Abstract
To assess the sensitivity of primary non-Hodgkin lymphoma cells to rituximab-mediated cytotoxicity, we compared the potency of several rituximab-mediated killing mechanisms on fresh lymphoma cells. All lymphoma cells tested were equally sensitive to antibody-dependent cell-mediated cytotoxicity (ADCC), antibody-mediated phagocytosis of tumor cells, and rituximab-induced apoptosis. However, they were differentially lysed by complement-dependent cytotoxicity (CDC). We found that taking into account both CD20 and complement regulatory protein expression on tumor cells could predict CDC sensitivity in vitro. Importantly, the sensitivity of lymphoma cells to CDC was consistent with the reported different clinical response rates of lymphomas: rituximab induced high CDC killing of follicular lymphoma cells, whereas mantle cell lymphoma and diffuse large cell lymphoma cells were moderately sensible to CDC, and small lymphocytic lymphoma cells were almost all resistant. We propose that CDC is a determinant mechanism of rituximab-induced killing in vivo. Poor sensitivity to CDC in vitro might predict a poor clinical response, whereas high sensitivity to CDC would only indicate a likelihood of response to rituximab treatment.
Introduction
Rituximab is a chimeric mouse/human antibody, bearing the human IgG1 and κ constant regions, and specific to the CD20 antigen expressed on mature B lymphocytes.1 CD20 is expressed on nearly 90% of B-cell non-Hodgkin lymphomas,2and therefore represents a quasi-universal target on lymphoma cells. However, in contrast with its broad expression, targeting CD20 by rituximab infusion has been successful in only a fraction of patients. Moreover, clinicals trials have shown differential response rates of distinct histologic types of lymphomas, low-grade/follicular lymphomas displaying the best response rate (about 50%) when rituximab was used as a single therapeutic agent.3,4 This raises the question of the mechanisms of rituximab-induced remissions and the extent to which they could explain these intergroup and intragroup differences. Several mechanisms have been proposed and tested in vitro, mainly on tumor cell lines. Through its human IgG1 Fc domain, rituximab can activate cellular effectors for antibody-dependent cell-mediated cytotoxicity (ADCC) or phagocytosis and recruit serum proteins for complement-dependent cytotoxicity (CDC).1 Moreover, cross-linking of CD20 molecules on tumor cell lines has been described as triggering their apoptotic death,5 as well as having an antiproliferative effect on some5 but not all, cell lines.6 However, the determinant mechanisms mediating tumor cell eradication in vivo are not known. It is speculated that ADCC and CDC may be important for in vivo efficacy of rituximab. In a murine model, Fcγ receptor (FcγR)–bearing effectors were mainly responsible for the rituximab-induced protection against implanted tumor cell growth, implying host cells as a major effector population.7 In another study, CDC was found to be the most potent mediator of tumor cell death in vitro, compared with cellular-mediated tumor cell killing.8 However, there has been no direct comparison of the intrinsic sensitivity of primary tumor cells to different effector mechanisms, especially when studying different histologic groups of lymphoma. Our aim was to evaluate the potency of described killing mechanisms of primary lymphoma cells by rituximab and to determine which can best explain the different response rates found in clinical studies.
Materials and methods
Cells
Lymphoma B cells were purified from lymph node biopsies of patients with B-cell lymphoma as described previously,9using a standard rosetting technique using 2-aminoethyl-isothiouronium bromide (AET)–sensitized sheep red blood cells. The purity (≥ 97%) of tumor suspensions was evaluated by flow cytometric analysis and cells were subsequently cryopreserved in liquid nitrogen. They included 7 follicular lymphomas (FLs), 7 mantle cell lymphomas (MCLs), 7 diffuse large cell lymphomas (DLCLs), 7 small lymphocytic lymphomas (SLLs), according to the Revised European-American Classification of Lymphoid Neoplasms (REAL) classification.10 Nontumor (NT) B cells were purified from hyperplastic lymph node biopsies according to the same procedure as for tumor cells.
Antibodies and flow cytometry
Mouse fluorochrome-conjugated isotype control antibodies, phycocyanine 5 (PC5)–coupled anti-CD19, phycoerythrin (PE)–coupled anti-CD20, fluorescein isothiocyanate (FITC)–coupled anti-CD59, and PE-coupled anti-CD55 were purchased from Immunotech (Marseille, France). FITC-coupled anti-CD46 was purchased from Becton Dickinson (Pont de Claix, France). Chimeric anti-CD20 antibody rituximab was purchased from Roche Pharmaceutical (Basel, Switzerland).
Three-color flow cytometry phenotyping of tumor cells was performed on a FACScan flow cytometer (Becton Dickinson, Mountain View, CA). Saturating amounts of antibodies were added to cells for 30 minutes at 4°C, before extensive washing and flow cytometry analysis. Mean fluorescence intensity (MFI) values were used as a semiquantitative measure of the expression of CD20 and of complement inhibitors. All phenotypes were performed on the same day as CDC assays.
CD20-induced apoptosis of tumor cells
Tumor cells (1 × 106/mL) were incubated in complete medium supplemented by 10% heat-inactivated human serum (obtained from voluntary donors at the Etablissement Français du Sang, La Tronche, France), in the presence or absence of 2 μg/mL rituximab. Apoptosis of cells was followed at day 0, day 1, and day 2 by staining with 2.5 μg/mL propidium iodide (PI; Immunotech) and flow cytometer analysis. Annexin V staining of apoptotic cells could not be reliably measured because rituximab induced cell clusters with ambiguous higher annexin V staining. PI staining was correlated to DiOC6(3) (Molecular Probes, Eugene, OR) or the TUNEL assay (Mebstain, Immunotech), but not to annexin V staining (see “Results and Discussion”). In preliminary experiments, graded doses of soluble rituximab (0, 0.02, 0.2, 2, 20 μg/mL) were added to the cells in test tubes, with or without cross-linking of rituximab by antihuman IgG goat antibody F(ab′)2 (Immunotech) or affinity-purified goat antihuman antibodies (Biosys, Compiègne, France) at 2 or 20 μg/mL, or rituximab was first immobilized on microplates before adding the cells. No significant effect of cross-linking was observed, and the optimal condition for apoptosis induction was chosen as 2 μg/mL soluble rituximab. Because of the high rate of spontaneous apoptosis of some tumor cells, CD20-induced apoptosis could not be determined as the percentage of remaining viable cells with anti-CD20 as compared to cells without antibody at the same time. It was therefore calculated according to the following formula: 100 × (% viable cells at day 1 without antibody − % viable cells with rituximab at day 1)/(% viable cells at day 0). Human pooled immunoglobulins (Tégéline, LFB, Les Ulis, France; containing 25% IgG1 antibodies and used as an isotype control for rituximab) had no effect at any concentration tested (not shown).
Phagocytosis
Tumor cells were opsonized for 30 minutes at 4°C by 2 μg/mL rituximab and then washed. Macrophages were generated as described11 and were added to tumor cells (1 macrophage for 5 tumor cells) in RPMI 10% heat-inactivated human serum. After 2 hours, cells were cytospun and stained for visual counting of phagocytosis. Results are presented as the percentage of phagocytosing macrophages (macrophages that have phagocytosed at least one tumor cell). For inhibition experiments, macrophages were incubated with 5 μg/mL CD16 (BD Pharmingen, Heidelberg, Germany), CD32 (Stem Cell Technologies, Meylan, France), or CD64 (Diaclone Research, Besançon, France) or their isotype control as blocking antibodies during phagocytosis.
ADCC
Monocytes and natural killer (NK) cells were purified from freshly collected blood using Rosette Sep isolation kits (Stem Cell Technologies). Polynuclear neutrophils were obtained by blood sedimentation on dextran and Ficoll-Hypaque density gradient centrifugation. Purity of effectors was determined by flow cytometry analysis and was 85% for NK cells, 70% for monocytes, 99% for neutrophils (based on forward and side scatter [FSC-SSC] profiles, CD14, CD16, and CD56 expression).
The cytotoxicity of effector cells in the presence or absence of 2 μg/mL rituximab was measured in a standard 4-hour51Cr-release assay, as previously described.12Briefly, 10451Cr-labeled tumor cells were mixed with the effector cells at different effector-target (E/T) ratios (25:1-0.01) in RPMI 10% heat-inactivated human serum. After a 4-hour incubation at 37°C in 5% CO2 in air, the radioactivity in the supernatants was counted. The percentage of specific lysis was calculated according to the following formula: % lysis = 100 × (ER − SR)/(MR − SR), where ER, SR, and MR represent experimental, spontaneous, and maximum51Cr-release, respectively. All expressed values are derived from averaged quadruplicate determinations.
Complement-mediated lysis
Rituximab was added to tumor cells (1 × 106 /mL) in complete medium supplemented by human serum, inactivated or not by incubation at 56°C for 30 minutes. After 2 hours at 37°C, cell lysis was determined by PI staining of cells and analysis by flow cytometry. Lysis was calculated according to the following formula: 100 × (% viable cells with inactivated serum − % viable cells with native serum)/(% viable cells with inactivated serum), all measures being taken after the 2-hour incubation.
Statistical analysis
All statistical analyses were performed using Statview software (Abacus Concepts, Berkeley, CA).
Results and discussion
In this paper, we examined cell autonomous factors that could account for rituximab efficacy. This was warranted by the observation that different lymphoma histologic types have different clinical outcomes, suggesting tumor-intrinsic influencing factors on rituximab efficacy. We therefore measured in vitro the intrinsic sensitivity of primary lymphoma cells to different mechanisms of antibody-mediated cytotoxicity.
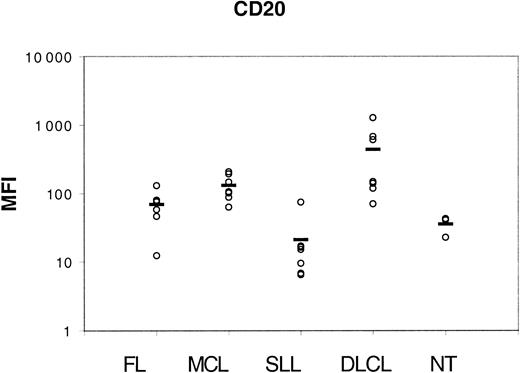
We first measured expression of CD20 molecules using a semiquantitative method on 28 tumor samples from distinct histologic types (7 FLs, 7 MCLs, 7 SLLs, and 7 DLCLs) and on 3 NT B-cell suspensions. As expected, Figure 1 shows that all tumor cells expressed CD20 antigen but with distinct intensities, depending on lymphoma groups. SLL cells expressed the lowest level of CD20, and DLCL cells the highest. CD20 expression was found at an intermediate level in FL cells and MCL cells.
CD20 expression.
Lymphoma cells were incubated with saturating amounts of PE-coupled anti-CD20 antibody for flow cytometry analysis of MFIs (displayed on the vertical axis). Horizontal bars indicate the mean value for each group.
CD20 expression.
Lymphoma cells were incubated with saturating amounts of PE-coupled anti-CD20 antibody for flow cytometry analysis of MFIs (displayed on the vertical axis). Horizontal bars indicate the mean value for each group.
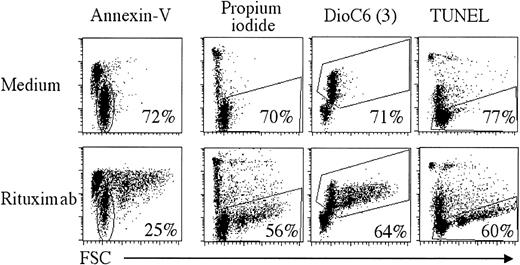
We then tested the rituximab-induced apoptosis for all tumor and NT samples. Several reports on lymphoma cell lines have shown a direct effect of rituximab in apoptosis induction, documented at the molecular level,5,13,14 and which is currently viewed as a major mechanism of rituximab efficacy.15 However, rituximab-induced apoptosis has not been extensively tested on primary lymphoma cells, and its importance remains to be evaluated. To determine the magnitude of CD20-induced apoptosis on fresh malignant cells, rituximab was added to lymphoma cells, and cell viability was followed by flow cytometry after 1 or 2 days. We used different techniques to analyze rituximab-induced cell death: annexin V staining to detect exposed phosphatidylserine, TUNEL method to reveal the 3′-OH DNA ends, DiOC6(3) labeling to monitor mitochondrial transmembrane potential disruption, and finally PI to allow discrimination of viable from nonviable cells. As illustrated in Figure2 for patient FL3, we found that rituximab induced annexin V staining, often accompanied by clustering of cells (cells with increased FSC), which could not be disrupted. However, in concordance with published results,16the significance of annexin V staining was not clear. Indeed, there was a good correlation between PI staining, TUNEL, and DiOC6(3) at day 2, but no correlation with annexin V staining. This indicates that annexin V cannot reliably be taken as a marker for rituximab-induced apoptosis, and this is why we used PI exclusion as a viability marker. Spontaneous apoptosis in absence of rituximab was highly variable during the 2-day observation period for the 28 tumor samples (between 0% and 77% at day 1; Table1), impairing accurate evaluation of apoptosis at later time points. Rituximab did induce apoptosis of lymphoma cells, but, in the majority of cases, this effect was modest; a mean of 10% CD20-induced apoptosis was found after 24 hours (Table1) and about 15% at 48 hours when evaluable (data not shown). However, this apoptosis was specific to CD20 because irrelevant immunoglobulins had no effect (data not shown). Interestingly, NT B cells were not sensitive to rituximab-induced apoptosis. In our hands, cross-linking of rituximab, either by plate coating or by addition of secondary antibodies, did not significantly alter apoptosis induction (data not shown). Therefore, primary lymphoma cells are weakly sensitive to rituximab-induced apoptosis. Moreover, no significant differences were observed between FL, MCL, SLL, and DLCL (Table 1), which questions its importance in vivo when considering the differential clinical responses of lymphomas.
Detection of rituximab-induced apoptosis.
B cells from patient FL3 were incubated in medium alone (upper panels) or with 2 μg/mL rituximab (lower panels) for 2 days. Cell death was analyzed using annexin V, PI, DiOC6(3), or the TUNEL assay. Percentages of gated cells are indicated.
Detection of rituximab-induced apoptosis.
B cells from patient FL3 were incubated in medium alone (upper panels) or with 2 μg/mL rituximab (lower panels) for 2 days. Cell death was analyzed using annexin V, PI, DiOC6(3), or the TUNEL assay. Percentages of gated cells are indicated.
Rituximab-induced death of lymphoma cells
| Case . | Viability . | % spontaneous death . | % CD20-induced death . |
|---|---|---|---|
| FL1 | 80 | ≤1 | 1 |
| FL2 | 83 | 28 | ≤1 |
| FL3 | 68 | ≤1 | 31 |
| FL4 | 82 | 13 | 5 |
| FL5 | 97 | 5 | 12 |
| FL6 | 92 | 49 | ≤1 |
| FL7 | 88 | 11 | 3 |
| MCL1 | 91 | 22 | ≤1 |
| MCL2 | 91 | 32 | 3 |
| MCL3 | 90 | ≤1 | 6 |
| MCL4 | 97 | 13 | 8 |
| MCL5 | 41 | 7 | 15 |
| MCL6 | 91 | 22 | 4 |
| MCL7 | 83 | 14 | 14 |
| SLL1 | 98 | 38 | 4 |
| SLL2 | 92 | 35 | ≤1 |
| SLL3 | 91 | 8 | 3 |
| SLL4 | 87 | 18 | ≤1 |
| SLL5 | 89 | 53 | 9 |
| SLL6 | 52 | 77 | 8 |
| SLL7 | 95 | 31 | 5 |
| DLCL1 | 76 | ≤1 | 17 |
| DLCL2 | 62 | 24 | ≤1 |
| DLCL3 | 85 | 19 | 2 |
| DLCL4 | 95 | 3 | 2 |
| DLCL5 | 49 | 63 | ≤1 |
| DLCL6 | 68 | 16 | 10 |
| DLCL7 | 61 | 46 | 11 |
| NT1 | 92 | 1 | ≤1 |
| NT2 | 95 | 42 | ≤1 |
| NT3 | 91 | 22 | ≤1 |
| Case . | Viability . | % spontaneous death . | % CD20-induced death . |
|---|---|---|---|
| FL1 | 80 | ≤1 | 1 |
| FL2 | 83 | 28 | ≤1 |
| FL3 | 68 | ≤1 | 31 |
| FL4 | 82 | 13 | 5 |
| FL5 | 97 | 5 | 12 |
| FL6 | 92 | 49 | ≤1 |
| FL7 | 88 | 11 | 3 |
| MCL1 | 91 | 22 | ≤1 |
| MCL2 | 91 | 32 | 3 |
| MCL3 | 90 | ≤1 | 6 |
| MCL4 | 97 | 13 | 8 |
| MCL5 | 41 | 7 | 15 |
| MCL6 | 91 | 22 | 4 |
| MCL7 | 83 | 14 | 14 |
| SLL1 | 98 | 38 | 4 |
| SLL2 | 92 | 35 | ≤1 |
| SLL3 | 91 | 8 | 3 |
| SLL4 | 87 | 18 | ≤1 |
| SLL5 | 89 | 53 | 9 |
| SLL6 | 52 | 77 | 8 |
| SLL7 | 95 | 31 | 5 |
| DLCL1 | 76 | ≤1 | 17 |
| DLCL2 | 62 | 24 | ≤1 |
| DLCL3 | 85 | 19 | 2 |
| DLCL4 | 95 | 3 | 2 |
| DLCL5 | 49 | 63 | ≤1 |
| DLCL6 | 68 | 16 | 10 |
| DLCL7 | 61 | 46 | 11 |
| NT1 | 92 | 1 | ≤1 |
| NT2 | 95 | 42 | ≤1 |
| NT3 | 91 | 22 | ≤1 |
Lymphoma cells or NT B cells were incubated with 2 μg/mL rituximab and cell death was measured by PI incorporation after 24 hours. Viability after cryopreservation/thawing is indicated as well as spontaneous death at day 1.
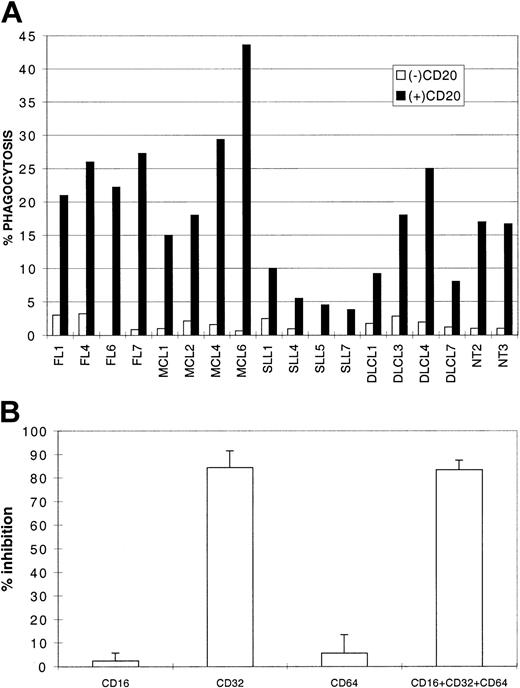
Because tumor cells did not appear to be intrinsically very sensitive to a direct effect of rituximab, we examined the involvement of cellular effectors that may be recruited to the tumor site. Tumor cell clearance by antibody-dependent phagocytosis of whole lymphoma cells was measured with macrophages as tissue cells that could infiltrate tumor lesions.17 We generated macrophages from blood monocytes11 and tested their capacity to phagocytose rituximab-opsonized lymphoma cells in the presence of human serum. As shown in Figure 3A, macrophages could engulf rituximab-opsonized tumor cells (4 FLs, 4 MCLs, 4 DLCLs, 4 SLLs, and 2 NTs), whereas tumor cells were only marginally internalized if not opsonized. In this assay, SLL cells were less readily phagocytosed, possibly due to their lower expression of CD20 (Figure 1). Inhibition experiments with blocking antibodies indicated that the low-affinity IgG receptor CD32/FcγRII was responsible for phagocytosis of tumor cells (about 80% of inhibition; Figure 3B) and that CD16 and CD64 did not participate in engulfment of B cells in these conditions. In conclusion, rituximab can induce lymphoma cell phagocytosis by tissue-scavenging cells.
Rituximab-mediated phagocytosis.
(A) Lymphoma cells (4 MCLs, 4 DLCLs, 4 FLs, 4 SLLs) and 2 NTs were opsonized (black bars) or not (empty bars) by 2 μg/mL rituximab, washed, and incubated with macrophages in RPMI 10% human serum for 2 hours. Phagocytosis is represented as the percentage of phagocytosing macrophages. (B) Inhibition represents pooled data (mean inhibition ± SD) from experiments with FL7, MCL6, NT3 and the same source of macrophages as in panel A, with anti-CD16, anti-CD32, anti-CD64 alone or in combination (CD16 + CD32 + CD64).
Rituximab-mediated phagocytosis.
(A) Lymphoma cells (4 MCLs, 4 DLCLs, 4 FLs, 4 SLLs) and 2 NTs were opsonized (black bars) or not (empty bars) by 2 μg/mL rituximab, washed, and incubated with macrophages in RPMI 10% human serum for 2 hours. Phagocytosis is represented as the percentage of phagocytosing macrophages. (B) Inhibition represents pooled data (mean inhibition ± SD) from experiments with FL7, MCL6, NT3 and the same source of macrophages as in panel A, with anti-CD16, anti-CD32, anti-CD64 alone or in combination (CD16 + CD32 + CD64).
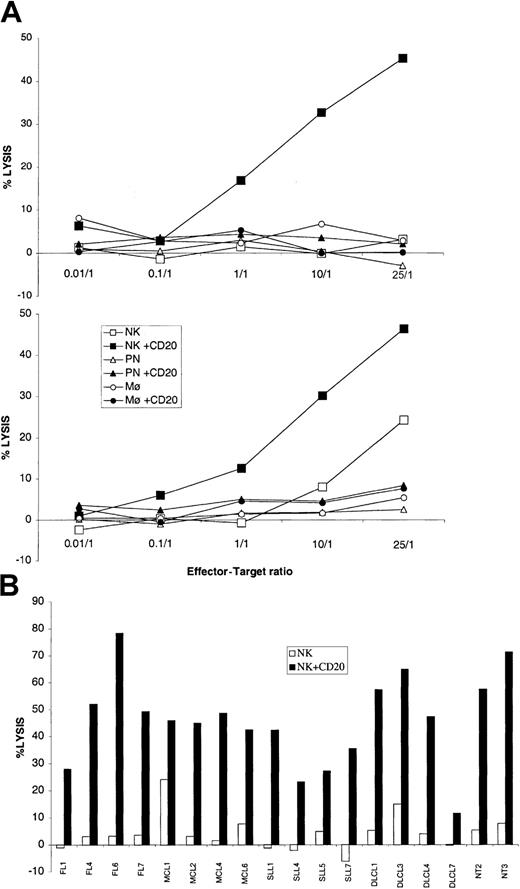
We then tested ADCC by different FcγR-expressing effectors. A recent study implicates FcγRIII allelic polymorphism as a determining factor for rituximab efficacy in FL, suggesting that ADCC is an important effector mechanism in vivo.18 Rituximab was originally described as a potent inducer of ADCC in the presence of peripheral blood mononuclear cells.1 Monocytes, NK cells, and polymorphonuclear leukocytes (PNs) have all been shown to kill opsonized tumor cells.19 20 They were purified from fresh blood and directly used in a 4-hour chromium-release assay against the same lymphoma cells as the ones used for phagocytosis, in the presence or absence of rituximab. As shown in Figure4A, only NK cells could lyse lymphoma cells from the 2 patients tested (MCL1 and MCL6) in the presence of rituximab. We then tested 14 additional samples from patients with distinct histologic types (4 FLs, 2 MCLs, 4 DLCLs, and 4 SLLs) and from 2 healthy donors for ADCC by NK cells, at a ratio of 25:1 (Figure 4B). In only 2 cases did NK cells lyse tumor cells without antibody. All tumor cells were lysed by NK cells in the presence of rituximab (between 11% and 78% lysis), with no evidence that one histologic group was less sensitive than the others.
ADCC.
(A) Lymphoma cells (MCL6 in upper panel, MCL1 in lower panel) were incubated at different ratios with NK cells (squares), polynuclear leukocytes (triangles), or monocytes (circles) in the presence (closed symbols) or absence (open symbols) of 2 μg/mL rituximab. Cytotoxicity was determined by chromium release and is represented as the percentage of specific lysis of tumor cells. (B) NK cell–mediated lysis of lymphoma cells (4 MCLs, 4 DLCLs, 4FLs, 4 SLLs) and 2 NTs, in presence (NK + CD20) or absence (NK) of rituximab at the 25:1 (E/T) ratio.
ADCC.
(A) Lymphoma cells (MCL6 in upper panel, MCL1 in lower panel) were incubated at different ratios with NK cells (squares), polynuclear leukocytes (triangles), or monocytes (circles) in the presence (closed symbols) or absence (open symbols) of 2 μg/mL rituximab. Cytotoxicity was determined by chromium release and is represented as the percentage of specific lysis of tumor cells. (B) NK cell–mediated lysis of lymphoma cells (4 MCLs, 4 DLCLs, 4FLs, 4 SLLs) and 2 NTs, in presence (NK + CD20) or absence (NK) of rituximab at the 25:1 (E/T) ratio.
Therefore, primary lymphoma cells can be cleared by cellular effectors in the presence of rituximab, either by phagocytosis or by ADCC, which are likely to be important in vivo.19 However, the magnitude of effector cell recruitment after rituximab infusion is unknown, and, as for rituximab-induced apoptosis, lymphoma cells were equally susceptible to rituximab-mediated phagocytosis or ADCC, raising doubts about the in vivo relevance of these mechanisms to explain the different response rates of lymphomas.
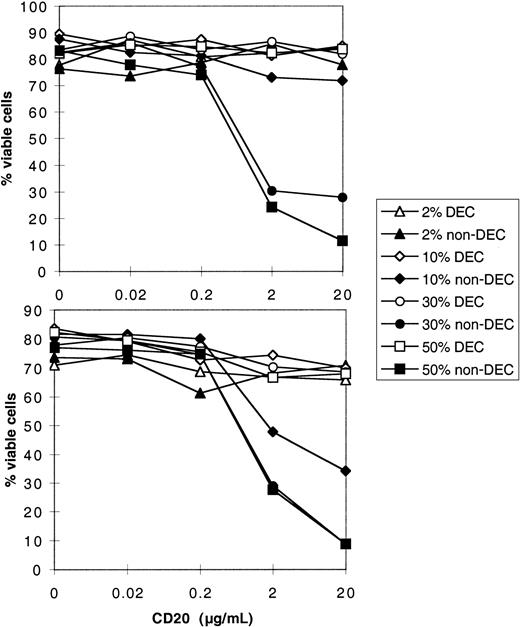
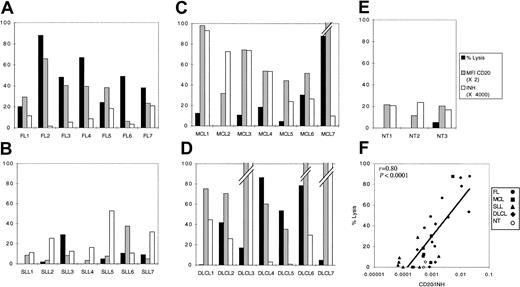
Besides cellular cytotoxicity, a potent mechanism of antibody-dependent tumor cell killing is complement-mediated lysis.8 Human cells are normally protected against spontaneous cytolytic activity of complement by a battery of regulatory proteins, present in a soluble form in the serum, or expressed on the cell surface.21However, in the presence of rituximab linked to the tumor cell, activation of the classical pathway may overrun these regulatory mechanisms and lead to cell lysis. Therefore, expression of these inhibitory proteins by the tumor cells may be predictive of the outcome of complement activation by rituximab, either death or survival.6,21 As preliminary studies, we performed kinetic and dose-response experiments, with cells from 2 patients, FL3 and FL4 (Figure 5). In the presence of rituximab, CDC (abrogated after heat-inactivation of serum) was very efficient in killing tumor cells, because up to 90% of cells were found to incorporate PI after only 2 hours at 37°C, in optimal conditions. These 2 patients displayed an identical expression of CD20; however, they did not have the same sensitivity to CDC. Indeed, rituximab induced dose-dependent killing of both cells, but cells from patient FL3 were killed in the presence of 10% serum, whereas cells from patient FL4 were not (Figure 5). Therefore, there seem to be other factors regulating complement-dependent cell death, the effect of which depends on rituximab and serum concentration. To test the implication of complement regulatory proteins (CRPs) on tumor cells, we used a semiquantitative method to determine CRP expression on cells from a number of patients (Figure 6), and CDC was measured in the presence of a fixed concentration of serum and rituximab. To appreciate regulatory mechanisms, we chose nonsaturating concentrations of rituximab (2 μg/mL) with sufficient serum concentration (30%) and we analyzed CDC sensitivity in a 2-hour assay of a panel of lymphoma cells (Figure 7). Complement-dependent lysis ranged from 0% to 90% for the cells tested. Cells that were resistant at 2 hours were still resistant at further incubation times (data not shown). Cells were classified according to their histologic type and it appeared that all FLs showed some sensitivity to CDC (> 20% lysis, ranging from 20% to 90%; Figure 7A). In contrast with a previous study,22 we found that SLLs were almost all resistant, with 25% lysis in only one case (Figure 7B), and MCLs were resistant except for 3 cases (lysis ≥ 20%; Figure 7C). DLCLs were lysed, but with some cells being resistant (3 of 7 patients; Figure 7D). Compared with tumor cells, NT B cells were not lysed at all (Figure 7E). We conclude that primary lymphoma cells exhibit different responses to CDC in this assay and that histologic groups constitute distinct entities with regard to their CDC sensitivity.
Dose-response curves for CDC.
Lymphoma cells (FL4 in upper panel, FL3 in lower panel) were incubated for 2 hours with graded doses of rituximab (horizontal axis) in the presence of increasing amounts of human serum (2%, 10%, 30%, and 50%), either heat-decomplemented (DEC) or native (non-DEC). Cell viability was determined by PI exclusion and is represented on the vertical axis.
Dose-response curves for CDC.
Lymphoma cells (FL4 in upper panel, FL3 in lower panel) were incubated for 2 hours with graded doses of rituximab (horizontal axis) in the presence of increasing amounts of human serum (2%, 10%, 30%, and 50%), either heat-decomplemented (DEC) or native (non-DEC). Cell viability was determined by PI exclusion and is represented on the vertical axis.
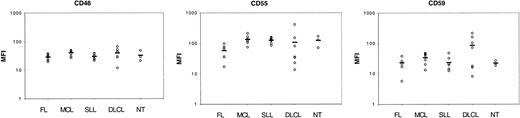
Expression of CRPs on lymphoma cells.
Lymphoma cells (FL, MCL, SLL, DLCL) or nontumor B cells (NT) were incubated with saturating amounts of fluorescent antibodies against CD46, CD55, or CD59 and analyzed by flow cytometry. MFIs are plotted for each cell (circles). Horizontal bars indicate the mean value for each group.
Expression of CRPs on lymphoma cells.
Lymphoma cells (FL, MCL, SLL, DLCL) or nontumor B cells (NT) were incubated with saturating amounts of fluorescent antibodies against CD46, CD55, or CD59 and analyzed by flow cytometry. MFIs are plotted for each cell (circles). Horizontal bars indicate the mean value for each group.
CDC.
Lymphoma cells (A-D) or NT B cells (E) were incubated for 2 hours with 2 μg/mL rituximab in the presence of 30% human serum, and cell lysis was determined by PI incorporation (black bars). Gray bars represent relative CD20 expression. INH values (defined as the product of MFI values for CD46, CD55, CD59) are represented by white bars. (F) Correlation between CDC and CD20/INH. MFI values of CD20 were divided by INH values for each cell and plotted against CDC.
CDC.
Lymphoma cells (A-D) or NT B cells (E) were incubated for 2 hours with 2 μg/mL rituximab in the presence of 30% human serum, and cell lysis was determined by PI incorporation (black bars). Gray bars represent relative CD20 expression. INH values (defined as the product of MFI values for CD46, CD55, CD59) are represented by white bars. (F) Correlation between CDC and CD20/INH. MFI values of CD20 were divided by INH values for each cell and plotted against CDC.
We then attempted to correlate CDC sensitivity to the expression of CD20 and of the CRPs CD46, CD55, and CD59. First, SLLs expressed very low levels of CD20 (Figure 1), which could explain on its own their resistance to CDC (Figure 7B). However, when comparing FLs and MCLs, both expressed high amounts of CD20, but FLs were sensitive to CDC, whereas MCLs were resistant (Figures 1 and 7A,C). Globally, there was no direct correlation between lysis and expression of CD20 nor between lysis and expression of any CRP (data not shown). However, because CRPs may act synergistically to control complement activation (due to amplification loops in the complement activation cascade), we plotted the product of MFI values of CRP (INH; defined as the product of MFI values for CD46, CD55, CD59) against CD20 expression and CDC (Figure 7). This allowed distinguishing easily between FLs and MCLs; both substantially express CD20, but MCLs display high INH values, whereas FL had low INH values. There was no correlation between lysis and INH values (data not shown). On the other hand, cells expressing low levels of CD20 were poorly lysed (NT and SLL). This suggested that one might take the ratio of CD20/INH as an indication of sensitivity to CDC, and there was a significant correlation between the order of magnitude of CD20/INH and CDC (r = 0,8;P < .0001, t test; Figure 7F). Multivariate analysis using CD20, CD46, CD55, and CD59 expression as regressors gave a very similar regression (P < .0001 and r = 0.816).
It has been suggested previously that CDC was directly correlated to CD20 expression, hence irrespective of CRP expression.22,23 However, this conclusion seems paradoxical with the claim that CDC is regulated by CRP, which is evidenced by the use of blocking antibodies,6,22 and is supported by this current study. We suggest that reported direct correlation between CD20 expression and CDC sensitivity rely on the saturating conditions used that do not allow regulation mechanisms to be evidenced. Based solely on CD20 expression, it is difficult to discriminate FL, MCL and DLCL cells. With the restriction that any correlation found in vitro depends on the experimental conditions used, we define a combination of 4 tumor-specific parameters as determining CDC lysis of lymphoma cells. However, augmenting rituximab dosing might induce better lysis of tumor cells, due to saturation of CD20 sites and overrunning of regulatory mechanisms (Figure 5), which could be translated as a therapeutic strategy.23-26
Our approach aimed at finding tumor cell–specific factors influencing rituximab killing in vitro. No striking differences were observed between primary lymphoma cells with regard to their sensitivity to rituximab-induced apoptosis, ADCC, and phagocytosis. However, CDC induced very different killing of tumor cells, with a pattern of sensitivity consistent with clinical data. In clinical studies, patients with FL display the best response rate to rituximab,3,4 whereas patients with MCL and DLCL have a moderate response,27 and SLL is associated with a poor or no clinical response to rituximab.28 Given that the other potential mechanisms of rituximab studied here (apoptosis, ADCC, phagocytosis) could not easily discriminate lymphoma cells, it is tempting to correlate in vitro CDC sensitivity to the probability of response according to the lymphoma histologic type; that is, poor sensitivity to in vitro CDC might predict a poor clinical response to rituximab. In the case of cells killed by CDC in vitro, this does not preclude the involvement of other factors finally determining the clinical efficacy of rituximab, and, indeed, a recent study found no correlation between sensitivity to CDC in vitro and clinical outcome within the FL histologic group.29 It is likely that supracellular factors, like tumor burden, or tumor vascularization are important parameters in determining rituximab efficacy. Moreover, complement activation is coupled to release of inflammatory chemotactic factors,30-33 recruiting cellular effectors for amplification and complexing of the antitumor response. In this perspective, CDC activation could be a necessary initial step (not necessarily massive) for recruitment of cells that may be critically involved, which is also consistent with its short kinetics. Intriguing clinical data are the occasional lag between rituximab infusion and therapeutic effect,34 which cannot easily be reconciled with immediate killing in short-term in vitro assays, but may indicate the involvement of late effectors. In line with this reasoning, rituximab-induced cross-presentation of tumor-derived antigens by dendritic cells was recently reported.35
Prepublished online as Blood First Edition Paper, October 3, 2002; DOI 10.1182/blood-2002-02-0469.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Joël Plumas, Etablissement Francais du Sang-Grenoble, 29, Ave du Maquis du Gresivaudan, La Tronche Cedex 38701, France; e-mail: joel.plumas@wanadoo.fr andjoel.plumas@efs.sante.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal