean chimerism of primary recipients used as donor cells for secondary transplants are shown below each bar. Secondary recipient donor chimerism was measured 8 months after transplantation. Error bars represent SEM.
Donor chimerism of secondary recipients transplanted with BM cells from one of the 4 primary recipient experimental groups.
Mean chimerism of primary recipients used as donor cells for secondary transplants are shown below each bar. Secondary recipient donor chimerism was measured 8 months after transplantation. Error bars represent SEM.
Consistent with previously published data,18 chimerism of most secondary recipients transplanted with BM cells from mice in the −/− control, low chimerism experimental group decreased significantly compared to primary recipient chimerism (Figure 7). However, all recipients transplanted with BM cells from one mouse in this transplant group displayed a progressive increase in donor chimerism (primary recipient chimerism, 28%; secondary recipient chimerism, 66%-68%). These data are analogous to observations in primary competitive repopulation experiments where transplantation resulted in a progressive increase in donor chimerism in one third of transplanted mice (Figure 3, upper bracket). Collectively, these data suggest that the selective pressure induced by transplantation resulted in the evolution of abnormal proliferation and/or survival in someFancc−/− HSCs.
To evaluate secondary recipients for evidence of hematologic defects, peripheral blood counts (RBCs, WBCs with differentials, and platelets), BM cellularity, spleen weight, and BM and spleen histology were examined in 3 secondary recipients from each of the 4 experimental groups (−/− control with low chimerism, −/− control with high chimerism, −/− rFancc, and +/+ control) 8 months following secondary transplantation. Peripheral blood counts, BM, and spleen cellularity were not significantly different between the 4 experimental groups (data not shown). However, significant differences were detected in splenic histology, as scored by a hematopathologist blinded to the experimental groups (Table 3). The expected predominance of lymphoid cells was evident in all mice transplanted with either −/− rFancc or +/+ control cells. However, 3 of 6 −/− control secondary recipients examined displayed a significant increase in the proportion of immature myeloid cells in the spleen (2 mice in the high chimerism group and 1 in the low chimerism group). These data suggest a spectrum of evolving hematopoietic defects in secondary recipients transplanted with −/− control cells. Collectively, these data suggest that uncorrectedFancc−/− HSC acquired an altered HSC phenotype after ex vivo culture and transplantation.
Proportion of secondary recipients that displayed abnormal splenic histology
| Transduction group . | No. mice with histologic changes/total mice evaluated . |
|---|---|
| −/− control | 3/6 |
| −/− rFancc | 0/3 |
| +/+ control | 0/3 |
| Transduction group . | No. mice with histologic changes/total mice evaluated . |
|---|---|
| −/− control | 3/6 |
| −/− rFancc | 0/3 |
| +/+ control | 0/3 |
Discussion
Infusing autologous gene-corrected HSCs is a potential therapy for multiple diseases affecting the hematopoietic system. FA is an excellent candidate disease to assess the feasibility and effectiveness of gene therapy, since gene-corrected HSC should have a selective advantage over mutant endogenous stem cells. Theoretically, this competitive advantage should allow progeny from the gene-corrected HSC to reconstitute the entire hematopoietic system, thus preventing the hematologic manifestations of FA.
In initial studies, we addressed the improvement inFancc−/− hematopoietic cell function by evaluating progenitor MMC sensitivity 6 months after transplantation with Fancc−/− cells transduced with a retrovirus encoding rFancc. MMC sensitivity ofFancc−/− progenitors transduced with either MSCV-Fancc or MFG-FAC was corrected to WT levels, indicating that the genotoxin-induced hypersensitivity was reversible in long-term reconstitution studies, consistent with previous studies using a similar model.15 16 These encouraging results led us to evaluate the potential of gene therapy to improve critically relevant HSC phenotypes, proliferation potential, and self-renewal capacity, using competitive repopulation studies.
Expression of rFancc in Fancc−/− HSCs significantly enhanced primary repopulating ability compared to controlFancc−/− HSCs, albeit not to WT levels (Figure3). In addition, secondary recipients transplanted withFancc−/− HSCs transduced with a retrovirus encoding rFancc maintained chimerism levels similar to primary recipients. In contrast, most secondary recipients transplanted with uncorrected Fancc−/− cells exhibited decreased chimerism compared to primary recipients, consistent with previous studies.18 Together these data suggest that introduction of rFancc into Fancc−/− HSCs significantly improves HSC proliferation potential and self-renewal capacity.
An intriguing observation, reproducible in 4 independent experiments but not detected in competitive repopulation experiments conducted with fresh BM cells (Figure 4, and Haneline et al17), was thatFancc−/− reconstituting cells incurred significant environmental stress after ex vivo culture and transplantation, which resulted in selection of HSCs with aberrant reconstituting ability. Of 22 primary recipients, 7 (32%) transplanted with Fancc−/− donor cells transduced with a control retrovirus (−/− control cells) exhibited a marked increase in donor chimerism. In addition, 3 of 4 primary recipients and 3 of 6 secondary recipients transplanted with −/− control cells displayed hematologic defects including BM aplasia, myelofibrosis, and altered splenic histology. In striking contrast, only 1 of 22 primary recipients and none of the secondary recipients transplanted with rFancc-transduced cells displayed these pathologic manifestations. The single exception in this group had only 1%-2% of total peripheral blood cells that contained proviral DNA. Together, these data show the potential benefit of gene transfer in offering an enhancement of long-term repopulating ability. However, the data also show that when repopulating cells were not transduced or transduced at a low frequency with rFancc, Fancc−/− HSCs had an increased risk of developing aberrant repopulating ability, which may predispose to the development of BM failure and myelodysplasia.
Since the loss of Fancc leads to a profound proapoptotic phenotype in human and murine hematopoietic cells,23,33and Fancc−/− HSC function is already impaired,17,18 ex vivo culture may promote the acquisition of adaptive mutations that protect abnormal HSC from apoptosis. Our data showing that Fancc−/− progenitors from multiple long-term reconstituted mice with hematologic defects were more resistant, and in some cases, totally resistant to the suppressive effects of IFN-γ, TNF-α, and MIP-1α would support this concept. In addition, these data suggest that the loss of inhibitory cytokine hypersensitivity in human cells may be one important initiating event leading to the predisposition of FA patients to develop clonal hematopoietic disorders. Furthermore, ex vivo culture may select for HSCs that have a proliferation and/or survival advantage, while transplantation may provide an additional pressure for the expansion of abnormal clones in vivo. The concept that selective pressure of cells has a critical role in the 5- to 10 000-fold increased risk of developing myelodysplasia or acute myeloid leukemia in FA patients has been proposed previously.34
It is also conceivable that the chromosomal instability inherent to FA cells led to secondary mutations during ex vivo culture with pharmacologic doses of cytokines that stimulate cells to proliferate. Several laboratories have shown that an accumulation of mutations is required for progression to myelodysplasia or leukemia.35Our data show that while ex vivo culture increased the risk of transplanted mice to develop aberrant hematopoietic phenotypes, an extended period of evaluation in primary and/or secondary recipients (16 months) was required before detecting histologic changes. These data suggest that either additional mutations are occurring in vivo, allowing for an accumulation of mutations over time, or alternatively that the mutations incurred during in vitro culture required several months after transplantation before altering normal BM/spleen histology. Collectively, these data provide an experimental model system to utilize Fancc−/− mice to further examine the pathogenesis of clonal hematopoietic disorders in FA, including myelodysplasia and leukemia.
Additionally, Fancc−/− mice and competitive repopulation studies provide a preclinical model system to evaluate the biologic consequences of gene transfer protocols with the goal of optimizing Fancc−/− HSC transduction without enhancing deleterious selection pressures on untransducedFancc−/− HSCs. The major strengths of this experimental approach compared to previously reported preclinical gene transfer studies15,16 are that it allows for both a sensitive evaluation of long-term HSC repopulating function as well as a means to examine genomic stability in an in vivo setting. Our data support using transduction protocols that require limited ex vivo manipulation. One strategy would be to use viral vectors that transduce resting cells such as lentiviruses.36-39
In summary, our data show that transduction of rFancc intoFancc−/− HSCs significantly improved HSC proliferation and self-renewal capacity determined by competitive repopulation studies. An unpredicted observation suggests that ex vivo culture and transplantation increases the risk of uncorrected HSCs to develop abnormal repopulating ability suggestive of clonal hematopoiesis. Further studies are planned to examine molecular and biochemical mechanisms responsible for these observations and to optimize the transduction procedure to decrease the risk of aberrant reconstituting ability in uncorrected Fancc−/−HSCs and subsequent development of altered hematopoiesis. These data have important implications for understanding the potential of retroviral-mediated gene transfer to suppress clonal evolution in FA patients as well as potential untoward effects if ex vivo manipulated HSCs are not transduced. Moreover, these data using a murine model of FA provide the first demonstration of the characteristic hematopoietic phenotypes reminiscent of the human disease without genotoxin treatment, which will assist in identifying potential mechanisms involved in the pathogenesis of the hematologic manifestations of FA.
The authors gratefully acknowledge Dr Manuel Buchwald (Hospital for Sick Children, University of Toronto) for providing the Fancc +/− mice. We thank Lee Ann Baldridge for her excellent technical expertise in conducting all histologic and immunohistologic analyses. In addition, we thank Scott Cooper for conducting inhibitory cytokine progenitor assays. We are also grateful to Drs Yoder, Dinauer, Pollok, Ingram, and Kapur (Indiana University) for many valuable discussions and thoughtful critique of the manuscript. In addition, we thank Marsha Hippensteel for exceptional administrative support.
Prepublished online as Blood First Edition Paper, October 10, 2002; DOI 10.1182/blood-2002-08-2404.
Supported by US Public Health Services grants P01 HL53586, P30 DK49218, R01 HL63219, R01 HL56416, and K08 HLDK04071-01 and the American Cancer Society no. IRG-84-002-16.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Laura S. Haneline, Cancer Research Institute, 1044 W Walnut Street, Room 476, Indianapolis, IN 46202-5254; e-mail: lhanelin@iupui.edu.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal