Abstract
Primary central nervous system lymphoma (PCNSL) is a rare but often rapidly fatal form of non-Hodgkin B-cell lymphoma that arises within the central nervous system (CNS) and has a low propensity to metastasize. We performed immunohistochemistry on formalin-fixed, paraffin-embedded brain biopsy specimens from 24 patients with PCNSL to investigate the expression of B cell–attracting chemokine 1 (BCA-1, CXCL13), a lymphoid chemokine involved in B-cell compartmental homing within secondary lymphoid organs and recently implicated in the pathogenesis of inflammatory and malignant lymphocyte-mediated diseases. Whereas BCA-1 was not detected in normal human brain, all 24 brain biopsy specimens containing PCNSL were positive for BCA-1. Double immunostaining on selected specimens localized BCA-1 to malignant B lymphocytes and vascular endothelium. In contrast, 2 chemokines implicated particularly in T-cell movement, secondary lymphoid tissue chemokine (SLC, CCL21) and Epstein-Barr virus–induced molecule 1 ligand chemokine (ELC, CCL19), were expressed only by occasional stromal cells in 2 and 4 of the 24 specimens, respectively. Tumor cells stained positively for CXCR5, the primary receptor for BCA-1. In situ hybridization verified the expression of BCA-1 mRNA by malignant B cells, but not vascular endothelium, within the tumor mass, suggesting that vascular endothelial BCA-1 expression may be consequent to transcytosis. In PCNSL, expression of BCA-1 by malignant lymphocytes and vascular endothelium may influence tumor development and localization to CNS.
Introduction
Primary central nervous system lymphoma (PCNSL) is an unusual form of non-Hodgkin lymphoma that involves the brain, spinal cord, meninges, and eye. Although PCNSL occurs rarely, morbidity and mortality are high. Current incidence of the tumor is estimated at approximately 1:100 000 persons per year.1 This figure has been increasing dramatically since the 1970s, in part due to the AIDS epidemic, but also due to an unexplained increased occurrence of the tumor in immunocompetent persons.2 Patients with PCNSL may experience a variety of rapidly progressive neurologic deficits, and, if untreated, generally die within 3 months as a result of progressive intracranial disease.3 Prognosis may be improved with aggressive chemotherapy and radiotherapy regimens, but most clinical series report a 5-year survival rate below 10%.4
Reviews spanning the past 10 years point out the paucity of information regarding the pathogenesis of PCNSL.5-7 Many studies have confirmed that the vast majority of cases of PCNSL are B-cell neoplasms.5,6,8 Particularly intriguing is why a lymphoma should involve the central nervous system (CNS), a site that does not contain resident lymphocytes under normal circumstances. Furthermore, although the lymphoma spreads aggressively within the CNS, it rarely metastasizes elsewhere in the body. In 1988, Hochberg and Miller proposed 2 hypotheses to explain these observations.5Although hard evidence for either one is lacking, these theories continue to be considered in the literature.7 One hypothesis suggests that the lymphoma represents malignant transformation of an inflammatory lesion in situ. Epstein-Barr virus infection has been linked to PCNSL that occurs in association with AIDS. Conversely, it is postulated that malignant B lymphocytes acquire selective homing receptors for ligands expressed by the cerebral vascular endothelium. Consistent with this theory is the observation that tumor cells often localize around blood vessels, although, to date, no unique adhesion molecules have been identified.
The so-called “lymphoid chemokines” and their counterreceptors are constitutively expressed and control homeostatic trafficking of B and T cells to and within secondary lymphoid organs.9 B cell–attracting chemokine 1 (BCA-1, CXCL13), which is expressed on putative follicular dendritic cells, is responsible for compartmental homing of CXCR5-bearing B lymphocytes and also directs T-helper cells into the lymphoid follicle. Expression of this chemokine is considered critical for lymphoid neogenesis.10 Other lymphoid chemokines, secondary lymphoid tissue chemokine (SLC, CCL21) and Epstein-Barr virus–induced molecule 1 ligand chemokine (ELC, CCL19), are expressed within T-cell areas in lymphoid organs where they are chemotactic for T cells via CCR7. Recently, several research groups have recognized a role for the lymphoid chemokines in disease.11-16 In particular, BCA-1 has been identified in tissue specimens collected from patients with Helicobacter pylori–induced chronic gastritis and gastric lymphoma,11 rheumatoid arthritis12,13 and Sjögren syndrome.14-16 We have used immunohistochemistry and in situ hybridization to investigate the expression of BCA-1 protein and mRNA in PCNSL, postulating that this chemokine may play a role in attracting malignant lymphocytes into or trapping them within the CNS.
Materials and methods
Tissue specimens
Brain biopsy specimens, originally taken for diagnosis of PCNSL over a period spanning 1982 to 2000, were obtained for 21 men and 3 women, aged 31 to 77 years (median, 55.5 years). It was possible to obtain information regarding infection with HIV for 20 of the 24 individuals. Of these 20 patients, 17 persons were negative for HIV and 3 persons were infected with HIV. The Oregon Health and Science University Institutional Review Board approved the use of these archived specimens for the purposes of this study. Original pathologic reports were reviewed to verify the diagnosis of non-Hodgkin B-cell PCNSL by immunophenotyping, and routine hematoxylin and eosin staining was used to characterize the tumors according to the current World Health Organization (WHO) classification.17 The specimens had been fixed in 10% buffered formalin and embedded in paraffin at the time of diagnostic assessment. Tissue cross-sections were cut at 3 or 5 μm thickness for immunostaining or in situ hybridization, respectively, mounted on Superfrost Plus glass slides (Fisher Scientific, Pittsburgh, PA) and stored at room temperature. Before immunostaining, the slides were heated at 60°C for up to 60 minutes to ensure firm adhesion between tissue and glass. Normal human tonsil was used to establish the appropriate working concentrations for the antibodies used in the immunostaining protocols. Normal human brain, obtained from autopsy cases, and tonsil were used as control tissues for the immunostaining and in situ hybridization experiments.
Primary antibodies
Polyclonal goat antihuman BCA-1, SLC, and ELC antibodies were purchased from R & D Systems (Minneapolis, MN), and used for immunostaining at concentrations of 2.5 μg/mL, 1 μg/mL, and 2.5 μg/mL, respectively. Goat IgG (Sigma Chemical, St Louis, MO), in appropriate concentration, was used as the negative control for these antibodies. Mouse monoclonal antibodies directed against human CD20 (B cells; clone L26, IgG2aκ), CD31 (vascular endothelium; clone JC/70A, IgG1κ), and CD21 (follicular dendritic cells; clone 1F8, IgG1κ) were obtained from Dako (Carpinteria, CA) and used in working dilutions of 1:1000 (approximately 0.4 μg/mL), 1:500 (approximately 0.7 μg/mL), and 1:20 (approximately 18 μg/mL), respectively. Corresponding irrelevant negative control mouse monoclonal antibodies, appropriately diluted, were directed against an undetermined hapten (clone MOPC-21, IgG1κ) and β-2-6 linked fructosam (clone UPC-10, IgG2aκ) (Sigma Chemical). Rat anti-CXCR5 monoclonal antibody (clone RF8B2, isotype IgG2b) has been described elsewhere.18 Activity of this anti-CXCR5 antibody has been demonstrated for Western blot analysis, flow cytometry, and immunohistochemistry. In the present study, the antibody was used for immunostaining at a dilution of 1:50 cell culture supernatant. Rat IgG2b (Southern Biotechnology Associates, Birmingham, AL) was used as the negative control antibody, at a concentration of 10 μg/mL.
Immunohistochemistry
To detect the lymphoid chemokines, tissue cross-sections were initially deparaffinized in xylene and rehydrated through graded alcohol solutions. Once hydrated, the sections were boiled in a microwave for 10 minutes in 10 mM citrate buffer at pH 6.0. After cooling, they were washed 3 times in a buffer of 50 mM Tris (tris(hydroxymethyl)aminomethane) and 0.15 M sodium chloride at pH 7.5 (TBS). Nonspecific binding was blocked for 1 hour with a solution of TBS, with 0.1% bovine serum albumin, 0.3% Triton X-100 and 2% vol/vol normal rabbit serum. The sections were then incubated overnight at 4°C with primary antibody that had been diluted in blocking solution. Following 3 washes with TBS, they were incubated with biotinylated rabbit antigoat immunoglobulin (Vector Laboratories, Burlingame, CA) diluted 1:200 in TBS, with the aforementioned additives, for 45 minutes at room temperature. Subsequently, they were washed 3 times with TBS, incubated with streptavidin-biotin complex conjugated to alkaline phosphatase (Vector Laboratories), and washed again 3 times with TBS. To visualize antibody complexes, the sections were incubated with Fast Red (Biogenex, San Ramon, CA), as described by the manufacturer. For detection of the cell surface markers, CD20, CD21, and CD31, a similar protocol was followed, with the exception that antigen retrieval for CD21 was performed by a 30-minute incubation in 0.1% trypsin (Sigma Chemical) in TBS with 0.1% calcium chloride. In addition, a biotinylated horse antimouse secondary antibody (Vector Laboratories) was used, and, consequently, horse serum was substituted for rabbit in the blocking solution.
Immunostaining for CXCR5 was performed according to an indirect method, but with the following variations. After deparaffinization and rehydration of the tissue, sections were heated for 30 minutes at 95°C in commercially available target retrieval solution (Dako, product no. S1700). After cooling, the sections were washed 3 times in phosphate-buffered saline (PBS), and nonspecific binding was blocked with a commercial blocking solution (Dako, product no. X0909). The sections were incubated for 2 hours at room temperature with primary antibody, diluted in commercial diluent (Dako, product no. S3022), and then washed twice with TBS. Subsequently, they were incubated for 30 minutes at room temperature with rabbit antirat secondary antibody, at a concentration of 1:200 in the same commercial diluent, and again washed twice with TBS. They were then treated for 30 minutes with streptavidin-biotin complex/alkaline phosphatase (Dako) in TBS. Finally, the sections were developed in 5-bromo-4-chloro-3-inodolyl phosphate/nitro blue tetrazolium (BCIP/NBT; Dako), according to the manufacturer's recommendations.
Double immunostaining was undertaken to confirm our impression from results of the immunohistochemical studies that BCA-1 was expressed by B lymphocytes and vascular endothelium. To detect BCA-1, the described protocol was followed, with the exception that development of the sections was performed using streptavidin-biotin conjugated to horseradish peroxidase (Vector Laboratories) and 3,3′-diaminobenzidine (DAB; Dako). Subsequently, the sections were washed 3 times in TBS, and the same protocol was repeated, staining for CD20 or CD31, which was developed with streptavidin-biotin conjugated to alkaline phosphatase and Fast Red.
In situ hybridization
35S-labeled sense and antisense BCA-1 mRNA probes, 368 bp in length and corresponding to position −24 to −344 of the BCA-1 sequence, were generated by in vitro transcription, using a transcription kit (Roche Molecular Biochemicals, Indianapolis, IN). Tissue sections were dewaxed, rehydrated in graded ethanol solutions, and subjected to in situ hybridization, according to a previously described method.11 Finally, the sections were dipped in Kodak photo emulsion NTB-2 (Rochester, NY) and exposed in complete darkness for 5 weeks at 4°C. Development and fixation were performed according to the instructions provided by Kodak, and counterstaining was with hematoxylin.
Results
Specimen histopathology
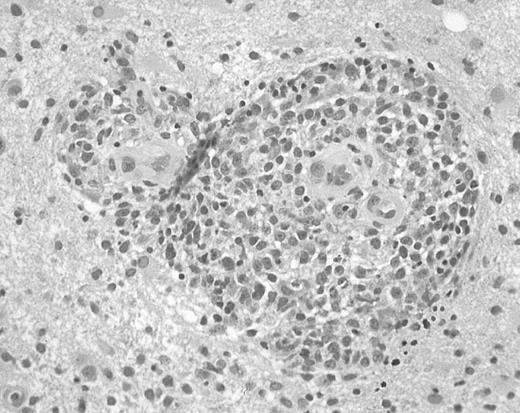
Brain biopsy specimens from 24 patients with non-Hodgkin B-cell PCNSL were studied. Specimens varied in size, most consisting of multiple pieces that varied from fragments measuring less than 0.1 × 0.1 cm to pieces measuring 2.0 × 0.9 cm. Microscopic examination revealed diffuse invasion of brain parenchyma by medium-sized or large atypical lymphocytes. The characteristic pattern of preferential concentric perivascular cuffing by malignant lymphocytes was observed in most cases, particularly in less densely infiltrated tissue or at the tumor periphery (Figure1). According to WHO criteria, 23 tumors were classified as diffuse large B-cell lymphomas and one tumor was classified as an atypical Burkitt lymphoma.
Histopathology of PCNSL.
Brain biopsy specimen from a patient with PCNSL shows infiltration of brain tissue by large malignant lymphocytes, with characteristic perivascular collections of tumor cells (hematoxylin and eosin, original magnification × 400).
Histopathology of PCNSL.
Brain biopsy specimen from a patient with PCNSL shows infiltration of brain tissue by large malignant lymphocytes, with characteristic perivascular collections of tumor cells (hematoxylin and eosin, original magnification × 400).
Immunohistochemistry
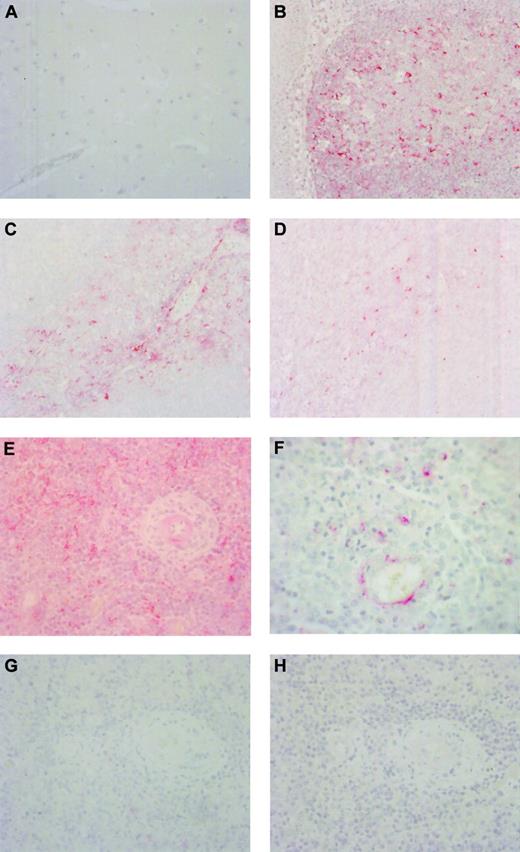
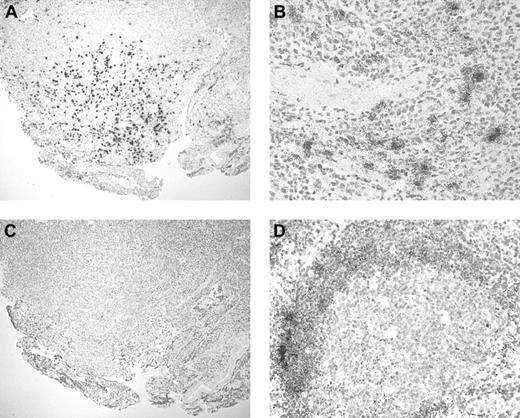
None of the lymphoid chemokines, including BCA-1 (Figure2A), were detected in normal human brain. Normal tonsil showed expression of BCA-1, SLC, and ELC in the expected distribution. In the lymphoid follicles there were numerous BCA-1+ cells, many of which had dendritiform morphology suggestive of follicular dendritic cells (Figure 2B). In contrast, SLC was present in T-cell areas, in some cases in association with vascular channels, assumed to represent high endothelial venules (Figure 2C). Cells expressing ELC were also localized to the T-cell areas (Figure2D). In brain biopsy specimens from patients with PCNSL, 24 of the 24 samples showed expression of BCA-1 (Figure 2E). Staining for BCA-1 appeared to be localized to the malignant lymphocytes (Figure 2F). The percentage of BCA-1+ tumor cells varied across the specimens. In areas where antigen was well preserved and the percentage of BCA-1+ cells was highest (ie, those areas of each specimen that were optimally penetrated with the formalin fixative), 13 specimens contained up to 25% positively stained cells, 4 specimens contained more than 25%, but no more than 50%, positively stained cells, and 7 specimens showed more than 50% positively stained cells. When antigen preservation was good, there was both membrane and cytoplasmic staining, whereas in less well-preserved areas, only diffuse or dotlike cytoplasmic staining was observed. In addition, the endothelium of some vessels stained positively for BCA-1 (Figure 2F). In the cases of SLC and ELC, positive staining was seen only in occasional stromal cells in 2 and 4 of the 24 specimens, respectively, and tumor cells were notably negative (Figure 2G-H). In a small number of specimens, there was antibody uptake by apoptotic cells including degenerate neurons that was not interpreted as positive. Negative control sections stained with goat IgG in place of the primary antibodies showed no positive staining.
BCA-1 expression in tonsil, brain, and PCNSL.
Immunohistochemical studies demonstrate that BCA-1 is not present in normal human brain (A) but is expressed in lymphoid follicles of tonsil (B) (Fast Red, hematoxylin counterstain, original magnification × 400). In tonsil, expression of SLC (C) and ELC (D) is localized to T-cell areas (Fast Red, hematoxylin counterstain, original magnification × 400). Brain biopsy specimen from a patient with PCNSL reveals widespread expression of BCA-1 (E), but not SLC (G) or ELC (H) within the tumor substance (Fast Red, hematoxylin counterstain, original magnification × 400). (F) Location of positive staining suggests that BCA-1 is expressed by malignant B cells and vascular endothelium (Fast Red, hematoxylin counterstain, original magnification × 1000).
BCA-1 expression in tonsil, brain, and PCNSL.
Immunohistochemical studies demonstrate that BCA-1 is not present in normal human brain (A) but is expressed in lymphoid follicles of tonsil (B) (Fast Red, hematoxylin counterstain, original magnification × 400). In tonsil, expression of SLC (C) and ELC (D) is localized to T-cell areas (Fast Red, hematoxylin counterstain, original magnification × 400). Brain biopsy specimen from a patient with PCNSL reveals widespread expression of BCA-1 (E), but not SLC (G) or ELC (H) within the tumor substance (Fast Red, hematoxylin counterstain, original magnification × 400). (F) Location of positive staining suggests that BCA-1 is expressed by malignant B cells and vascular endothelium (Fast Red, hematoxylin counterstain, original magnification × 1000).
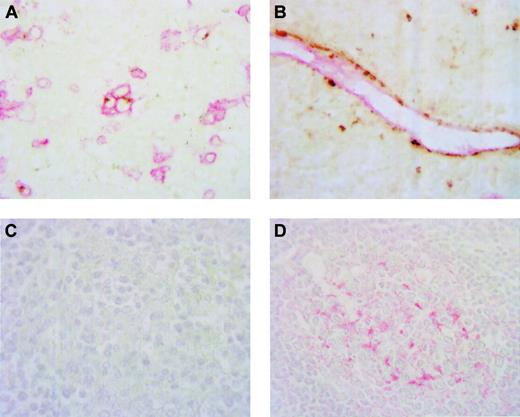
To confirm the pattern of expression observed on immunostaining for BCA-1 alone, 5 brain biopsy specimens that contained sufficient tumor were chosen for double immunostaining using antibodies directed against BCA-1 and CD20 or CD31. This double-immunostaining study confirmed that BCA-1 was expressed by CD20+ B cells and CD31+vascular endothelium (Figures 3A-B). Negative control sections stained with goat IgG and irrelevant mouse monoclonal antibody as primary antibodies showed no positive staining.
Although there was no apparent expression of BCA-1 by dendritiform cells within the tumors, because follicular dendritic cells are a potential source of BCA-1 and are found in some lymphomas, PCNSL specimens were examined for the presence of these cells. Sections of the same 5 brain biopsy specimens that were used for the double-immunostaining studies were stained for the presence of CD21. Within these tumor specimens, no CD21+ cells were observed (Figure 3C). Sections of normal tonsil stained in parallel with the brain biopsy specimens demonstrated CD21+ dendritiform cells within lymphoid follicles (Figure3D).
BCA-1 is expressed by B lymphocytes and vascular endothelium in PNCSL.
Double immunostaining confirms expression of BCA-1 by CD20+B cells in panel A (DAB, BCA-1; Fast Red, CD20; no counterstain, original magnification × 1000) and CD31+ vascular endothelium in panel B (DAB, BCA-1; Fast Red, CD31; no counterstain, original magnification × 1000). Brain biopsy specimen from a patient with PCNSL does not contain CD21+ cells (C), whereas, in panel D, CD21+ cells are detected in a lymphoid follicle of a normal tonsil (Fast Red, hematoxylin counterstain, original magnification × 600).
BCA-1 is expressed by B lymphocytes and vascular endothelium in PNCSL.
Double immunostaining confirms expression of BCA-1 by CD20+B cells in panel A (DAB, BCA-1; Fast Red, CD20; no counterstain, original magnification × 1000) and CD31+ vascular endothelium in panel B (DAB, BCA-1; Fast Red, CD31; no counterstain, original magnification × 1000). Brain biopsy specimen from a patient with PCNSL does not contain CD21+ cells (C), whereas, in panel D, CD21+ cells are detected in a lymphoid follicle of a normal tonsil (Fast Red, hematoxylin counterstain, original magnification × 600).
Six brain biopsy specimens were stained for the presence of CXCR5. Within the tumor mass, many CXCR5+ cells were identified, with stain frequently concentrated at the cell membrane (Figure4A). Although the proportion of positive cells varied across a given section, in some areas of positive staining, virtually all cells stained positively for the receptor. In other areas of tumor, there was no specific staining. Often positive cells appeared to be localized around blood vessels. In normal tonsil, CXCR5 was localized to cells in the mantle zone and germinal centers of secondary lymphoid follicles (Figure 4B). Although some uptake was noted in control sections stained with rat IgG, the pattern of staining was nonspecific.
CXCR5 expression in tonsil and PCNSL.
(A) Immunohistochemical examination of brain biopsy specimen from a patient with PCNSL demonstrates expression of CXCR5 by cells of perivascular malignant infiltrates (BCIP/NBT, no counterstain, original magnification × 400). (B) Normal tonsil shows CXCR5-expressing cells in lymphoid follicles, particularly in the mantle zone (BCIP/NBT, no counterstain, original magnification × 400).
CXCR5 expression in tonsil and PCNSL.
(A) Immunohistochemical examination of brain biopsy specimen from a patient with PCNSL demonstrates expression of CXCR5 by cells of perivascular malignant infiltrates (BCIP/NBT, no counterstain, original magnification × 400). (B) Normal tonsil shows CXCR5-expressing cells in lymphoid follicles, particularly in the mantle zone (BCIP/NBT, no counterstain, original magnification × 400).
In situ hybridization
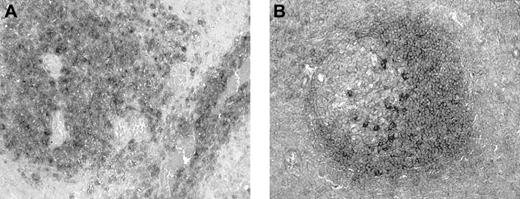
Expression of BCA-1 transcript was demonstrated in 8 of the 8 tumor samples examined by in situ hybridization (Figure5A). Although simultaneous immunophenotyping was not performed, it appeared that tumor cells were positive, whereas vascular endothelial cells were negative (Figure 5B). However, the pattern of expression varied across the section, and some tumor cells did not express BCA-1 mRNA. No hybridization was detected in sections incubated with the BCA-1 sense mRNA probe (Figure 5C). The BCA-1 mRNA was also detected in the lymphoid follicles of human tonsil (Figure 5D), whereas no hybridization was observed when the sense probe was applied.
BCA-1 mRNA expression in PCNSL.
In situ hybridization confirms BCA-1 expression in brain biopsy specimen from a patient with PCNSL. Antisense (A) and sense (C) hybridizations are shown (hematoxylin counterstain, original magnification × 40). (B) Vascular endothelium does not appear to synthesize BCA-1 because BCA-1 mRNA is localized solely to B lymphocytes (hematoxylin counterstain, original magnification × 200). (D) In human tonsil, BCA-1 mRNA is present in the lymphoid follicles (hematoxylin counterstain, original magnification × 200).
BCA-1 mRNA expression in PCNSL.
In situ hybridization confirms BCA-1 expression in brain biopsy specimen from a patient with PCNSL. Antisense (A) and sense (C) hybridizations are shown (hematoxylin counterstain, original magnification × 40). (B) Vascular endothelium does not appear to synthesize BCA-1 because BCA-1 mRNA is localized solely to B lymphocytes (hematoxylin counterstain, original magnification × 200). (D) In human tonsil, BCA-1 mRNA is present in the lymphoid follicles (hematoxylin counterstain, original magnification × 200).
Discussion
PCNSL is a malignant B-cell neoplasm that arises within and remains localized to the CNS. Using immunohistochemistry, we have demonstrated the presence of lymphoid chemokine, BCA-1, in brain biopsy specimens obtained from 24 patients with this tumor. Although expressed in secondary lymphoid tissue, where it participates in B-cell movement, BCA-1 was not present in normal brain. Significantly, BCA-1 expression was observed in all biopsy specimens. Although the majority of our patients were HIV−, 3 individuals were HIV+. The finding that BCA-1 expression occurred independently of HIV infection in PNCSL is interesting because different pathogenic processes are believed to lead to development of the lymphoma in these distinct patient subgroups.7 Clearly some disease mechanisms are common to both forms of PCNSL. In contrast, but not unexpectedly, SLC and ELC, lymphoid chemokines that have major influence on T-cell migration, were not detected in the lymphoma cells of any PCNSL, but were seen only in occasional stromal cells in a small number of specimens. In secondary lymphoid tissue, the predominant source of BCA-1 is believed to be the dendritic cell population located in the lymphoid follicle. These cells are present in some lymphomas, including the diffuse large B-cell subtype.19 However, follicular dendritic cells were notably absent from the PCNSL specimens, although our methods identified these cells in normal tonsil. Double immunostaining localized expression of BCA-1 to B lymphocytes and vascular endothelium within the tumor mass. Many infiltrating B cells expressed CXCR5, the primary receptor for BCA-1. In situ hybridization provided confirmation of our immunohistochemical results, by identifying BCA-1 mRNA in a proportion of the tumor specimens.
Expression of BCA-1 in PCNSL is consistent with data from recent publications suggesting that this molecule may be involved in the pathogenesis of several lymphocyte-mediated diseases.11-16Inducible expression of BCA-1 was first described in relation to gastric lymphomas of the mucosa-associated lymphoid tissue (MALT) type, arising in association with H pylori–induced gastritis and mucosal lymphoid aggregates.11 BCA-1 is also present in inflamed synovial biopsies from patients with rheumatoid arthritis.12,13 Furthermore, in a multivariate analysis, Takemura and colleagues showed that expression of BCA-1 independently predicted the occurrence of lymphoid neogenesis in joints.13 Similar studies have implicated BCA-1 in the formation of lymphoid follicular aggregates in the salivary glands of patients with Sjögren syndrome.14-16 Interestingly, B-lymphocyte chemoattractant (BLC), the mouse homologue of human BCA-1, has been detected in renal inflammatory infiltrates of (NZB × NZW)F1 mice, a crossbreed that spontaneously develops lupus nephritis.20
In PCNSL specimens, malignant B lymphocytes were observed to be the primary source of BCA-1. Previous investigations relating to H pylori–associated gastric lymphoma were strongly suggestive that malignant B cells could express this chemokine.11 Benign B cells may also express BCA-1 in the context of inflammation.11,14 In our study, the proportion of positively stained malignant cells varied across a given tissue section and between different biopsy specimens, both by immunohistochemistry and in situ hybridization. Although differences in antigen preservation across the tissue and variation in the stage of cell cycle could account for this observation, there may be heterogeneity between cells at a molecular level. Gene microarray analysis has identified genetic differences between diffuse large B-cell systemic lymphomas that could not be differentiated at a cellular level.21 Molecular heterogeneity within any given diffuse large B-cell lymphomas is also recognized.22
We identified vascular endothelium as an additional source of BCA-1 in PCNSL. The malignant lymphocytes of PCNSL frequently infiltrate vascular structures. Yet, double staining verified that vascular endothelium also expressed BCA-1. In situ hybridization observations suggested that although malignant B cells produced BCA-1 mRNA, the vascular endothelium did not. Using immunostaining, other groups have demonstrated expression of BCA-1 by follicular high endothelial venules in normal tonsil,23 as well as inflamed salivary gland and joint.13,15 However, whether vascular endothelial cells can synthesize BCA-1 is unknown. The possibility that the molecule is taken up by the process of transcytosis has been considered by other researchers.15This was proposed as an explanation for apparently paradoxical findings from independent studies of salivary gland from patients with Sjögren syndrome that transcript is not expressed by endothelial cells,14 whereas expressed product is localized to these cells.14 Other lymphoid chemokines, SLC24 and ELC,25 when injected intradermally, are translocated to the surface of high endothelial venules in draining lymph nodes where they may exert chemotactic activity. Transcytosis of other molecules is known to occur in the cerebral circulation.26 Further, in PNCSL the tumor cells characteristically aggregate in close proximity to vessels, which would facilitate transfer of BCA-1 from these cells to vascular endothelium.
Given that the malignant B cells express CXCR5, the primary BCA-1 receptor, it is tempting to speculate about the role of BCA-1 in the pathogenesis of PCNSL, a lymphoma that arises in a tissue that does not normally contain lymphocytes. Because the specimens we studied were taken after the development of substantial tumor mass, our work gives no information concerning the regulation and time course of secretion. Studies in mice have revealed that production of BLC by putative follicular dendritic cells is controlled by a positive feedback loop involving the CXCR5-bearing B cells that have been attracted to the site by BLC.27 B cell–derived lymphotoxin α/β (LTα/β) and tumor necrosis factor α (TNF-α) interact with LTβ receptor (LTβR) and TNF receptor 1 (TNFR1), respectively, on follicular dendritic cells to induce BLC expression. B cells express TNFR2,28 but not TNFR128 or LTβR,29 implying that aberrant regulatory mechanisms must exist to induce the malignant cells to produce BCA-1. Whether the chemokine influences tumor cell proliferation is also unknown. However, it is clear that the BCA-1/CXCR5 interaction is capable of triggering the extracellular signal-regulated kinase/mitogen-activated protein kinase (ERK/MAPK) signal transduction pathway, which participates in lymphocyte activation.30 Related lymphoid chemokines, ELC and SLC, can promote cell proliferation under certain circumstances.31
Cell adhesion molecules regulate the tissue-specific dissemination of malignant lymphocytes in other lymphomas,32 and recently, chemokines and their receptors have been implicated in tissue-specific metastasis.33 The possibility of malignant B cells homing to cerebral vasculature, as a consequence of specificity of cell adhesion molecule expression, has been suggested to occur in PCNSL,5 although the putative molecules have not been identified.7 Malignant lymphocytes typically cluster around vessels. Because vascular endothelium does not appear to produce BCA-1, it seems unlikely that the molecule could act as a specific homing signal in PNCSL, unless benign B cells, already present within the CNS, were induced to synthesize and secrete the molecule. It is intriguing that some patients with PCNSL may demonstrate benign inflammatory intraparenchymal brain lesions that resolve prior to the development of tumor.34 Conversely, PCNSL may represent malignant transformation within a benign lymphocytic proliferation, a scenario that may explain many MALT lymphomas11,15 In this setting, activated CD4+ T-helper cells promote low-grade B-cell neoplasia in response to chronic antigen stimulation.35 Most B-cell lymphomas show variable infiltration by benign T cells. Immunostaining of selected specimens confirmed the presence of T cells in PCNSL, with CD4+ and CD8+ cells present in normal, reversed, and increased CD4/CD8 ratios in different biopsies (data not shown). Because CXCR5 is expressed by recently activated memory CD4+ T cells,36 it is possible that some of the CXCR5 expression we observed is associated with CD4+ T cells in the tumors. Although possibly implying local activation of antigen-specific B cells and in situ tumorigenesis, the CD4+ T-cell infiltration in the high-grade lymphomas of the CNS may also reflect an antitumor immune response or nonspecific infiltration.37 While the site of origin of PCNSL remains uncertain, it is probable that BCA-1 contributes to the localization of this tumor, which virtually always remains isolated to the CNS.
Prepublished online as Blood First Edition Paper, October 3, 2002; DOI 10.1182/blood-2002-05-1576.
Supported by grants from Fight for Sight (J.R.S., GA20025), the National Institutes of Health (J.T.R., EYO6484), Research to Prevent Blindness (Casey Eye Institute), and the Helmut Horten Foundation (Institute for Research in Biomedicine). J.R.S. was a recipient of a National Health and Medical Research Council of Australia Neil Hamilton Fairley Postdoctoral Fellowship (997099) and is currently a recipient of a Research to Prevent Blindness Career Development Award.
A portion of this work was presented at the American Society of Hematology 42nd Annual Meeting in San Francisco, CA, in December 2000.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Justine R. Smith, Casey Eye Institute, Oregon Health and Science University, 3375 SW Terwilliger Blvd, Portland, OR 97201; e-mail: smithjus@ohsu.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal