Glucocorticoid hormones (GCHs) regulate normal and neoplastic lymphocyte development by exerting antiproliferative and/or apoptotic effects. We have previously shown that dexamethasone (DEX)–activated thymocyte apoptosis requires a sequence of events including interaction with the glucocorticoid receptor (GR), phosphatidylinositol-specific phospholipase C (PI-PLC), and acidic sphingomyelinase (aSMase) activation. We analyzed the mechanisms of GCH-activated apoptosis by focusing on GR-associated Src kinase, cytochrome c release, and caspase-8, -9, and -3 activation. We show here that PI-PLC binds to GR-associated Src kinase, as indicated by coimmunoprecipitation experiments. Moreover, DEX treatment induces PI-PLC phosphorylation and activation. DEX-induced PI-PLC phosphorylation, activation, and apoptosis are inhibited by PP1, a Src kinase inhibitor, thus suggesting that Src-mediated PI-PLC activation is involved in DEX-induced apoptosis. Caspase-9, -8, and -3 activation and cytochrome c release can be detected 1 to 2 hours after DEX treatment. Caspase-9 inhibition does not counter cytochrome crelease, caspase-8 and caspase-3 activation, and apoptosis. Caspase-8 inhibition counters cytochrome c release, caspase-9 and caspase-3 activation, and apoptosis, thus suggesting that caspase-8 inhibitor can directly inhibit caspase-9 and/or that DEX-induced caspase-8 activation is upstream to mitochondria and can regulate caspase-3 directly or through cytochrome c release and the consequent caspase-9/caspase-3 activation. DEX-induced caspase-8 activation, like ceramide-induced caspase-8 activation, correlates with the formation of Fas-associated death domain protein (FADD)/caspase-8 complex. Caspase-8 activation is countered by the inhibition of macromolecular synthesis and of Src kinase, PI-PLC, and aSMase activation, suggesting it is downstream in the DEX-activated apoptotic pathway of thymocytes.
Introduction
Glucocorticoid hormones (GCHs) are used in a number of inflammatory and autoimmune diseases, in organ transplantation, and in the treatment of leukemia and lymphomas.1-6 GCHs induce the apoptosis of thymocytes, particularly in stress conditions, when higher levels of circulating hormones are present in the blood. They also induce cytotoxicity against normal and neoplastic lymphocytes during pharmacologic therapy. However, their therapeutic use is limited because of several unwanted side effects, which can lead to the suspension of treatment, and because of resistance, as may occur in leukemia and lymphoma. Thus, a more profound analysis of the molecular mechanisms implicated in apoptosis induction appears necessary.
Thymocytes are highly sensitive to GCH-induced apoptosis, and, though they have been investigated in many studies, the molecular mechanisms responsible for GCH-induced cell death have not yet been fully clarified. GCH interaction with the glucocorticoid receptor (GR), endonuclease activation, Ca++ mobilization, cytochromec release, and proteasome and caspase activation have been shown to participate in GCH-induced apoptosis.7-12
In a previous work13 we showed that activation of a sequence of events is required for dexamethasone (DEX)–induced apoptosis of thymocytes. In particular, DEX-induced apoptosis can be attributed to early (5-15 minutes) ceramide generation due to activation of acidic sphingomyelinase (aSMase). Moreover, DEX treatment rapidly (1-5 minutes) activates phosphatidylinositol-specific phospholipase C (PI-PLC) through a mechanism mediated by protein kinase C (PKC) activity and induces diacylglycerol (DAG) generation, which precedes, and is required for, aSMase activation and ceramide generation.13
Caspase activity plays a crucial role in and is downstream to PI-PLC and aSMase activation, insofar as inhibition of early ceramide generation blocks caspase activation and thymocyte death. All these events, including early PKC and PI-PLC activation, require GCH interaction with the GR and are countered by GR antagonists such as mifepristone.13
The GCH/GR interaction is required for GCH-induced genomic and nongenomic effects, GR release from other components of the cytoplasm macromolecular complex, including HSP90 and GR-associated Src kinase, and its translocation to the nucleus.14 15
In the present study, we further analyzed the molecular mechanisms involved in DEX-induced apoptosis of thymocytes to understand how GCH activates PI-PLC and to elucidate the role of different caspases in apoptosis. In particular, we performed experiments aimed at defining the role of GR-associated Src kinase, the role of activation of different caspases such as caspase-8, -9, and -3, and the role of cytochrome c release. Our results indicate that (1) PI-PLC binds GR-associated Src in the HSP90/GR/Src complex; (2) GR-associated Src kinase inhibition blocks DEX-induced PI-PLC phosphorylation, activation, and apoptosis; and (3) DEX treatment, like ceramide treatment, induces Fas-associated death domain protein (FADD)/caspase-8 complex formation and activation of caspase-8, -9, and -3. In addition, we found that caspase-9 inhibition does not counter cytochrome c release, caspase-8 and caspase-3 activation, or apoptosis. On the other hand, caspase-8 inhibition counters cytochrome c release, caspase-9 and caspase-3 activation, and apoptosis. We also found that DEX-induced caspase-8 activation is downstream of early events such as Src, PI-PLC, and aSMase activation and is inhibited by Src, PI-PLC, aSMase, and macromolecular synthesis inhibitors. These results indicate that a complex of molecular mechanisms, including GR-associated Src kinase and caspase-8/caspase-3 activation, is involved in DEX-induced apoptosis of thymocytes.
Materials and methods
Cell system and treatments
Thymocytes from 4- to 6-week-old C3H/HeN mice were enriched after passage through nylon wool columns. The effect of several agents on DEX-induced apoptosis was evaluated. These were U73122 (Calbiochem, La Jolla, CA), a PI-PLC inhibitor16; D609 (Kamiya, Thousand Oaks, CA), a PC-PLC inhibitor17; monensin (Sigma-Aldrich, St Louis, MO), an aSMase inhibitor18; and the Src kinase inhibitor PP1 (Alexis, San Diego, CA).19Caspase inhibitors were Z-DEVD-FMK (Calbiochem), a caspase-3 inhibitor, Z-IETD-FMK (Calbiochem), a caspase-8 inhibitor, and Z-LEHD-FMK (Calbiochem), a caspase-9 inhibitor. In general, cells were preincubated with inhibitors, at the concentrations indicated in the legends to the figures, for 30 minutes before DEX treatment. Time of DEX treatment varied depending on the experiment, as indicated in “Materials and methods” and in the legends to the figures.
Apoptosis evaluation
Apoptosis was measured by flow cytometry as described elsewhere.20 After culturing, cells were centrifuged, and the pellets were gently resuspended in 1.5 mL hypotonic propidium iodide (PI) solution (50 μg/mL PI in 0.1% sodium citrate plus 0.1% Triton X-100; Sigma, St Louis, MO) before overnight storage at 4°C in the dark. The PI fluorescence of individual nuclei was measured by flow cytometry with standard FACScan equipment (Becton Dickinson Biosciences, San Jose, CA). Nuclei traversed the light beam of a 488-nm argon laser. A 560-nm dichroid mirror (DM 570) and a 600-nm bandpass filter (bandwidth, 35 nm) were used to collect the red fluorescence resulting from PI DNA staining, and the data were recorded in logarithmic scale in a Hewlett Packard (HP 9000, model 310; Palo Alto, CA) computer. The percentage of apoptotic cell nuclei (subdiploid DNA peak in the DNA fluorescence histogram) was calculated with specific FACScan research software (Lysis II; Becton Dickinson).
Immunofluorescence staining and nuclear translocation assay
To evaluate the nuclear translocation of the GR, thymocytes were processed for immunofluorescence using the paraformaldehyde-saponin procedure.21 Cells (2 × 106/mL) were preincubated for 30 minutes with PP1 (10 μM) or U73122 (2.5 μM); thymocytes were then incubated for 30 minutes with DEX 10−7 M, and the treatment was stopped by the immersion of samples in methanol at −20°C for 30 seconds.
After extensive washing in phosphate-buffered saline (PBS) with 1% HEPES (N-2-hydroxyethylenepiperazine-N′-2-ethanesulfonic acid), cells were fixed in 4% formaldehyde for 20 minutes on ice, washed again, and incubated at 4°C for 1 hour with blocking buffer (PBS with 3% bovine serum albumin [BSA] and 1% glycine). For staining, cells were incubated for 45 minutes at 4°C with 100 ng polyclonal rabbit anti-GR antibodies (Santa Cruz Biotechnology, CA), in buffer containing 0.1% saponin and were washed and incubated for 45 minutes at 4°C with Texas Red-conjugated goat antirabbit immunoglobulin G (IgG; Molecular Probes, Eugene, OR) in PBS-saponin. Cells were then washed, stuck on slides coated with poly-L-lysine, and mounted in buffered glycerol for fluorescence microscopic analysis. Photographs were taken on a Leitz Dialux 20 microscope (Wetzlar, Germany).
PI-PLC activity assay
PI-PLC activity was determined in vitro by its capacity to hydrolyze 14C-PI vesicles to generate DAG. Cells were treated for 5 minutes with DEX in the presence or absence of the PC-PLC inhibitor D609 (50 μg/mL), the PI-PLC inhibitor U73122 (2.5 μM), or the Src kinase inhibitor PP1 (10 μM). Treatment was stopped by the immersion of samples in methanol/dry ice (−70°C) for 10 seconds, followed by centrifugation at 4°C in a microfuge. Pellets were then resuspended in 250 mM Tris (tris[hydroxymethyl]aminomethane)–HCl buffer, pH 7.4, containing 10 μM phenylmethylsulfonyl fluoride (PMSF), 100 μM bacitracin, 1 mM benzamidine, 1 μM aprotinin, 10 μM leupeptin, 10 μM pepstatin, and 5 μg/mL soybean trypsin inhibitor. Cells were lysed by sonication with a cell sonifier. Radiolabeled PI vesicles were prepared by sonicating (5 minutes, 5W, 80% output) L-3-phosphatidylinositol-1stearoyl-2[14C]arachidonoyl (NEN Life Science Products, Boston, MA) for the detection of released DAG through PI-PLC. Vesicles were resuspended at 10 μM in the reaction buffer (50 mM Tris-HC1, pH 7.4, 5 mM CaCl2, 5 mM MgCl2, 0.01% fatty acid free-BSA). Whole cell lysate (50-100 μg protein) was added to 250 μL reaction buffer containing the vesicles and incubated at 37°C for 1 hour, and the reaction buffer was stopped by the addition of 250 μL chloroform/methanol/acetic acid (4:2:1, by volume). To separate the organic phase from the aqueous phase, 250 μL H20, 250 μL CHCl3, and 100 μL KCl were added, and the mixture was centrifuged at 4.000 rpm in a microfuge for 5 minutes. The organic phase was removed, dried under nitrogen, resuspended in 200 μL chloroform, and applied to a silica gel thin-layer chromatography (TLC) plate (Merck, Darmstadt, Germany), with an automatic applicator Linomat IV (Camag, Muttens, Switzerland). Samples were chromatographed in chloroform/methanol/acetic acid/water (100:60:16:8) to separate the parent phospholipids from the PI-PLC product (ie, DAG). Authentic standards were chromatographed with the lipid extracts to locate the compounds of interest by exposure to iodine vapor. Radioactive spots, as visualized by autoradiography that corresponded to standards, were scraped from the plate and counted by liquid scintillation. Radioactive measurements were converted to picomole product by using the specific activity of substrate. Blank values obtained from controls lacking cell proteins were subtracted from the experimental values. PI-PLC activity was expressed as pmol DAG produced/106 cells.
In vitro aSMase assay
Aliquots of 6 × 106 cells/mL were treated for 5 minutes with DEX or U73122 or monensin or any combination of them. Treatment was stopped by the immersion of samples in methanol/dry ice (−70° C) for 10 seconds, followed by centrifugation at 4°C in a microfuge. To measure aSMase, the cells were washed after treatment, and the pellet was resuspended in 200 μL 0.2% Triton X-100 and was incubated for 15 minutes at 4°C. The cells were sonicated, and the protein concentration was assayed. Then 50 to 100 μg protein was incubated for 2 hours at 37°C in a buffer (50 μL final volume) containing 250 mM sodium acetate, 1 mM EDTA (ethylenediaminetetraacetic acid), pH 5.0, and 0.32 μL N-methyl-14C sphingomyelin (0.04 μCi/mL [0.00148 MBq], specific activity 56.6 mCi/mM [2094.2 MBq]; Amersham). The reaction was stopped by the addition of 250 μL chloroform/methanol (2:1, by volume). Lipids were extracted as described above. The organic phase, obtained through the different extraction steps, was collected and washed once with 1 mL chloroform/methanol/water (3:48:47, by volume) to totally remove free radioactive phosphorylcholine. Aqueous phases were collected, transferred to scintillation vials, and routinely counted by liquid scintillation. Counts per minute represented the choline phosphate generated from sphingomyelin (SM) hydrolysis. The organic phase was analyzed on TLC plates by using chloroform/methanol/ammonia hydroxide (7N)/water (85:15:0.5:0.5, by volume). The hydrolysis of SM was quantitated by autoradiography and liquid scintillation and was expressed as pmol SM hydrolysed/106 cells.
Immunoprecipitation and Western blotting of PI-PLC
Thymocytes were incubated for 1 hour with the Src inhibitor PP1 (10 μM), PI-PLC inhibitor U73122 (2.5 μM), and PC-PLC inhibitor D609 (50 μg/mL) and were treated alone or with DEX 10−7M for 5 minutes. After treatment, cells were harvested, and whole cell lysates were prepared in an extraction buffer containing 50 mM Tris-HCl, pH 8.0, 137 mM NaCl, 10% glycerol, 1% Nonidet P-40 (NP-40), 1 mM sodium vanadate, 10 mM sodium pyrophosphate, 50 mM sodium fluoride, 1 mM PMSF, 10 μg/mL leupeptin, and 2 μg/mL aprotinin.
Phosphotyrosine-containing proteins were immunoprecipitated with agarose-conjugated 4G-10 antibodies (Upstate Biotechnology, Waltham, MA), and PI-PLC abundance in the 4G-10 immunoprecipitates was assessed by Western blot analysis using monoclonal anti–PI-PLCγ antibodies (Upstate Biotechnology) in an enhanced chemiluminescence system SuperSignal (Pierce, Rockford, IL).
Coimmunoprecipitation of HSP90, GR, Src, PI-PLC, caspase-8, and FADD
Thymocytes were treated with DEX 10−7 M or C2 ceramide for the indicated times, and the lysates were prepared with RIPA buffer (50 mM Tris, pH 7.5, 150 mM NaCl, 1% NP-40, 0.5% deoxycholate, 0.1% sodium dodecyl sulfate [SDS], and 5 mM EDTA) supplemented with 1 mM PMSF, 1 mM sodium vanadate, 10 mM sodium pyrophosphate, 50 mM sodium fluoride, 10 μg/mL leupeptin, and 2 μg/mL aprotinin. In some experiments, groups were pretreated for 30 minutes with the specific Src inhibitor PP1 (10 μΜ).22 Proteins, 1 mg in RIPA buffer, were immunoprecipitated overnight with 3 μg monoclonal anti-FADD antibody (Upstate Biotechnology), monoclonal anti–caspase-8 antibody (Alexis), or monoclonal anti-HSP90 antibody (BD PharMingen, San Diego, CA) with continuous rocking. Antigen–antibody complexes were precipitated with protein A bound to Sepharose beads (Pharmacia, Piscataway, NJ) for 2 hours before SDS–polyacrylamide gel electrophoresis (SDS-PAGE). For coimmunoprecipitation experiments, Western blot analysis was performed using anti-FADD (Upstate Biotechnology), anti–caspase-8 (Alexis), monoclonal anti-HSP90 (BD PharMingen), polyclonal anti-GR (Santa Cruz Biotechnology), monoclonal anti–Src-1 (Upstate Biotechnology) able to recognize the GR-associated Src,22 or monoclonal anti–PI-PLCγ (Upstate Biotechnology) antibodies.
Determination of cytochrome c release
Thymus cells (3 × 107) treated with DEX (10−7 M) were washed in PBS and resuspended in 500 μL ice-cold buffer containing 20 mM HEPES, 250 mM sucrose, 2 mM EDTA, 20 μg/mL PMSF, 2 μg/mL leupeptin, and 10 μg/mL aprotinin, pH 7.1. Cells were disrupted on ice by a Teflon homogenizer and were centrifuged for 5 minutes at 3000g to remove nuclei and unbroken cells. Supernatants were then centrifuged for 20 minutes at 12 000g to isolate the mitochondrial fraction. For Western blot detection of cytochrome c, the supernatant from the last centrifugation and the mitochondrial fractions were subjected to 15% SDS-PAGE. After protein transfer, the membrane was blocked with a 5% solution of BSA in TBST buffer (25 mM Tris-HCl, 137 mM NaCl, 0.05% Tween 20, pH 7.4) and was incubated for 1 hour at room temperature with monoclonal antibody against cytochrome c (BD PharMingen). The membrane was then incubated with monoclonal antibody goat antimouse coupled to peroxidase (Pierce). Specific protein complexes were identified using the SuperSignal (Pierce) substrate chemiluminescence reagent.
Western blotting to evaluate activation of caspase-3, -8, and -9
Cells were washed once with ice-cold PBS and were lysed by incubation for 30 minutes on ice in 100 μL lysis buffer (20 mM Tris-HCl, 0.15 M NaCl, 5 mM EDTA, 100 mM PMSF, 2.5 mM leupeptin, 2.5 mM aprotinin). After centrifugation at 15 000 rpm for 15 minutes, extracted proteins were separated on a 12% or a 15% SDS-polyacrylamide gel and were electrophoretically transferred to a nitrocellulose transfer membrane (Schleicher & Schuell, Keene, NH). Membrane was blocked with TBST (20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.1% Tween 20) containing 5% skimmed milk for 1 hour at room temperature, and each antibody was applied overnight at 4°C in the same blocking solution. Anti–caspase-3 antibody and anti–caspase-8 antibody were purchased from Santa Cruz Biotechnology, and anti–caspase-9 antibody was purchased from New England Biolabs (Beverly, MA). After incubation, membranes were washed with TBST and incubated for 1 hour with horseradish peroxidase–labeled goat antirabbit (for anti–caspase-8 and -9) or antimouse (for caspase-3) IgG (Pierce). Antigen–antibody complexes were revealed by enhanced chemiluminescence.
Statistical analysis
All the experiments here shown were repeated at least 3 times. For data analysis, the Student t test was used with the STATPAC Computerized Program, and P < .05 was considered significant.
Results
Role of GR-associated Src kinase in DEX-mediated PI-PLC activation of apoptosis
We have previously reported that DEX-induced apoptosis requires a sequence of biochemical events: DEX/GR interaction is followed by a rapid (1-5 minutes) PI-PLC activation that can be inhibited by the specific PI-PLC inhibitor U73122, but not by the PC-PLC inhibitor D609. Moreover, this PI-PLC activation is under the control of PKC activity and is inhibited by PKC inhibitors.13 GR-associated Src kinase, following DEX/GR interaction, is activated and contributes to early nongenomic effects.22
We evaluated the role of GR-associated Src kinase in DEX-induced PI-PLC activation and apoptosis. For that purpose, thymocytes were treated with PP1, a specific Src kinase family inhibitor that stabilizes the HSP90/GR/Src cytoplasm complex and inhibits DEX-induced GR-associated Src activation.19,22 Results indicate that PP1, like the specific PI-PLC inhibitor U73122, inhibits PI-PLC activation (Figure1A) and apoptosis (Figure 1B). As previously reported, D609, a PC-PLC inhibitor, did not inhibit PI-PLC or DEX-activated apoptosis.13 DEX-induced apoptosis (Figure 1B) was evaluated 18 hours after DEX treatment, and similar results were obtained after 12 hours (P < .001 comparing percentage apoptosis of DEX-treated [52 ± 5] and untreated [14 ± 2] thymocytes).
Src activity involvement in DEX-induced thymocyte apoptosis.
Effect of PP1 on DEX-induced PI-PLC activity, apoptosis, GR assembly, and GR nuclear translocation. (A) Thymocytes were pretreated for 30 minutes with PP1 (10 μM), U73122 (2.5 μM), and D609 (50 μg/mL) and then with DEX (10−7 M, 5 minutes). To evaluate PI-PLC activity, cell extracts were reacted with radiolabeled PI vesicles, and then the DAG released was separated by TLC and visualized by autoradiography. *P < .001. (B) Effect of PP1, U73122, and D609 on DEX-induced thymocyte apoptosis, as detected after 18 hours of culture with DEX (10−7 M). Apoptosis was evaluated by PI staining and FACScan flow cytometer. Mean values ± SE of 3 different experiments in duplicate are reported. *P < .001. (C) Thymocytes were pretreated for 30 minutes with PP1 (10 μM) before treatment with DEX (10−7M, 30 minutes). Cell lysates were immunoprecipitated (IP) with anti-HSP90 antibody and then Western blotted with anti-HSP90, anti-GR, and anti-Src antibodies. (D) Immunofluorescence analysis of thymocytes untreated or treated for 30 minutes with DEX alone or in combination with PP1 and U73122. After treatment, cells were stained with anti-GR antibody using the paraformaldehyde-saponin procedure.
Src activity involvement in DEX-induced thymocyte apoptosis.
Effect of PP1 on DEX-induced PI-PLC activity, apoptosis, GR assembly, and GR nuclear translocation. (A) Thymocytes were pretreated for 30 minutes with PP1 (10 μM), U73122 (2.5 μM), and D609 (50 μg/mL) and then with DEX (10−7 M, 5 minutes). To evaluate PI-PLC activity, cell extracts were reacted with radiolabeled PI vesicles, and then the DAG released was separated by TLC and visualized by autoradiography. *P < .001. (B) Effect of PP1, U73122, and D609 on DEX-induced thymocyte apoptosis, as detected after 18 hours of culture with DEX (10−7 M). Apoptosis was evaluated by PI staining and FACScan flow cytometer. Mean values ± SE of 3 different experiments in duplicate are reported. *P < .001. (C) Thymocytes were pretreated for 30 minutes with PP1 (10 μM) before treatment with DEX (10−7M, 30 minutes). Cell lysates were immunoprecipitated (IP) with anti-HSP90 antibody and then Western blotted with anti-HSP90, anti-GR, and anti-Src antibodies. (D) Immunofluorescence analysis of thymocytes untreated or treated for 30 minutes with DEX alone or in combination with PP1 and U73122. After treatment, cells were stained with anti-GR antibody using the paraformaldehyde-saponin procedure.
As control for PP1 treatment efficacy, we used 2 different approaches. First, we performed coimmunoprecipitation experiments. GCH treatment induces GR and Src release from the cytoplasm macromolecular complex.14,15 Results in Figure 1C confirm previous observations indicating that an HSP90/GR/Src complex exists, that DEX treatment induces GR and Src release from the protein complex, and that release is inhibited by PP1.22 Second, DEX-induced GR nuclear translocation requires the GR release from the cytoplasm macromolecular complex.
We evaluated the effect of PP1 treatment on DEX-induced GR nuclear translocation. Results in Figure 1D indicate that the PP1-induced stabilization of the HSP90/GR/Src macromolecular complex was paralleled by an inhibition of GR nuclear translocation. Moreover, the specific PI-PLC inhibitor U73122 did not interfere with GR nuclear translocation.
PI-PLC associates with the HSP90/GR/Src macromolecular complex and is phosphorylated after DEX treatment
Overall the results illustrated in Figure 1 suggest that GR-associated Src kinase is involved in DEX-mediated PI-PLC activation and apoptosis. We determined whether PI-PLC could reside in the same molecular complex with GR-associated Src and HSP90. Results of a representative experiment, obtained by coimmunoprecipitation with anti-HSP90 antibodies, are shown in Figure2. Figure 2A shows that GR, Src PI-PLC, and HSP90 coimmunoprecipitated in the protein extract from untreated thymocytes. DEX treatment induced GR, Src, and PI-PLC release at 5 and 30 minutes after treatment. In particular, 5 minutes after DEX treatment, GR was not detectable, whereas PI-PLC and Src were still detectable but were significantly reduced; 30 minutes after DEX treatment GR, PI-PLC, and Src were not detectable (Figure 2A). These results indicate that PI-PLC was in the same macromolecular complex with HSP90, GR, and Src and that DEX treatment induced GR, Src, and PI-PLC release.
DEX induces displacement of PI-PLC, Src, and GR from HSP90 but does not affect PI-PLC/Src association.
Cellular extracts from murine thymocytes, untreated or treated with DEX (10−7 M) for the indicated times (5 or 30 minutes), were immunoprecipitated (IP) with anti-HSP90 (A) or anti-Src antibodies (B). Western blot analyses were performed with the indicated antibodies. (C) Murine thymocytes were treated with PP1 (10 μM), U73122 (2.5 μM), and D609 (50 μg/mL) 30 minutes before DEX treatment (10−7 M, 5 minutes). Tyrosine-phosphorylated proteins were immunoprecipitated with agarose-conjugated 4G-10 antibodies, and PI-PLC abundance in the 4G-10 immunoprecipitates was assessed by Western blot with anti–PI-PLC monoclonal antibody (C, bottom). Whole lysates were run to control the PI-PLC levels (C, top).
DEX induces displacement of PI-PLC, Src, and GR from HSP90 but does not affect PI-PLC/Src association.
Cellular extracts from murine thymocytes, untreated or treated with DEX (10−7 M) for the indicated times (5 or 30 minutes), were immunoprecipitated (IP) with anti-HSP90 (A) or anti-Src antibodies (B). Western blot analyses were performed with the indicated antibodies. (C) Murine thymocytes were treated with PP1 (10 μM), U73122 (2.5 μM), and D609 (50 μg/mL) 30 minutes before DEX treatment (10−7 M, 5 minutes). Tyrosine-phosphorylated proteins were immunoprecipitated with agarose-conjugated 4G-10 antibodies, and PI-PLC abundance in the 4G-10 immunoprecipitates was assessed by Western blot with anti–PI-PLC monoclonal antibody (C, bottom). Whole lysates were run to control the PI-PLC levels (C, top).
We also performed experiments to determine the possible binding of GR, Src, and PI-PLC in untreated and DEX-treated thymocytes. Results in Figure 2B, obtained by coimmunoprecipitation with anti-Src antibodies, showed that Src binds PI-PLC and GR in untreated thymocytes. In contrast, at 30 minutes after DEX treatment Src binds PI-PLC, but not GR. These data confirm that DEX treatment induces GR, Src, and PI-PLC release but does not affect Src/PI-PLC binding. These data further confirm the results reported in Figure 1C, which show DEX treatment induced the release of GR and Src from the HSP90 and which confirm previous reports indicating that Src associates directly with PI-PLC.23
PI-PLC activation is reported to be a consequence of phosphorylation, which is blocked by PP1.24 25 We evaluated whether DEX induces PI-PLC phosphorylation. In the same Src/PI-PLC coimmunoprecipitation experiment, we analyzed the presence of phosphorylated proteins. For that purpose, phosphorylated proteins were immunoprecipitated with agarose-conjugated antiphosphotyrosine monoclonal antibodies, and the presence of PI-PLC was evaluated by anti–PI-PLC monoclonal antibody. Results indicate that phosphorylated PI-PLC was present in DEX-treated thymocytes (Figure 2C). Moreover, treatment with the specific Src inhibitor, PP1, inhibited DEX-induced PI-PLC phosphorylation, suggesting that Src is involved in PI-PLC phosphorylation. As control, we also tested the effect of the specific PI-PLC inhibitor U73122. U73122 inhibited DEX-induced PI-PLC phosphorylation, whereas D609, a specific PC-PLC inhibitor, did not. These results indicate that Src and PI-PLC are in the same protein complex and that GR-associated Src may contribute to DEX-induced PI-PLC phosphorylation and activation.
DEX induces caspase activation
It has been reported that protease activation is involved in apoptosis.26-29 Two main caspase activation pathways can be considered the pathway activated by death receptors, which includes the sequential activation of caspase-8 and of caspase-3, and the cytochrome c–dependent pathway activated by nonreceptor signals, which includes the sequential activation of caspase-9 and caspase-3.30-33
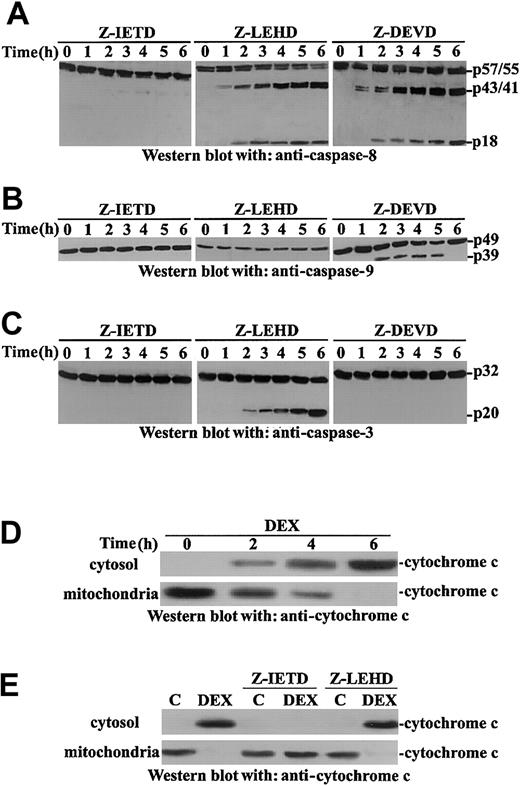
We analyzed the DEX-activated caspase pathway in thymocytes. Western blot experiments (Figure 3A) indicated that DEX treatment activates caspase-8 (detectable at 1-2 hours after treatment), caspase-9, and caspase-3.
DEX activates caspase-3, -8, and -9.
(A) Murine thymocytes were untreated or treated for the indicated times with DEX (10−7 M, 1-6 hours). Caspase activation was assayed by Western blot analysis with the appropriate antibodies. (B) Thymocytes were pretreated for 30 minutes with the indicated caspase inhibitor and were cultured in the presence or absence of DEX (10−7 M, 18 hours). Apoptosis was evaluated by PI staining. Mean values ± SE of 3 different experiments in duplicate are reported.
DEX activates caspase-3, -8, and -9.
(A) Murine thymocytes were untreated or treated for the indicated times with DEX (10−7 M, 1-6 hours). Caspase activation was assayed by Western blot analysis with the appropriate antibodies. (B) Thymocytes were pretreated for 30 minutes with the indicated caspase inhibitor and were cultured in the presence or absence of DEX (10−7 M, 18 hours). Apoptosis was evaluated by PI staining. Mean values ± SE of 3 different experiments in duplicate are reported.
Experiments were performed to elucidate the role of caspases in DEX-induced apoptosis and to determine whether the caspase-8/-3 or the caspase-9/-3 pathway is involved. We tested the influence of caspase-8, -9, and -3 inhibitors on apoptosis. Results in Figure 3B indicate the caspase-8 inhibitor inhibits apoptosis but the caspase-9 inhibitor, though able to inhibit caspase-9 activation, does not. As expected, the caspase-3 inhibitor blocks apoptosis. These results are in agreement with previous observations indicating that FADD or caspase-8 inhibition can result in caspase-9 and -3 inhibition.34
As further control, we evaluated the effect of caspase-8, -9, and -3 inhibitors on DEX-induced caspase-8, -9, and -3 activation. The caspase-9 inhibitor completely inhibits caspase-9 but not caspase-8 and only slightly caspase-3; the caspase-3 inhibitor inhibits caspase-3; and the caspase-8 inhibitor inhibits caspase-8, -9, and -3 (Figure 4A-C).
It has been previously reported that, as detected by using florigenic caspase substrates, an order of appearance of caspase activity can occur so that caspase-9 appears before caspase-8 activity in DEX-induced thymocytes.35 It has also been reported that caspase-8 activation can precede caspase-9 in Fas/FADD-dependent and also in Fas/FADD-independent apoptosis.35 36 We were unable to find a clear order in caspase-9, -8, and -3 activation (Figures 3-4), and this difference may be attributed to the different sensitivities of the assays used to detect caspase activation. These results suggest DEX treatment activates the caspase-8/-3 and the caspase-9/-3 pathways. Caspase-9 is dispensable, but caspase-8 activation is required for apoptosis induction.
DEX induces sequential activation of caspase-8, -9, and -3 and cytochrome
c release from mitochondria. Murine thymocytes were pretreated for 30 minutes with the indicated caspase inhibitor and then were untreated or treated with DEX (10−7 M) for different times (1-6 hours). Cell lysates were analyzed by Western blot for caspase cleavage with anti–caspase-8 (A), anti–caspase-9 (B), or anti–caspase-3 (C) antibodies. Cell lysates from thymocytes untreated or treated with DEX (10−7 M) for different times (2-6 hours; D) or pretreated with the indicated caspase inhibitor (30 minutes) before DEX treatment (6 hours; E) were assayed for cytochromec release in Western blot using an anti–cytochromec monoclonal antibody.
DEX induces sequential activation of caspase-8, -9, and -3 and cytochrome
c release from mitochondria. Murine thymocytes were pretreated for 30 minutes with the indicated caspase inhibitor and then were untreated or treated with DEX (10−7 M) for different times (1-6 hours). Cell lysates were analyzed by Western blot for caspase cleavage with anti–caspase-8 (A), anti–caspase-9 (B), or anti–caspase-3 (C) antibodies. Cell lysates from thymocytes untreated or treated with DEX (10−7 M) for different times (2-6 hours; D) or pretreated with the indicated caspase inhibitor (30 minutes) before DEX treatment (6 hours; E) were assayed for cytochromec release in Western blot using an anti–cytochromec monoclonal antibody.
DEX-induced cytochrome c release: inhibition of caspase-8 counters cytochrome c release
It has been reported that DEX induces cytochrome crelease that, in turn, mediates caspase-9 activation.37-41We evaluated the effect of DEX treatment on cytochrome crelease. Results show that DEX treatment induces cytochromec release, already detectable at 2 hours after treatment (Figure 4D).
Cytochrome c release is under the control of caspase-8.31,32 41 We evaluated the possible roles of caspase-8 and -9 in the regulation of DEX-induced cytochromec release in thymocytes. Although the inhibition of caspase-9 did not counter cytochrome c release, caspase-8 inhibition countered cytochrome c release (Figure 4E). These results indicate that in DEX-treated thymocytes, cytochromec release is under the control of caspase-8, and they explain, at least in part, why caspase-8 inhibition counters DEX-induced caspase-9 activation (Figure 3B).
These data suggest caspase-8 is important in DEX-induced thymocyte apoptosis because activated caspase-8 can directly activate caspase-3 and allow caspase-9 activation by regulating DEX-induced cytochromec release. Moreover, though caspase-9 inhibition does not block apoptosis (Figure 3B) because caspase-8 directly activates caspase-3, caspase-8 inhibition does block apoptosis because direct caspase-3– and caspase-9–mediated caspase-3 activation are inhibited (Figure 4A-C).
DEX and ceramide induce FADD/caspase-8 association
The above results indicate that DEX activates thymocyte caspase-8. Apoptotic stimuli are reported to induce the association of FADD with caspase-8. This association is necessary for caspase activation and can be detected in the FADD/caspase-8 complex in Fas receptor–dependent and receptor-independent apoptosis.42,43 We previously reported that DEX treatment induces rapid aSMase activation and ceramide generation that precedes caspases activation.13It has been reported that ceramide can induce caspase-8 and -3 activation in cardiomyocytes.44 We attempted to determine whether DEX and ceramide induce the FADD/caspase-8 complex, and our results showed DEX or ceramide treatment induces FADD/caspase-8 formation in thymocytes (Figure 5A-B). In addition, we evaluated whether ceramide, like DEX, activates caspase-8 in thymocytes. Our results showed that ceramide, when exogenously added to thymocytes, induced caspase-8 activation (Figure 5C). These findings indicate that ceramide contributes to DEX-induced caspase-8 activation in thymocytes, that caspase-8 activation is caused, at least in part, by induction of the FADD/caspase-8 complex, and that DEX-induced ceramide generation is upstream of caspase activation.
DEX and C2 ceramide induce caspase-8–FADD association.
(A) Thymocytes, untreated or treated for the indicated times (0.5-3 hours) with DEX (10−7 M) or C2 ceramide (50 μM), were immunoprecipitated with anti-FADD monoclonal antibody and immunoprecipitates revealed with anti–caspase-8 and anti-FADD antibodies. (B) Thymocytes, untreated or treated for different times (0.5-3 hours) with DEX (10−7 M) or C2 ceramide (50 μM), were immunoprecipitated with anti–caspase-8 antibody and immunoprecipitates revealed with anti-FADD and anticaspse-8 antibodies. (C) Thymocytes, untreated or treated for different times (1-6 hours) with C2 ceramide (50 μM), were assayed for caspase-8 cleavage by Western blot with anti–caspase-8 antibody.
DEX and C2 ceramide induce caspase-8–FADD association.
(A) Thymocytes, untreated or treated for the indicated times (0.5-3 hours) with DEX (10−7 M) or C2 ceramide (50 μM), were immunoprecipitated with anti-FADD monoclonal antibody and immunoprecipitates revealed with anti–caspase-8 and anti-FADD antibodies. (B) Thymocytes, untreated or treated for different times (0.5-3 hours) with DEX (10−7 M) or C2 ceramide (50 μM), were immunoprecipitated with anti–caspase-8 antibody and immunoprecipitates revealed with anti-FADD and anticaspse-8 antibodies. (C) Thymocytes, untreated or treated for different times (1-6 hours) with C2 ceramide (50 μM), were assayed for caspase-8 cleavage by Western blot with anti–caspase-8 antibody.
DEX-induced caspase-8 activation is countered by PI-PLC, aSMase, macromolecular synthesis, and Src kinase inhibitors
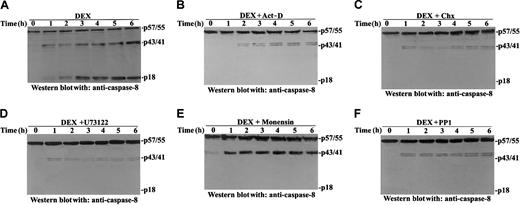
Many studies report DEX-induced apoptosis is a macromolecular, synthesis-dependent event.8,45 We have also demonstrated that sequential PI-PLC and aSMase activation precede caspase activation and contribute to DEX-induced apoptosis.13 Results shown in Figure 5 indicate that DEX, like ceramide, activates caspase-8. We assessed the effect of inhibitors of Src, PI-PLC, aSMase, mRNA, and protein synthesis on DEX-induced caspase-8 activation and apoptosis. Results shown in Figure 6 indicate apoptosis is inhibited by macromolecular synthesis inhibitors (Figure6A) and PI-PLC and aSMase inhibitors (Figure 6B), such as the Src inhibitor PP1 (Figure 1B).
Effects of protein synthesis inhibitors (act-D and Chx), monensin, and U73122 on DEX-induced apoptosis.
Thymocytes pretreated for 30 minutes with 2.5 μg/mL act-D or 50 μg/mL Chx (A) or with 2.5 μM U73122 or 10 μg/mL monensin (Mon.; B) were cultured for 18 hours in the presence of DEX (10−7 M). Apoptosis was evaluated by PI staining and FACScan flow cytometry. Hydrolysis of SM was evaluated in untreated and DEX-treated thymocytes, not pretreated or pretreated with U73122 or monensin (C). Mean values ± SE of 3 different experiments in duplicate are reported.
Effects of protein synthesis inhibitors (act-D and Chx), monensin, and U73122 on DEX-induced apoptosis.
Thymocytes pretreated for 30 minutes with 2.5 μg/mL act-D or 50 μg/mL Chx (A) or with 2.5 μM U73122 or 10 μg/mL monensin (Mon.; B) were cultured for 18 hours in the presence of DEX (10−7 M). Apoptosis was evaluated by PI staining and FACScan flow cytometry. Hydrolysis of SM was evaluated in untreated and DEX-treated thymocytes, not pretreated or pretreated with U73122 or monensin (C). Mean values ± SE of 3 different experiments in duplicate are reported.
As control we tested the effect of PI-PLC and aSMase inhibitors on aSMase activity. Results in Figure 6C concur with previous data indicating that PI-PLC precedes, and is required for, aSMase activation and ceramide generation and showing that DEX-induced apoptosis is under the control of macromolecular synthesis and PI-PLC/aSMase activation.8,9,13,45 46
We also tested the effect of those inhibitors on DEX-induced caspase-8 activation. Figure 7 shows that Src kinase, PI-PLC aSMase, and macromolecular synthesis inhibitors all counter DEX-induced caspase-8 activation, indicating DEX-induced caspase-8 activation is required for apoptosis induction and is downstream in the apoptotic signal pathway. In particular, results in Figure 7B-C indicate that actinomycin D (act-D) and cycloheximide (Chx) inhibit caspase-8 activation. We have reported that macromolecular synthesis inhibitors do not counter DEX-induced ProCPP32 cleavage.13 This apparent discrepancy can be attributed to the different assays used; a colorimetric assay was used for ProCPP32 cleavage detection, whereas caspase activation was evaluated here through a more specific assay, Western blot immunoassay with specific antibodies.
Effects of protein synthesis inhibitors (act-D and Chx), monensin, PP1, and U73122 on DEX-induced caspase 8 activation.
Thymocytes were treated for different times with DEX (10−7M; A) or were pretreated for 30 minutes with 2.5 μg/mL act-D (B), 50 μg/mL Chx (C), 2.5 μM U73122 (D), 10 μg/mL monensin (E), or 10 μM PP1 (F) before DEX treatment. Caspase-8 cleavage was evaluated by Western blot analysis with anti–caspase-8 antibody.
Effects of protein synthesis inhibitors (act-D and Chx), monensin, PP1, and U73122 on DEX-induced caspase 8 activation.
Thymocytes were treated for different times with DEX (10−7M; A) or were pretreated for 30 minutes with 2.5 μg/mL act-D (B), 50 μg/mL Chx (C), 2.5 μM U73122 (D), 10 μg/mL monensin (E), or 10 μM PP1 (F) before DEX treatment. Caspase-8 cleavage was evaluated by Western blot analysis with anti–caspase-8 antibody.
Discussion
The results of the present study provide new information concerning mechanisms of the GCH-mediated apoptotic pathway in thymocytes, and we show that GR-associated Src kinase and caspase-8 activation contribute to DEX-induced apoptosis. GCH-mediated regulation of apoptosis has been described in a number of different cells and tissues, including thymocytes, normal and neoplastic lymphocytes, granulocytes, neurones, epithelial cells, germ cells, and osteoblasts.7,8,46-52 GCH-induced apoptosis in cells of the lymphoid compartment may be partly responsible for its immunosuppressive and anti-inflammatory effects and its antileukemia efficacy in pharmacologic treatments. Transcription-dependent and -independent mechanisms have been described as mediating the GCH-activated death of lymphoma and leukemia cell lines, suggesting that different molecular mechanisms are involved in GCH-mediated apoptosis induction in different cells and tissues.4-6,48 53-55
We have reported that both transcription-dependent and transcription-independent signals—for example, PI-PLC activation and the consequent aSMase activation and ceramide generation—may be involved in DEX-induced thymocyte apoptosis.13 Here we show DEX-induced PI-PLC activity is regulated, at least in part, by the activation of GR-associated Src kinase and that PI-PLC itself associates with the GR macromolecular complex. Our results concur with previous reports indicating that Src and PI-PLC exist in the same molecular complex, which, as this study indicates, can be immunoprecipitated from thymocyte protein extracts. In particular, GR-associated Src kinase activation is reported to be responsible for nongenomic effects induced by DEX–GR interaction.22 This activation is inhibited by PP1, a Src family kinase inhibitor. As we show here PP1, like the specific PI-PLC inhibitor U73122, blocks DEX-induced PI-PLC activation and apoptosis. PI-PLC phosphorylation is required for activation.23-25 Our results show that though DEX treatment does not influence Src/PI-PLC complex formation, it does induce PI-PLC phosphorylation and activation. PI-PLC phosphorylation and apoptosis are inhibited by PP1 and by the specific PI-PLC inhibitor U73122, but not by the PC-PLC inhibitor D609.
These observations confirm previous findings that DEX–GR interaction is necessary for apoptosis induction, that PI-PLC activation is regulated by phosphorylation, and that DEX-induced PKC activity is required for PI-PLC activation and apoptosis.13 22-25Moreover, these data suggest that thymocyte PI-PLC phosphorylation and activation are mediated by DEX-induced Src kinase activity.
Many studies report GCH activates caspases and suggest caspase activation may play a role in DEX-induced thymocyte apoptosis.9,28,29 Sequential cleavage and activation of caspases is an important mechanism in most apoptosis models. Initiator caspases, including receptor-activated caspase-8 and cytochromec–dependent caspase-9, activate effector caspases, including caspase-3.33 The results of our experiments in the present study indicate DEX-treatment activates caspase-8, -9, and -3 in thymocytes. Caspase-8 may have a major role; its inhibition blocks apoptosis. The finding that inhibition of caspase-9 does not block apoptosis provides further evidence of the role of caspase-8 activity in DEX-induced thymocyte death. Caspase inhibitors might not be specific.56 Caspase-9 inhibitor does inhibit caspase-9 activation (Figure 3A) but does not inhibit caspase-3 activation and apoptosis (Figure 3B). Caspase-8 inhibitor counters caspase-8 and caspase-9 activation, possibly because of a nonspecific inhibiting activity but possibly also because caspase-8, but not caspase-9, inhibition counters the DEX-induced cytochromec release (Figure 4D-E) required for caspase-9 activation. These results are in agreement with data from other experimental models suggesting caspase-8 has a major role in apoptosis activation, and this effect is amplified through cytochrome c release and the consequent caspase-9 activation.34,41 Caspase-8 can directly activate caspase-3 or regulate cytochrome c release and, consequently, caspase-9 activation so that, though caspase-9 inhibition leaves the caspase-8/-3 pathway functional, caspase-8 inhibition can counter direct caspase-3 activation, cytochromec release, and the consequent cytochromec–dependent activation of the caspase-9/-3 pathway.33 37-41 As a consequence, caspase-8 inhibition can block DEX-induced caspase-9 and caspase-3 activation.
These data are in part in agreement with previous results obtained in caspase-9 null mice.57,58 In fact, it has been reported that DEX treatment of caspase-9 null thymocytes induces detectable—but reduced, compared with wild-type thymocytes—caspase-8, -3, and -2 activation, thus suggesting that caspase-9 is not necessary for caspase-8/-3 activation or for the activation of other executioner caspases such as caspase-2.57 Moreover, it has been reported that DEX treatment induces DNA fragmentation, though to a lesser extent than wild-type mice, in thymocytes of caspase-9 null mice, thus suggesting that the caspase-8/-3 pathway can mediate, at least in part, DEX-induced apoptosis.58 Our results show that the caspase-8/-3 pathway is involved in apoptosis induction and that differences, compared with results obtained with caspase-9 null mice, can be attributed to the different experimental models in that we used adult thymocytes that may have a different susceptibility to DEX-induced apoptosis compared with fetal thymocytes used in the experiments with caspase-9 null mice.57,58 As an example, fetal thymocytes are more sensitive to Fas-induced apoptosis58 than mature or adult thymocytes.59 60 Moreover, in the present study, the use of caspase-9 inhibitor could not result in total inhibition, as in caspase-9 null mice, and could leave, albeit at an undetectable level, enough caspase-9 activity to amplify caspase-8–activated apoptosis. Taken together, these data suggest DEX can activate a number of caspases, and an interplay between caspases may be important for the effective activation of apoptosis.
Our results in adult thymocytes concur with previous observations indicating that DEX activates caspase-8, that caspase-8 controls cytochrome c release and the consequent caspase-9 activation, and that caspase-8 can be activated without triggering membrane receptors.34,37,43,61,62 This receptor-independent caspase-8 activation may be mediated, in part, through an increase in DEX-induced ceramide levels, and indeed ceramide is reported to activate caspase-8.44 63 The data presented here indicate that exogenous ceramide induces the FADD/caspase-8 complex and caspase activation and that the aSMase inhibitor monensin, like the PI-PLC inhibitor U73122, counters DEX-induced caspase-8 activation.
Receptor-mediated caspase-8 activation may be mediated by FADD/caspase complex formation.42,43 Our results show that DEX treatment induces the FADD/caspase-8 complex. A similar effect is obtained with exogenous ceramide. These results confirm previous findings showing FADD/caspase-8 complex formation can be detected when caspase-8 activation occurs in the absence of membrane receptor–mediated apoptotic stimuli.43 64
Ceramide may be involved in DEX-induced FADD/caspase-8 complex formation, caspase-8 activation, and apoptosis. We have previously reported that the inhibition of PI-PLC and/or of the consequent aSMase and ceramide generation counters DEX-induced apoptosis.13We show that the inhibitors of PI-PLC or aSMase block DEX-induced caspase-8 activation.
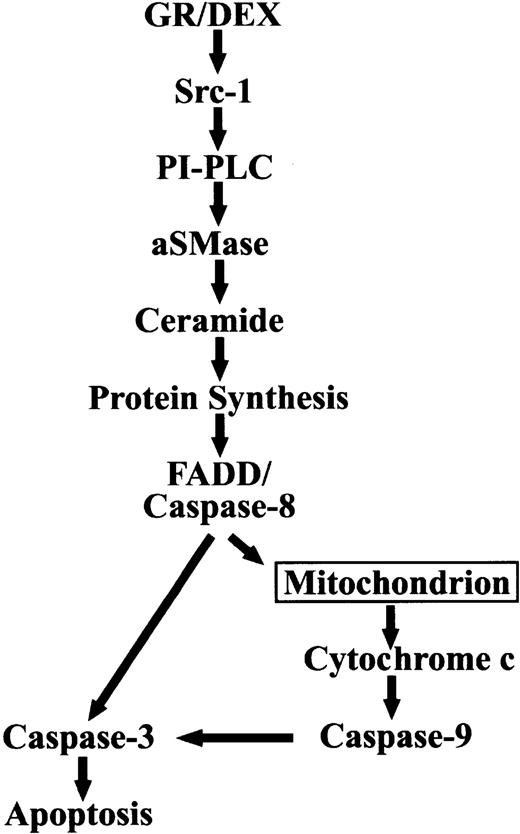
In conclusion, the results of the present study, together with our previous observations,13 indicate that a sequence of biochemical events is activated by DEX in thymocytes (Figure8). Although the experimental results indicate precise biochemical mechanisms are involved in thymocyte apoptosis, they may be only a part of a complex array of apoptotic signals activated by DEX and may not be relevant for other tissues, including neoplastic cells such as leukemia and lymphoma. This advance in knowledge of DEX-activated biochemical events may be the basis for further studies aimed at analyzing the complex pattern of resistance and susceptibility to GCH treatment. Accurate study of the GCH-activated biochemical events involved in apoptosis modulation, in all susceptible tissues, may contribute to a better understanding of some of the effects responsible for the therapeutic efficacy of GCH treatment and may be helpful in the development of new pharmacologic approaches aimed at controlling immune response and neoplastic cell growth.
Prepublished online as Blood First Edition Paper, August 29, 2002; DOI 10.1182/blood-2002-06-1779.
Supported by Associazione Italiana Ricerca sul Cancro (AIRC), Milan, Italy.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Carlo Riccardi, Department of Clinical and Experimental Medicine, Pharmacology Section, University of Perugia, Via del Giochetto, 06122 Perugia, Italy; e-mail:riccardi@unipg.it.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal