Abstract
This study examined the prognostic value of circulating peripheral blood plasma cells (PBPCs) in patients with primary systemic amyloidosis (AL). A sensitive slide-based immunofluorescence technique was used to assess 147 patients for circulating PBPCs. Circulating monoclonal plasma cells were quantified as a percentage of circulating cytoplasmic immunoglobulin-positive cells (PBPC%). The absolute circulating plasma cell count was also determined. When analyzed retrospectively, 24 (16%) of 147 patients were found to have detectable circulating PBPCs. Overall survival for patients with high PBPC%'s (> 1%) was poorer (median survival, 10 vs 29 months;P = .002). Similarly, overall survival for patients with high PBPC counts (> 0.5 × 106/L) was significantly poorer (median, 13 vs 31 months;P = .003). Increased percentages of bone marrow plasma cells (BMPC%; P = .0004), increased levels of serum β2-microglobulin (P = .04), and dominant cardiac amyloid involvement (P = .03) also predicted poorer survival. The combined consideration of circulating PBPCs and BMPC% identified low-, intermediate-, and high-risk groups with median survivals of 37.5, 15.5, and 10 months, respectively (P = .0003). Multivariate analysis revealed circulating PBPCs and BMPC% to be independent prognostic factors for survival. Patients with PBPC%'s of 2% or higher were significantly more likely to have a coexisting clinical diagnosis of multiple myeloma (50% vs 12%, P = .008). The prognostic value of circulating PBPCs may help select treatment for patients with AL.
Introduction
Primary amyloidosis (AL) is a monoclonal plasma cell disorder characterized by the excess production and deposition of monoclonal immunoglobulin light chain fragments in various organs and tissues of affected individuals. The pattern of organ involvement varies among individuals and determines the clinical presentation of the patient. The 4 most common presentations of AL patients are nephrotic-range proteinuria with or without renal insufficiency, congestive heart failure resulting from restrictive cardiomyopathy, unexplained hepatomegaly, and idiopathic peripheral neuropathy.1 Although the clinical course depends on the dominant organ system involved by amyloidosis, the cause of death in most patients is cardiac related and usually reflects the extent of cardiac involvement by amyloidosis.1 Patients with AL can respond to chemotherapy; however, the overall prognosis for these patients is poor, with median survival for nonresponding patients reported to be a year.2 Response to conventional alkylating agent–based chemotherapy is slow (median time to response is a year) and seen in a minority of patients (18%), but responders have improved survival (90 months vs 12 months).2
Approximately 10% of AL occurs with concurrent multiple myeloma (MM), that is, with anemia caused by plasma cell replacement of bone marrow hematopoietic precursors or lytic bone lesions. Similarly, a fraction of patients with clinical MM have associated AL. A minority of AL cases have 30% or more plasma cells in the bone marrow in the absence of clinical features of MM. Evolution of MM from AL is rare, occurring in 6 of 1600 patients over 20 years.3
The presence of high levels of circulating plasma cells has independent prognostic value in MM.4 We have previously shown that circulating monoclonal plasma cells are detected in a subset of patients with AL.5 The purpose of this retrospective study was to determine the prognostic significance of increased levels of circulating monoclonal plasma cells in AL.
Patients, materials, and methods
Patients were considered to have AL if they had biopsy-proven amyloid tissue deposits associated with a monoclonal plasma cell population in the bone marrow or evidence of a monoclonal (M) protein in serum or urine. Clinical MM was defined as the presence of an M protein in serum or urine associated with lytic bone disease or 30% or more monoclonal plasma cells in the bone marrow.
Patients and data collection
From 1991 to 1996, 694 patients with AL were evaluated at Mayo Clinic. Testing for the presence of circulating plasma cells, using a specialized slide-based immunofluorescent technique, was introduced in 1991 on a limited basis and then expanded in 1994. The study population consisted of 147 patients with biopsy-proven AL who underwent testing for circulating plasma cells by this assay between 1991 and 1996. The majority (90%) of patients underwent testing from 1994 to 1996, during which time 368 patients with amyloidosis were evaluated. During this period, the test was done primarily to assess its validity in AL patients. All patients with AL who underwent such testing during the specified period are included in this study. These patients underwent a general medical and laboratory evaluation that included a complete blood cell count, serum chemistry panel, serum and urine protein electrophoresis with immunofixation, serum β2-microglobulin evaluation, serum C-reactive protein measurement, skeletal survey for metastatic lesions, bone marrow aspiration and biopsy, and a bromodeoxyuridine bone marrow plasma cell labeling index (PCLI). To exclude patients with familial, senile, or secondary amyloidosis, we included only patients who had a documented M protein in serum or urine, a monoclonal plasma cell population in the bone marrow specimen, or both. The determination of dominant organ involvement at presentation was made on clinical grounds on the basis of the primary source of the patient's symptoms. Patients defined as having dominant cardiac amyloidosis had symptoms of cardiac amyloid involvement and had increased ventricular septal wall thickness. Almost all patients had involvement of 2 or more organs and received conventional-dose chemotherapy, with the exception of 9 patients (6%) who underwent high-dose therapy with stem cell rescue (SCT). Clinical and laboratory data were reviewed with permission from the clinic's institutional review board, and data from all patients were used in analysis of clinical outcome.
Detection and quantification of circulating plasma cells
The detection and quantification of peripheral blood plasma cells (PBPCs) and the PCLI were done with a sensitive, slide-based immunofluorescence technique, as previously described.6 By this assay, which is far more sensitive at detecting circulating PBPCs than a standard white blood cell count and differential count, a high PBPC count was defined as more than 0.5 × 106 plasma cells per liter. A high percentage of circulating cytoplasmic immunoglobulin-positive cells (PBPC%) was defined as more than 1% cytoplasmic immunoglobulin-positive cells, which are considered plasma cells morphologically.
Statistical methods
Patients were followed up until death or, for those still alive, until most recent follow-up. The major goal of this study was to retrospectively review the correlation of circulating PBPCs with patient overall (all-cause) survival. Patient characteristics, including age, serum albumin level, serum creatinine level, serum β2-microglobulin level, serum C-reactive protein level, serum M protein level, PBPC count, PBPC%, PCLI, bone marrow plasma cell percentage (BMPC%), and dominant cardiac amyloid involvement, were examined for their ability to predict overall survival. Cutoffs or threshold values for the various parameters were defined on the basis of known normal ranges or previously published results. Survival curves were generated by the Kaplan-Meier method, and differences between pairs of curves were analyzed by the log-rank test. The interaction between each potential patient characteristic or prognostic factor and its effect on survival was analyzed by the Cox proportional hazards model. All analyses were performed with the StatView software package from SAS (Cary, NC).
Results
The 147 patients ranged in age from 37 to 86 years. Patient characteristics are listed in Table 1. Follow-up data were available for all patients.
Characteristics of patients with AL (n = 147)
| Characteristic . | No. . |
|---|---|
| Age, median (range), y | 61 (37-86) |
| Sex, no. (%) | |
| Male | 88 (60) |
| Female | 59 (40) |
| Serum M protein level, median (range), g/dL* | |
| All patients | 0.4 (0.0-4.1) |
| Patients with MM† | 0.6 (0.0-4.1) |
| Patients without MM | 0.4 (0.0-3.0) |
| Primary organ involved (% of patients)‡ | |
| Kidney | 55 (38.0) |
| Heart | 54 (38.0) |
| Nerve | 12 (8.0) |
| Liver | 9 (6.0) |
| Gastrointestinal | 4 (3.0) |
| Other | 10 (7.0) |
| Albumin level, median (range), g/dL‡ | 2.8 (1.0-4.1) |
| C-reactive protein level, median (range), mg/mL1-153 | 0.3 (0.1-7.3) |
| β2-microglobulin level, median (range), μg/mL1-155 | 3.0 (1.2-24.0) |
| Creatinine level, median (range), mg/dL1-154 | 1.1 (0.2-7.8) |
| PBPC%, median (range) | 0.0 (0.0-87) |
| PBPCs, median (range), 106/L | 0.0 (0.0-262) |
| BM PCLI, median (range), %# | 0.0 (0.0-20) |
| BMPC%, median (range) | 7.0 (0.0-85) |
| Characteristic . | No. . |
|---|---|
| Age, median (range), y | 61 (37-86) |
| Sex, no. (%) | |
| Male | 88 (60) |
| Female | 59 (40) |
| Serum M protein level, median (range), g/dL* | |
| All patients | 0.4 (0.0-4.1) |
| Patients with MM† | 0.6 (0.0-4.1) |
| Patients without MM | 0.4 (0.0-3.0) |
| Primary organ involved (% of patients)‡ | |
| Kidney | 55 (38.0) |
| Heart | 54 (38.0) |
| Nerve | 12 (8.0) |
| Liver | 9 (6.0) |
| Gastrointestinal | 4 (3.0) |
| Other | 10 (7.0) |
| Albumin level, median (range), g/dL‡ | 2.8 (1.0-4.1) |
| C-reactive protein level, median (range), mg/mL1-153 | 0.3 (0.1-7.3) |
| β2-microglobulin level, median (range), μg/mL1-155 | 3.0 (1.2-24.0) |
| Creatinine level, median (range), mg/dL1-154 | 1.1 (0.2-7.8) |
| PBPC%, median (range) | 0.0 (0.0-87) |
| PBPCs, median (range), 106/L | 0.0 (0.0-262) |
| BM PCLI, median (range), %# | 0.0 (0.0-20) |
| BMPC%, median (range) | 7.0 (0.0-85) |
Data available for 141 patients (96%).
Twenty patients (14%) had concurrent MM.
Data available for 144 patients (98%).
Data available for 106 patients (72%).
Data available for 118 patients (80%).
Data available for 83 patients (56%).
#Data available for 113 patients (77%).
In our series, 20 of 147 patients had concurrent MM. Fourteen patients had 30% or more bone marrow plasma cells (BMPC%). Six patients were classified as having MM on the basis of lytic bone disease. AL patients with concurrent MM have a worse prognosis than other AL patients (median survival of 14 months vs 32 months;P = .02), and a higher proportion had detectable circulating PBPCs (30% vs 13%). Of the subset of patients with PBPC%'s of 2% or more, 50% had clinical MM, compared with 12% of patients with PBPC%'s lower than 2% (P = .008).
Survival
Of the 147 patients, 109 died; the median overall (all-cause) survival of the cohort was 25 months. By univariate analysis (Table2), overall survival was significantly poorer for patients with high PBPC counts (> 0.5 × 106/L), with median survival of 13 months vs 31 months (P = .003; Figure1A). Similarly, patients with a high circulating PBPC% (> 1%) had poorer overall survival (median survival of 10 months vs 29 months; P = .002; Figure 1B). As a dichotomized variable (cutoff of 2.7 μg/mL), β2-microglobulin level was significant for survival (P = .04; Figure 1C), as was dominant cardiac amyloid involvement (P = .03; Figure 1D). Patients with increased bone marrow monoclonal plasma cell burden (BMPC% > 10%) also had poorer survival, with median survival of 14 vs 33 months (P = .04; Figure 1E). Age, sex, serum C-reactive protein level, serum albumin level, serum creatinine level, serum M protein level, and bone marrow PCLI were not significant for survival. By multivariate analysis, high circulating PBPC count and BMPC% were significant determinants of survival (Table3).
Prognostic factors for overall survival of AL patients: univariate analysis
| Prognostic factor . | P* . |
|---|---|
| PBPC% higher than 1% | .002 |
| PBPC count higher than 0.5 × 106/L | .003 |
| BMPC% higher than 10% | .04 |
| Serum β2-microglobulin level of 2.7 μg/mL or higher | .04 |
| No dominant cardiac amyloid involvement | .03 |
| Prognostic factor . | P* . |
|---|---|
| PBPC% higher than 1% | .002 |
| PBPC count higher than 0.5 × 106/L | .003 |
| BMPC% higher than 10% | .04 |
| Serum β2-microglobulin level of 2.7 μg/mL or higher | .04 |
| No dominant cardiac amyloid involvement | .03 |
The difference between pairs of survival curves for categorical variables was assessed with a log-rank test. For continuous variables, a Cox proportional hazards P is reported.
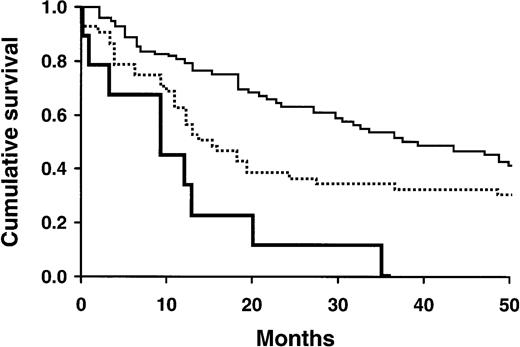
Survival of AL patients in relation to various prognostic factors.
(A) Survival of patients based on PBPC count higher than 0.5 × 106/L (lower curve) or less than or equal to 0.5 × 106/L (upper curve; P = .003). (B) Survival of patients based on PBPC% higher than 1% (lower curve) or less than or equal to 1% (upper curve;P = .002). (C) Survival of patients based on serum β2-microglobulin levels of 2.7 μg/mL or higher (lower curve) or less than 2.7 μg/mL (upper curve;P = .04). (D) Survival of patients based on presence (lower curve) or absence (upper curve) of dominant cardiac amyloid involvement (P = .03). (E) Survival of patients based on BMPC% higher than 10% (lower curve) or less than or equal to 10% (upper curve; P = .04).
Survival of AL patients in relation to various prognostic factors.
(A) Survival of patients based on PBPC count higher than 0.5 × 106/L (lower curve) or less than or equal to 0.5 × 106/L (upper curve; P = .003). (B) Survival of patients based on PBPC% higher than 1% (lower curve) or less than or equal to 1% (upper curve;P = .002). (C) Survival of patients based on serum β2-microglobulin levels of 2.7 μg/mL or higher (lower curve) or less than 2.7 μg/mL (upper curve;P = .04). (D) Survival of patients based on presence (lower curve) or absence (upper curve) of dominant cardiac amyloid involvement (P = .03). (E) Survival of patients based on BMPC% higher than 10% (lower curve) or less than or equal to 10% (upper curve; P = .04).
Prognostic factors for overall survival of AL patients: multivariate analysis
| Prognostic factor . | Hazard ratio (95% confidence interval) . | P . |
|---|---|---|
| PBPC count higher than 0.5 × 106/L | 1.86 (1.04-3.3) | .04 |
| BMPC% higher than 10% | 1.02 (1.0-1.03) | .004 |
| Prognostic factor . | Hazard ratio (95% confidence interval) . | P . |
|---|---|---|
| PBPC count higher than 0.5 × 106/L | 1.86 (1.04-3.3) | .04 |
| BMPC% higher than 10% | 1.02 (1.0-1.03) | .004 |
Nine of the 147 patients underwent SCT. When these patients were excluded from the analysis, the study findings were unchanged. In the remaining 138 patients, overall survival was poorer in those with high circulating PBPC%'s (> 1%), with median survival of 10 months vs 31 months (P = .02).
Additionally, when patients with concurrent MM were excluded from the analysis, the effect of high PBPC count on survival remained unchanged. Of 127 AL patients without MM, those with high circulating PBPC%'s (> 1%) had significantly poorer survival (10 months vs 32.5 months;P = .04).
Survival analysis using various combinations of prognostic factors
A combined consideration of PBPC% and BMPC% separated patients into high-, intermediate-, and low-risk groups, with median survival times of 10, 15.5, and 37.5 months, respectively (P = .0003; Table4; Figure2).
Survival of AL patients estimated by the combined consideration of prognostic factors
| Prognostic factors4-150 . | Both favorable . | Mixed favorable . | Both unfavorable . | P . | |||
|---|---|---|---|---|---|---|---|
| No. . | MS, mo . | No. . | MS, mo . | No. . | MS, mo . | ||
| PBPC% and BMPC% | 88 | 37.5 | 50 | 15.5 | 9 | 10.0 | .0003 |
| Serum β2-microglobulin level and BMPC% | 31 | 54.0 | 55 | 19.0 | 32 | 13.5 | .008 |
| Prognostic factors4-150 . | Both favorable . | Mixed favorable . | Both unfavorable . | P . | |||
|---|---|---|---|---|---|---|---|
| No. . | MS, mo . | No. . | MS, mo . | No. . | MS, mo . | ||
| PBPC% and BMPC% | 88 | 37.5 | 50 | 15.5 | 9 | 10.0 | .0003 |
| Serum β2-microglobulin level and BMPC% | 31 | 54.0 | 55 | 19.0 | 32 | 13.5 | .008 |
PBPC% of 1% or less is defined as favorable; PBPC% higher than 1%, unfavorable; BMPC% of 10% or less, favorable; BMPC% higher than 10%, unfavorable; serum β2-microglobulin level less than 2.7 μg/mL, favorable; serum β2-microglobulin level 2.7 μg/mL or higher, unfavorable.
Survival of AL patients in relation to various combinations of favorable and unfavorable PBPC% and BMPC%.
Favorable PBPC% is defined as 1% or less; favorable BMPC% is defined as 10% or less. Both favorable, upper curve; mixed favorable, middle curve; both unfavorable, lower curve.
Survival of AL patients in relation to various combinations of favorable and unfavorable PBPC% and BMPC%.
Favorable PBPC% is defined as 1% or less; favorable BMPC% is defined as 10% or less. Both favorable, upper curve; mixed favorable, middle curve; both unfavorable, lower curve.
Discussion
There is a substantial basis for the study of circulating monoclonal plasma cells in AL. The number and labeling index of circulating clonal PBPCs are markers for disease activity and have been shown to be prognostic for the clinical course of smoldering MM7 and MM.4,6,8 In MM, PBPC% and bone marrow PCLI can be used to reliably stratify patients into low-, intermediate-, and high-risk groups, with statistically significant differences in survival.4 Although the presence of a circulating clonal plasma cell population has been demonstrated previously in AL patients by several different techniques, the prognostic significance of this finding has been unclear.5 9
The cause of death in most patients with AL is cardiac related, with clinical outcome determined by the extent of cardiac involvement with amyloidosis.1 In a previous multivariate analysis for prognostic factors in AL, presence of symptomatic congestive heart failure emerged as the most powerful adverse predictor for survival.10 Serum β2-microglobulin level has been controversial in predicting survival.1,11 Other prognostic factors, such as serum creatinine and serum albumin levels, were identified in specific amyloid-related organ syndromes.1 12
Our study is a retrospective analysis of 147 patients with AL (of 694 patients evaluated between 1991 and 1996) who underwent testing for circulating PBPCs, on a random basis, at presentation. The results indicate that, in this group of patients, high levels of circulating PBPCs are associated with poorer survival. This establishes a new prognostic factor for AL patients at diagnosis.
A lower PBPC% threshold (> 1% vs > 4%) is associated with worse prognosis in AL patients than in MM patients.4Consistent with previous reports,5,9 circulating PBPCs were detected in a significantly smaller proportion of patients with AL than had previously been reported for MM patients (16% vs 80%).4 Median absolute PBPC count (0 vs 4.4 × 106/L) and PBPC% (0% vs 6.0%) were lower in newly diagnosed AL patients than in newly diagnosed MM patients.4 These likely reflect the lower proliferative capacity of the neoplastic plasma cell clone in AL than in MM.
This study confirms the prognostic value of bone marrow plasma cell burden (BMPC%), serum β2-microglobulin concentration, and dominant cardiac involvement by amyloidosis in AL patients. The combined consideration of the level of circulating clonal plasma cells and bone marrow plasma cell burden allows AL patients to be stratified into low-, intermediate-, and high-risk groups (Table 4; Figure 2). We recognize that the assay for circulating PBPCs may not be widely available or widely used. Alternatively, the combined consideration of serum β2-microglobulin concentration and BMPC% also effectively separates AL patients into low-, intermediate-, and high-risk groups, with median survivals of 54, 19, and 13.5 months, respectively (P = .008; Table 4). The optimal combination of prognostic variables to allow for the most reliable stratification of AL patients needs further study in a larger cohort.
Detection of circulating plasma cells in AL may facilitate treatment decisions, which are frequently difficult in these patients. Myeloablative chemotherapy with stem cell rescue has been offered to a highly select group of AL patients. Further studies are needed to establish whether, as seen in MM, monitoring of PBPCs helps determine the appropriate time to harvest peripheral blood stem cells and select candidates for stem cell rescue.13,14 Such studies will also establish whether, as in MM, the presence of circulating PBPCs predicts a shorter relapse-free survival.15 Additionally, eradication of circulating PBPCs may serve as a surrogate marker for response to therapy in a minority of AL patients.
Although roughly 10% of AL patients have concurrent MM and vice versa, such a diagnosis is frequently difficult to establish. Some patients are diagnosed primarily with MM with lytic bony lesions and are incidentally found to have amyloid deposits on tissue examination, whereas the situation is reversed in the remaining patients with concurrent disease. Although AL patients with concurrent MM have a greater likelihood of having circulating PBPCs and have a worse prognosis than AL patients without concurrent MM, exclusion of this patient subset did not change the overall study conclusions. Our study indicated that the subgroup of patients with 2% or more circulating PBPCs had a higher likelihood of concurrent MM.
In summary, we found that a high level of circulating clonal plasma cells was associated with a poorer prognosis in AL, as has been found for other plasma cell dyscrasias. This is likely due to a higher proliferative rate of the abnormal plasma cell clone and, consequently, a propensity to involve the peripheral blood.
Prepublished online as Blood First Edition Paper, September 5, 2002; DOI 10.1182/blood-2002-06-1698.
Supported by grants CA85818 and CA62242 from the National Cancer Institute, Bethesda, MD. S.V.R. and R.F. are supported in part by Leukemia and Lymphoma Society Translational Research Awards.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
S. Vincent Rajkumar, Division of Hematology and Internal Medicine, Mayo Clinic, 200 First St SW, Rochester, MN 55905; e-mail: rajks@mayo.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal