Autoimmune hemolytic anemia (AIHA) is a disease in which autoantibodies against red blood cells (RBCs) lead to their premature destruction. Most clinically significant autoantibodies are of the immunoglobulin G (IgG) type, which leads primarily to the uptake and destruction of RBCs by splenic and hepatic macrophages. Therapies such as corticosteroids and splenectomy are directed at interfering with this process. Liposomally encapsulated clodronate (dichloromethylene diphosphonate) has previously been found to be a potent antimacrophage agent. It selectively depletes animals of macrophages within 24 hours of administration by inducing apoptosis in these cells. Therefore, we hypothesized that liposomal clodronate would be a useful agent for treating AIHA. We tested this hypothesis in a mouse model of AIHA in which animals were given either anti-RBC antibodies or preopsonized RBCs. In either case, liposomal clodronate substantially decreased RBC destruction. This drug formulation was effective within hours by first blocking and then depleting phagocytic macrophages, and its action lasted for 1 to 2 weeks. Thus, in AIHA, liposomal clodronate therapy may act like a temporary, medicinal splenectomy. As such, it may prove useful in situations where rapid response to therapy is critical or other medical therapies are inadequate.
Introduction
Autoimmune hemolytic anemia (AIHA) is an autoimmune disease in which antibodies directed against the patient's own red blood cells (RBCs) lead to their premature destruction.1Anemia can be sudden and life threatening, or more gradual in onset. Although most cases are idiopathic, association with other forms of autoimmunity, malignancy, or infection is common.2-5 AIHA occurs in both children and adults, with a wide age distribution.
AIHA can be mediated by immunoglobulin G (IgG), IgM, or, rarely, IgA antibodies.1 Most clinically significant cases, however, are caused by IgG antibodies.1,3 In these patients, autoantibodies bind to RBCs and promote their uptake by splenic and hepatic macrophages via Fc receptors.6Although IgG antibodies can fix complement, the principal means of destruction of RBCs in these cases is via phagocytosis.1,7-9 Therefore, even though B cells (often with T-cell help) are producing the offending autoantibody, macrophages are the essential effector cells for the development of anemia. This fact is reflected in the therapy for AIHA. Splenectomy and corticosteroids, mainstays of treatment, both ultimately interfere with the phagocytosis of opsonized RBCs.1 Experimental therapy in animal models has also used the specific blocking of Fc-mediated uptake of RBCs by either genetic means or the use of anti-Fc antibodies.10-14
Clodronate (dichloromethylene diphosphonate) is a bisphosphonate used for treating osteolytic bone diseases and osteoporosis. Bisphosphonates alleviate these disorders by inhibiting the function of osteoclasts. There is evidence for both a direct blocking effect on these cells as well as an apoptosis-inducing effect.15,16 Over a decade ago, it was found that incorporation of this drug into liposomes allowed it to become a potent antimacrophage agent both in vivo and in vitro.17,18 The liposomal drug is taken up by macrophages and rapidly causes apoptosis.19,20 Its effects in vivo are influenced principally by its route of administration. Injection into tissues leads to the depletion of macrophages from the tissue itself and from draining lymph nodes. Intravenous injection of liposomally encapsulated clodronate leads to nearly complete depletion of splenic and hepatic macrophages as well as marginal zone dendritic cells within 24 hours.21 Unlike other methods of macrophage depletion, however, this treatment does not lead to the secretion of proinflamatory cytokines by the dying macrophages.22Moreover, liposomal clodronate appears to have a very selective effect on macrophages and phagocytic dendritic cells. Neutrophils and lymphocytes have not been found to be directly affected by the drug.21,23,24
Due to the well-established, critical role of macrophages in AIHA, we wondered whether the potent and specific antimacrophage effects of liposomal clodronate could be harnessed to treat this disease. To study this question, we generated a model of AIHA in mice. In this model, we treated mice with monoclonal anti-RBC antibodies derived from either rats or autoimmune mice. These antibodies produced significant red blood cell destruction and anemia, which we believe mimics spontaneous AIHA. We found that liposomal clodronate was a very potent therapy to prevent or alleviate anemia in this model.
Materials and methods
Mice
Female C57BL/6 and male A/J mice were obtained from The Jackson Laboratory (Bar Harbor, ME). All mice used were 6 to 12 weeks of age. Intravenous injections were performed via the retro-orbital plexus in mice anesthetized with tribromoethanol. If mice received 2 different injections sequentially, they were performed on opposite sides. All bleeding of mice was via the tail veins into EDTA (ethylenediaminetetraacetic acid) saline. A minimum of 3 mice per experimental group were used in all experiments. In time-course experiments, no mouse was bled more than once, so each time point represents at least 3 unique mice.
Antibodies
The antimurine red blood cell antibodies used in these studies were as follows: TER-119 (a generous gift of Dr I. Weissman), a rat IgG2b monoclonal antibody25; 34-3C, a mouse IgG2a monoclonal antibody8; and 4C8, a monoclonal mouse IgM.7 TER-119 and 34-3C were affinity-purified via protein G and protein A chromatography, respectively. The antibody 4C8 was purified by means of a HiTrap IgM purification column (Amersham Pharmacia, Uppsala, Sweden) per the manufacturer's protocol. Except where otherwise specified, the following doses of these antibodies were administered to experimental animals: TER-119, 50 μg intraperitoneally; 34-3C, 150 μg intraperitoneally; 4C8, 150 μg intravenously. These doses were determined empirically to produce significant anemia in healthy C57BL/6 mice. For flow cytometry and microscopy, anti-CD68 (FA-11) and F4/80 were obtained from Serotec (Oxford, United Kingdom). FA-11 was either biotinylated or coupled to Oregon green with the use of a commercial dye-coupling kit (Molecular Probes, Eugene, OR).
Liposomes
Clodronate (dichloromethylene diphosphonate) was provided by Roche Diagnostics (Mannheim, Germany). Clodronate liposomes were prepared as previously described.22 Briefly, 86 mg phosphatidylcholine (Lipoid EPC; LIPOID, Ludwigshafen, Germany) and 8 mg cholesterol (Sigma Chemical, St Louis, MO) were combined with 10 mL clodronate (0.7 M) solution and sonicated gently. The resulting liposomes were then washed to eliminate free drug. Empty liposomes were prepared under the same conditions with the use of phosphate-buffered saline (PBS) instead of the clodronate solution. All liposomes were passed through a 12-μm filter immediately prior to use in order to eliminate large lipid aggregates. Unless otherwise specified, 10 mL liposomes per kilogram of body weight (or 0.1 mL/10 g) were administered intravenously in all experiments. This dose has been previously shown to eliminate splenic and hepatic macrophages within 24 hours.17 PKH26 labeling of liposomes was performed by incubating the liposomal solution with an equal volume of diluent C (Sigma Chemical) containing 16 μM PKH26 (Sigma Chemical) for 5 minutes. Liposomes were then washed by centrifugation prior to injection.
Flow cytometry/microscopy
For flow cytometry of whole blood, animals were bled via a tail vein into EDTA saline. Circulating carboxyfluorescein succimidyl ester (CFSE)–labeled RBC numbers were determined via flow cytometry of whole blood. RBCs were defined by forward- versus side-scatter gating. Labeled cells were easily distinguished from unlabeled RBCs by CFSE fluorescence. Reticulocyte counts were determined by flow cytometry after staining with Auramine-O (Sigma Chemical). An absolute reticulocyte number was obtained by multiplying this percentage by the total number of RBCs per microliter. Quantification of RBC-bound antibody was determined by a flow cytometry–based method. RBCs were washed repeatedly in EDTA saline and then incubated with a cyanin 5 (Cy-5)–conjugated F(ab′)2 goat anti–mouse/rat IgG reagent (Jackson Immunoresearch, West Grove, PA) and analyzed on a Facscaliber (Becton Dickinson, San Jose, CA).
For examination of spleen or liver cells, tissues were dissected and treated with collagenase as follows: Organs were minced with sharp scissors and placed in a solution (2 mL) of collagenase D (100 U/mL) (Boehringer Mannheim, Mannheim, Germany) and DNAse (0.1 mg/mL) (Sigma Chemical) for 30 minutes at 37°C. Then, 1 mL of 0.1 M EDTA in PBS was added, and cells were incubated for another 5 minutes. The resulting cells and fragments were passed through a 100-μm strainer. RBCs were subsequently lysed with buffered ammonium chloride. After immunostaining, cells were either analyzed via flow cytometry or cytospun onto slides for microscopy. For flow cytometry, Oregon green–coupled anti-CD68 was used after permeabilizing cells with saponin. For microscopy, biotinylated primary antibodies against CD68 or F4/80 were used, followed by a streptavidin Cy-5 secondary reagent. Intracellular staining for RBCs was achieved in permeabilized cells by staining with trinitrophenyl (TNP)–coupled Ter-119, followed by a (hamster) anti-TNP antibody (BD Pharmingen, San Diego, CA), and then a Cy-3–coupled rabbit antihamster polyclonal antibody (Jackson Immunoresearch). Nuclei were counterstained with Hoechst 33342 (Molecular Probes).
Preparation of RBCs
RBCs were opsonized in vitro with Ter-119 by incubating 109 cells per milliliter with 2 μg/mL antibody for 30 minutes. This dose was determined empirically as a nonagglutinating dose that labeled all cells (data not shown). Cells were then washed to remove any unbound antibody. Dye labeling with CFSE (Molecular Probes) was performed as follows: 109 washed cells per milliliter were incubated with 50 μM CFSE in saline for 15 minutes at 37°C. They were then washed again prior to reinjection. For labeling of RBCs with 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindodicarbocyanine, 4-chlorobenzenesulfonate (DiD) (Molecular Probes), cells were incubated at 109/mL in diluent C (Sigma Chemical) with 2 μM DiD for 5 minutes at room temperature. They were then reinjected into donor mice 2 or 3 times until approximately 60% of the donor mouse's RBCs were labeled with dye. This mouse was then bled to provide a source of labeled RBCs. This passage through a second donor mouse was performed because it led to a significant decrease in spontaneous dye uptake by splenocytes of untreated mice injected with nonopsonized RBCs.
Determination of hemoglobin and RBC counts
Blood hemoglobin concentrations were determined by a spectrophotometric method on a Sysmex NE1500 (Sysmex Corporation of America, Long Grove, IL). These values were correlated with RBC counts obtained on the same machine and on a Coulter Z1 (Miami, FL).
Results
Liposomal clodronate alleviates acute IgG-induced anemia
To induce anemia, C57BL/6 mice were injected with either Ter-119, an IgG2b monoclonal rat antimouse red blood cell antibody, or 1 of 2 different monoclonal autoantibodies derived from autoimmune NZB mice, 34-3C (IgG2a) or 4C8 (IgM). After a single injection of these antibodies, anemia progressed for approximately 48 to 72 hours in otherwise untreated mice (data not shown).
As shown in Figure 1A, mice pretreated with PBS-containing liposomes develop severe anemia, as documented by a marked decrease in blood hemoglobin values, 36 hours after injection with TER-119. This anemia was identical to antibody-challenged animals, which received no pretreatment (data not shown). In contrast, pretreatment with liposomal clodronate largely prevented the development of this anemia. Injection of liposomal clodronate into otherwise unperturbed mice had no significant effect on the blood hemoglobin concentration, lymphocyte, neutrophil, or platelet counts (Figure 1A and data not shown).
Alleviation of acute IgG-induced anemia by liposomal clodronate.
(A) Liposomal clodronate prevents the development of anemia induced by TER-119, a rat antimouse RBC antibody. Mice were injected with either liposomal clodronate or PBS (empty) liposomes. At 36 hours later, they were injected with anti-RBC antibody (TER-119). Mice were bled 36 hours after the antibody injection in order to determine blood hemoglobin concentration. One representative experiment out of 3 is shown. (B) Clodronate prevents the development of anemia induced by 34-3C (a mouse IgG2a autoantibody against murine RBCs), but not by 4C8, an autoreactive mouse IgM. Mice were injected as described in the legend to panel A, except with 150 μg 34-3C intraperitoneally, or 150 μg 4C8 intravenously. One representative experiment out of 3 is shown. Data are presented as means ± standard errors. (C) This effect of liposomal clodronate occurs despite a large amount of IgG bound to all circulating RBCs. RBCs from normal animals and animals injected with both clodronate and the indicated antibodies were washed 3 times in EDTA saline. The cells were then incubated with a goat anti–mouse/rat secondary antibody and analyzed by flow cytometry.
Alleviation of acute IgG-induced anemia by liposomal clodronate.
(A) Liposomal clodronate prevents the development of anemia induced by TER-119, a rat antimouse RBC antibody. Mice were injected with either liposomal clodronate or PBS (empty) liposomes. At 36 hours later, they were injected with anti-RBC antibody (TER-119). Mice were bled 36 hours after the antibody injection in order to determine blood hemoglobin concentration. One representative experiment out of 3 is shown. (B) Clodronate prevents the development of anemia induced by 34-3C (a mouse IgG2a autoantibody against murine RBCs), but not by 4C8, an autoreactive mouse IgM. Mice were injected as described in the legend to panel A, except with 150 μg 34-3C intraperitoneally, or 150 μg 4C8 intravenously. One representative experiment out of 3 is shown. Data are presented as means ± standard errors. (C) This effect of liposomal clodronate occurs despite a large amount of IgG bound to all circulating RBCs. RBCs from normal animals and animals injected with both clodronate and the indicated antibodies were washed 3 times in EDTA saline. The cells were then incubated with a goat anti–mouse/rat secondary antibody and analyzed by flow cytometry.
Liposomal clodronate also efficiently prevented the development of anemia after injection of a true autoantibody, 34-3C (Figure 1B). In contrast, injection of a monoclonal IgM, 4C8, produced an anemia that was not affected by liposomal clodronate. This finding is consistent with the fact that the 4C8 IgM anti-RBC autoantibody causes anemia in a “macrophage-independent” way, as a result of a massive agglutination of RBCs in liver and spleen.5
Liposomal clodronate was effective at preventing the development of anemia, despite high levels of RBC-bound IgG (Figure 1C). At the same time that animals were bled to assess anemia, an aliquot of blood was obtained to perform a flow cytometry–based Coombs assay. Representative assays from clodronate-treated mice, challenged with either TER-119 or 34-3C, are shown. The amount of RBC-bound antibody on circulating RBCs correlated with the dose of antibody administered and with the degree of anemia in animals not treated with clodronate (data not shown). Coombs assays performed on TER-119–treated animals were consistently higher than 34-3C–treated animals, despite a higher administered dose of 34-3C. This fact is consistent with the higher affinity of TER-119. Coombs assays performed on mice in the PBS liposome group revealed similar results (data not shown). Interestingly, however, the amount of RBC-bound antibody was moderately lower, consistent with the ongoing clearance of opsonized RBCs in these animals.
Liposomal clodronate alleviates antibody-induced anemia when administered chronically
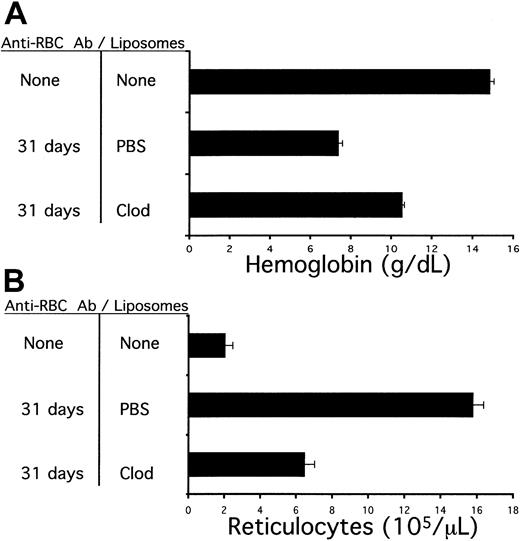
To assess whether liposomal clodronate could be a useful reagent in a more chronic setting, the following experiment was performed. Increasing doses of 34-3C were administered to A/J mice over a 31-day period. The weekly antibody doses were as follows: week 1, 70 μg; week 2, 105 μg; week 3, 140 μg; week 4, 175 μg; and week 5, 105 μg (half-week only). The weekly dose was split into 2 intraperitoneal injections administered every 3 or 4 days. At the same time that antibody injections were started, weekly liposome treatments were begun. Mice were given injections of either PBS or clodronate liposomes once per week, for a total of 5 injections. At the end of a month, animals were bled to assess hemoglobin and reticulocyte counts (Figure2). Because their IgG allotype matches that of 34-3C, A/J mice were chosen for this experiment in order to avoid the development of a neutralizing antiallotypic antibody response.
Alleviation of antibody-induced anemia by chronically administered liposomal clodronate.
Mice were injected with escalating doses of 34-3C (a murine antimurine RBC antibody) over a 31-day period. Mice were concurrently treated with weekly injections of liposomes containing either clodronate or PBS. Animals were bled at the end of this time to assess blood hemoglobin and reticulocyte counts. (A) Blood hemoglobin values (mean ± standard error). (B) Reticulocyte counts (mean ± standard error).
Alleviation of antibody-induced anemia by chronically administered liposomal clodronate.
Mice were injected with escalating doses of 34-3C (a murine antimurine RBC antibody) over a 31-day period. Mice were concurrently treated with weekly injections of liposomes containing either clodronate or PBS. Animals were bled at the end of this time to assess blood hemoglobin and reticulocyte counts. (A) Blood hemoglobin values (mean ± standard error). (B) Reticulocyte counts (mean ± standard error).
All animals developed anemia in this experiment, but liposomal clodronate–treated animals developed substantially less anemia. Furthermore, the difference in hemoglobin values of the 2 treatment groups could lead to an underestimate of the difference between them in RBC consumption, because there was a significant compensatory reticulocytosis. Animals in the PBS-treated group had 2- to 3-fold higher absolute reticulocyte counts (Figure 2B). Similarly to the experiments described in Figure 1, all circulating RBCs in mice from either treatment group were positive for surface-bound antibody (data not shown).
Liposomal clodronate rapidly prevents the clearance of opsonized RBCs
To further investigate how liposomal clodronate inhibited the development of anemia, we labeled RBCs with the fluorescent dye CFSE and opsonized them in vitro with anti-RBC antibody (Ter-119). These RBCs were then injected into mice that had been treated with either PBS or clodronate-containing liposomes 36 hours previously. Control mice received an identical number of dye-labeled, but not opsonized, RBCs. After 1 hour was allowed for the RBCs to equilibrate in the peripheral circulation, mice were bled at various time points. The RBCs that were opsonized in vitro with antibody were found to be rapidly cleared from the circulating pool (Figure 3A). Within 1 hour, more than 70% of these cells were removed from circulation. In contrast, mice pretreated with clodronate failed to clear any measurable number of antibody-coated RBCs from circulation for the length of the experiment (48 hours).
Rapid halt of the clearance of opsonized red blood cells (RBCs) by liposomal clodronate.
(A) Pretreatment with liposomal clodronate prevents the clearance of opsonized, circulating RBCs. Mice were injected with either PBS- or clodronate-containing liposomes. At 36 hours later, they were injected with identical numbers (109) of CFSE-labeled RBCs with or without in vitro incubation with anti-RBC antibody (Ter-119). Clearance of labeled RBCs from peripheral circulation was assessed by flow cytometry of blood specimens obtained at the indicated times. Data are expressed as percentages of circulating RBCs that are CFSE+ (± standard errors). Data presented are from 1 experiment representative of 2. (B) Liposomal clodronate acts extremely rapidly to halt clearance of opsonized RBCs. Mice were injected with liposomal clodronate either simultaneously or prior to (at the time indicated) injection with CFSE-labeled/antibody-opsonized RBCs (5 × 108). Control mice were injected either with an identical number of nonopsonized RBCs (and no liposomes) or with PBS liposomes (and opsonized RBCs). Clearance of labeled RBCs from peripheral circulation was assessed by flow cytometry of whole blood, obtained 4 hours after RBC injection. Data presented are from 1 experiment representative of 3; data are presented ± standard error.
Rapid halt of the clearance of opsonized red blood cells (RBCs) by liposomal clodronate.
(A) Pretreatment with liposomal clodronate prevents the clearance of opsonized, circulating RBCs. Mice were injected with either PBS- or clodronate-containing liposomes. At 36 hours later, they were injected with identical numbers (109) of CFSE-labeled RBCs with or without in vitro incubation with anti-RBC antibody (Ter-119). Clearance of labeled RBCs from peripheral circulation was assessed by flow cytometry of blood specimens obtained at the indicated times. Data are expressed as percentages of circulating RBCs that are CFSE+ (± standard errors). Data presented are from 1 experiment representative of 2. (B) Liposomal clodronate acts extremely rapidly to halt clearance of opsonized RBCs. Mice were injected with liposomal clodronate either simultaneously or prior to (at the time indicated) injection with CFSE-labeled/antibody-opsonized RBCs (5 × 108). Control mice were injected either with an identical number of nonopsonized RBCs (and no liposomes) or with PBS liposomes (and opsonized RBCs). Clearance of labeled RBCs from peripheral circulation was assessed by flow cytometry of whole blood, obtained 4 hours after RBC injection. Data presented are from 1 experiment representative of 3; data are presented ± standard error.
To determine how rapidly liposomal clodronate exerts its effects, we performed the following experiment: Mice were injected with identical numbers of dye-labeled, antibody-opsonized RBCs after receiving liposomal clodronate at various time points. They were bled 4 hours after the RBC injection. In our previous experiment, we had chosen to pretreat with clodronate 36 hours prior to injecting opsonized RBCs because this interval allowed for efficient depletion of splenic and hepatic macrophages before challenge. As is apparent from Figure 3B, liposomal clodronate very rapidly halts clearance of opsonized RBCs. It completely prevented clearance when given only 3 hours before the RBCs. Furthermore, even if given at the same time as opsonized RBCs (sequentially, in opposite retro-orbital injection sites), it was still able to prevent clearance of more than 60% of these cells. Together, these data (Figure 3A-B) indicate that liposomal clodronate causes a significant decrease in opsonized RBC clearance in less than 1 hour.
Liposomal clodronate blocks phagocytosis by macrophages
We expected that liposomal clodronate was able to prevent the clearance of opsonized RBCs in these experiments because it destroyed the macrophages that would normally take up antibody-coated RBCs. However, in our studies, the clodronate acted very quickly, within 1 hour, even though clodronate has not been reported to deplete macrophages from the spleen and liver of treated animals within this time frame.17-23 To investigate this issue further, we performed the following experiment.
Mice were injected with either PBS or clodronate liposomes that had been labeled with the lipophilic dye PKH26. At 2 hours later, they were injected with RBCs that had been labeled with another dye, DiD. At 3 hours after the RBC injection, the animals were killed, and their liver and spleen cells were stained for CD68, a marker specific for mononuclear phagocytes (macrophages, dendritic cells, and monocytes). Most CD68+ cells in the spleen or liver, however, are macrophages because they are more abundant than either dendritic cells or monocytes in these tissues.26 27
As shown in Figure 4A, liposomal clodronate does not significantly deplete macrophages (CD68+ cells) from the spleen within 5 hours. Similar results were seen in the liver (data not shown). This is in contrast to later time points (24 hours), where liposomal clodronate very thoroughly depletes spleen and liver macrophages17-23 (and data not shown). Even though liposomal clodronate does not deplete macrophages within the first few hours, it has a potent ability to prevent the clearance of opsonized RBCs within this same time frame (Figure 3). This apparent contradiction is explained in Figure 4B.
Liposomal-clodronate blocking of phagocytosis of antibody-opsonized RBCs by splenic macrophages.
Mice were injected with PKH-labeled liposomes (containing either clodronate or PBS), and 2 hours later, DiD-labeled, antibody-opsonized RBCs (109). At 3 hours after RBC injection, animals were killed, and spleen and liver cells were stained for CD68. (A) CD68+ spleen cells (macrophages, dendritic cells, and monocytes) are not yet significantly depleted within this 5-hour time frame. Data are presented as percentages of total spleen cells. (B) Liposomal clodronate uptake blocks subsequent ingestion of opsonized RBCs by macrophages. Only CD68+ spleen or liver cells are shown. The uptake of PKH-labeled liposomes and the uptake of DiD-labeled RBCs are indicated by fluorescence in their respective channels. Percentage values represent the mean percentages (± standard errors) of CD68+ cells that are positive for both liposomes (PKH) and RBCs (DiD). MFI indicates mean DiD fluorescence of cells positive for both liposomes and RBCs. Data presented are from 1 experiment representative of 3.
Liposomal-clodronate blocking of phagocytosis of antibody-opsonized RBCs by splenic macrophages.
Mice were injected with PKH-labeled liposomes (containing either clodronate or PBS), and 2 hours later, DiD-labeled, antibody-opsonized RBCs (109). At 3 hours after RBC injection, animals were killed, and spleen and liver cells were stained for CD68. (A) CD68+ spleen cells (macrophages, dendritic cells, and monocytes) are not yet significantly depleted within this 5-hour time frame. Data are presented as percentages of total spleen cells. (B) Liposomal clodronate uptake blocks subsequent ingestion of opsonized RBCs by macrophages. Only CD68+ spleen or liver cells are shown. The uptake of PKH-labeled liposomes and the uptake of DiD-labeled RBCs are indicated by fluorescence in their respective channels. Percentage values represent the mean percentages (± standard errors) of CD68+ cells that are positive for both liposomes (PKH) and RBCs (DiD). MFI indicates mean DiD fluorescence of cells positive for both liposomes and RBCs. Data presented are from 1 experiment representative of 3.
Both PBS and clodronate liposomes are taken up efficiently by CD68+ spleen and liver macrophages within 5 hours, as indicated by PKH fluorescence. Some macrophages have also taken up opsonized RBCs as indicated by DiD fluorescence. These double-positive cells are particularly evident in the mice that were treated with PBS liposomes. In contrast, far fewer macrophages from liposomal clodronate–treated mice have taken up RBCs. Furthermore, the cells in these mice, which have ingested RBCs, have a much lower MFI for DiD. This implies that they have each taken up fewer RBCs. These data indicate that the macrophages that have taken up liposomal clodronate are subsequently blocked from ingesting antibody-coated RBCs. This blocking effect, however, is observed only within a short window of time because within another 18 hours these macrophages will all be destroyed by the liposomal clodronate. The combined effects of initially blocking, then depleting, macrophages explains the rapid and complete onset of action for liposomal clodronate in our model.
Engulfed RBCs are readily seen in the macrophages of untreated mice
To confirm that what we were seeing by flow cytometry represented actual phagocytosis of RBCs and not some other process of dye acquisition, we performed microscopy on dispersed splenocytes. We examined dispersed cells because in the intact spleen, red pulp macrophages are in close contact with RBCs and it is very difficult to clearly determine whether any are intracellular.
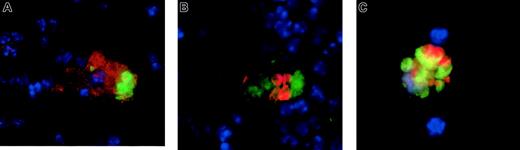
We injected antibody-opsonized RBCs into mice that either were untreated or had received liposomal clodronate 24 hours previously. At 4 to 8 hours later, mice were killed; spleens were collagenase treated; RBCs were lysed; and cells were fixed and permeabilized, stained with the indicated antibodies, and cytospun onto slides for microscopy. After RBC lysis, no free RBCs could be found on slides. As expected, the only RBCs that were seen were those that appeared to be within other cells. Few macrophages remained 24 hours after clodronate treatment. Those that were found did not appear to have ingested RBCs (data not shown). In control mice, however, macrophages were found to have ingested RBCs by 3 different immunofluorescent stains. These stains each reveal intact RBCs within macrophages. Figure5A reveals an example of surface staining with F4/80, a macrophage-specific marker (in red), followed by intracellular staining with Ter-119 (anti-RBC, green; nuclei are counterstained blue). Figure 5B is an example of intracellular staining with anti-CD68 (green) and Ter-119 (red). Finally, 5C is an example of ingested CFSE-labeled RBCs' being visualized (in green) owing to CFSE labeling prior to injection, along with intracellular staining for CD68. CD68 can be seen colocalizing with the RBCs because it is found largely in endosomes.
Ingested RBCs in splenic macrophages of untreated (but not clodronate-treated) mice.
Ingested RBCs are readily apparent in splenic macrophages of untreated mice (but not in clodronate-treated mice). Mice were injected with antibody-opsonized RBCs (109). At 4 to 8 hours later, they were killed and the splenocytes were dispersed, stained, and cytospun onto slides. RBCs were lysed prior to staining until no free RBCs were visible on the slides. (A) Cells were stained for F4/80, a macrophage-specific cell surface molecule, (in green) then fixed and permeabilized for intracellular staining with Ter-119, an RBC specific antibody (in red). (B) Cells were intracellularly stained with anti-CD68 (in red) and anti-RBC (Ter-119, in green). (C) Cells were stained intracellularly for CD68 (in red), and RBCs (in green) are visualized because they were labeled with CFSE prior to administration. In all images, nuclei were counterstained in blue. No ingested RBCs were visible in mice pretreated with liposomal clodronate (data not shown). Original magnifications × 100.
Ingested RBCs in splenic macrophages of untreated (but not clodronate-treated) mice.
Ingested RBCs are readily apparent in splenic macrophages of untreated mice (but not in clodronate-treated mice). Mice were injected with antibody-opsonized RBCs (109). At 4 to 8 hours later, they were killed and the splenocytes were dispersed, stained, and cytospun onto slides. RBCs were lysed prior to staining until no free RBCs were visible on the slides. (A) Cells were stained for F4/80, a macrophage-specific cell surface molecule, (in green) then fixed and permeabilized for intracellular staining with Ter-119, an RBC specific antibody (in red). (B) Cells were intracellularly stained with anti-CD68 (in red) and anti-RBC (Ter-119, in green). (C) Cells were stained intracellularly for CD68 (in red), and RBCs (in green) are visualized because they were labeled with CFSE prior to administration. In all images, nuclei were counterstained in blue. No ingested RBCs were visible in mice pretreated with liposomal clodronate (data not shown). Original magnifications × 100.
The effects of liposomal clodronate last 1 to 2 weeks
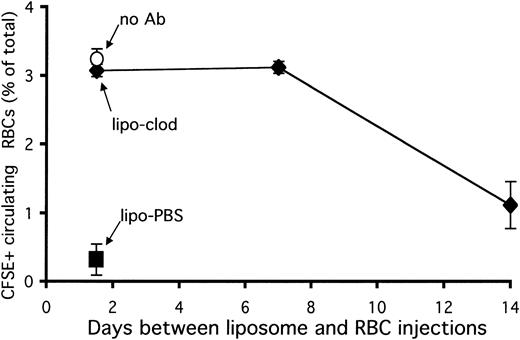
A single dose of liposomal clodronate has previously been found to deplete splenic (red pulp) macrophages for 1 to 2 weeks in mice.21 After this time, natural turnover of red pulp macrophages leads to the replenishment of this population. This led us to wonder how long a single dose of liposomal clodronate would be effective in halting consumption of antibody-opsonized RBCs. To examine this, mice were injected with clodronate liposomes and then left undisturbed for 1 or 2 weeks. They were then injected with CFSE-labeled, antibody-opsonized RBCs. Mice were bled 4 hours later to assess survival of circulating RBCs. Control mice received either PBS or clodronate liposomes 36 hours before RBC injection. An additional control was the injection of an identical number of nonopsonized RBCs into an untreated mouse. As can be seen in Figure6, treatment with clodronate 1 week prior to challenge with opsonized RBCs was as effective as treatment 36 hours before challenge. Treatment given 2 weeks prior to challenge was not as effective, however. These results are consistent with the persistent effects of liposomal clodronate on RBC uptake being due to macrophage depletion. As the red pulp macrophage population is replenished (in 1 to 2 weeks), the effects of liposomal clodronate on RBC clearance diminish.
Effect of a single dose of liposomal clodronate on clearance of opsonized RBCs.
A single dose of liposomal clodronate affects clearance of opsonized RBCs for 1 to 2 weeks. Mice were injected at the indicated times with either PBS or clodronate liposomes. RBCs were labeled with CFSE and opsonized with anti-RBC antibody in vitro. Control mice received an identical number of nonopsonized RBCs (5 × 108). Persistence of labeled RBCs was assessed by tail bleeding 4 hours later. Data are expressed as a percentage of circulating RBCs which were CFSE+ (± standard error). Data presented are from 1 representative experiment out of 2.
Effect of a single dose of liposomal clodronate on clearance of opsonized RBCs.
A single dose of liposomal clodronate affects clearance of opsonized RBCs for 1 to 2 weeks. Mice were injected at the indicated times with either PBS or clodronate liposomes. RBCs were labeled with CFSE and opsonized with anti-RBC antibody in vitro. Control mice received an identical number of nonopsonized RBCs (5 × 108). Persistence of labeled RBCs was assessed by tail bleeding 4 hours later. Data are expressed as a percentage of circulating RBCs which were CFSE+ (± standard error). Data presented are from 1 representative experiment out of 2.
Liposomal clodronate is effective over a wide range of doses
The standard dose that we chose for treating animals in the experiments described so far in this paper was 10 mL per kilogram of body weight (or 0.1 mL/10 g). This dose was chosen because it has previously been shown to deplete splenic macrophages efficiently.22 This dose, however, may not be very practical for use in humans or large animals, because it represents a rather large infusion of liposomal drug, 700 mL for an average adult. Therefore, we wondered whether a smaller dose would be useful for preventing the uptake of opsonized RBCs.
To examine this question, we injected mice with decreasing doses of liposomal clodronate 36 hours prior to injection with opsonized, dye-labeled RBCs. Control mice received either PBS liposomes (at 10 mL/kg) or an identical number of nonopsonized RBCs (and no liposomes). Mice were bled 4 hours after injection to assess survival of circulating RBCs. Figure 7 reveals that liposomal clodronate worked equivalently from 1 to 10 mL/kg and that its efficacy dropped off below that dose. Although liposomal clodronate may not completely deplete splenic macrophages at these lower doses, it appears to be depleting (or blocking) the most actively phagocytic ones at these doses. Therefore, it maintains its efficacy over a wide range of doses.
Liposomal clodronate effectiveness and dosage.
Liposomal clodronate is effective over a wide dose range. Mice were injected with the indicated doses of clodronate or PBS liposomes. At 36 hours later, they were injected with CFSE-labeled, antibody-opsonized RBCs (5 × 108). Control mice received an identical number of nonopsonized RBCs. Persistence of circulating, labeled RBCs was assessed by tail bleed 4 hours later. Data are expressed as percentages of circulating RBCs that were CFSE+ (± standard errors). Data presented are from 1 experiment representative of 3.
Liposomal clodronate effectiveness and dosage.
Liposomal clodronate is effective over a wide dose range. Mice were injected with the indicated doses of clodronate or PBS liposomes. At 36 hours later, they were injected with CFSE-labeled, antibody-opsonized RBCs (5 × 108). Control mice received an identical number of nonopsonized RBCs. Persistence of circulating, labeled RBCs was assessed by tail bleed 4 hours later. Data are expressed as percentages of circulating RBCs that were CFSE+ (± standard errors). Data presented are from 1 experiment representative of 3.
Discussion
Liposomal clodronate has previously been well characterized as a potent antimacrophage agent.17 For this reason, it has also been investigated as a potentially useful drug for treating autoimmune disorders such as adjuvant arthritis, uveitis, and experimental autoimmune encephalitis in animal models.28,29 More recently, it was found to be useful in a mouse model of immune thrombocytopenic purpura.24 This article demonstrates for the first time that it also appears to be a useful agent for treating antibody-induced anemia and, potentially, spontaneous AIHA.
The experiments detailed in this paper show that liposomal clodronate consistently prevents or halts red blood cell destruction. It is effective when given prior to antibody challenge, as well as when given concurrently in a more chronic fashion. It protects against the development of anemia, despite the fact that virtually all circulating RBCs are antibody coated. Not surprisingly, liposomal clodronate failed to protect against IgM-mediated anemia. In addition to its striking potency, it has an extremely rapid onset of action. Furthermore, a single depleting dose is sufficient to protect mice from antibody-induced RBC destruction for 1 to 2 weeks. All of these characteristics are very desirable for a new agent to treat AIHA.
Autoimmune hemolytic anemia is a highly variable disease. It can be caused by a variety of IgG and/or IgM antibodies. Numerous underlying conditions have been associated with it. Furthermore, its natural history is also quite variable. While many patients present with a slowly developing anemia, others, including the majority of patients in pediatric case series, present with sudden, severe anemia.30-32 We have attempted to model this disorder by administering a variety of anti-RBC antibodies in both a chronic and an acute fashion. We used a xenogeneic rat-derived antibody and a mouse-derived IgG monoclonal antibody with similar results. While this model does not mimic every aspect of the disease, we believe it is an appropriate one for studying antimacrophage agents in this disease.
Current mainstays of therapy for AIHA include transfusion, corticosteroids, and, eventually, splenectomy.1 Each of these treatments has drawbacks. Transfusions have well-described risks that accompany their use in AIHA patients. Corticosteroids have a multitude of well-known acute and chronic side effects. They also have another shortcoming. The onset of action of corticosteroids is variable, frequently taking many hours (or even days).1 In patients presenting with very severe anemia, this shortcoming is a major source of concern and potential morbidity. In multiple case series, patients such as these, presenting with severe anemia (hemoglobin below 60 g/L [6 g/dL]), are quite common.30,32 33
Splenectomy, another mainstay of treatment, has several drawbacks as well. The most obvious one is routine surgical morbidity and mortality. Another drawback of splenectomy is its association with a life-long risk of fatal sepsis from encapsulated micro-organisms.34,35 Finally, splenectomy does not always alleviate AIHA, because hepatic macrophages may be responsible for a significant proportion of RBC consumption.36,37 In fact, the substantial role of hepatic Kupffer cells in the development of AIHA has been repeatedly shown in mice.6-9 The unpredictable efficacy of splenectomy is of particular concern in light of the short-term (surgical) and life-long (infectious) morbidities of this procedure.
Liposomal clodronate, with its swift and potent ability to shut off RBC consumption, may reasonably be thought of as a temporary medicinal splenectomy. In our experimental system, it completely halted uptake of opsonized RBCs by the spleen, as a splenectomy would be expected to do. However, because the spleen is not removed, its function is eventually restored by the natural replenishment of macrophages. Liposomal clodronate offers many potential advantages over current therapy. Its speed of onset appears to be superior to that of corticosteroids. This may prove to be particularly useful in cases of severe, life-threatening anemia where cardiovascular compromise is evident. While liposomal clodronate is unlikely to replace corticosteroids, it may be useful as an adjunct therapy, allowing more rapid, reliable cessation of RBC destruction. Another potential advantage of clodronate is its ability to block or deplete phagocytic macrophages outside of the spleen. Macrophages in the liver and bone marrow, which are not accessible by surgical means, are affected as well.22 Liposomal clodronate may ultimately be useful as an intermittent therapy for patients who continue to experience significant anemia after splenectomy.
Another important aspect of liposomal clodronate, which can be viewed as both an advantage and a disadvantage, is its temporary (1 to 2 weeks for a single dose) duration of action. While cases of AIHA in adult patients are typically chronic, most pediatric case series report that approximately half of all warm autoantibody (IgG-mediated) AIHA cases are acute, lasting less than 6 months.33 32 Accordingly, there may be situations in both adult and pediatric patients where temporary ablation of splenic function is desirable, but where life-long loss of splenic function is not necessary. If the use of liposomal clodronate improves medical management of AIHA, some patients may thereby avoid splenectomy and its life-long consequences.
Our experimental data indicate that liposomal clodronate is effective when given repeatedly, and concurrently with anti-RBC antibody challenge. This “chronic” administration raises the possibility that liposomal clodronate could be administered repeatedly over a long period of time and thus supplant other medical (and surgical) therapies. Indeed, liposomal clodronate could conceivably be used intermittently as a steroid-sparing agent in chronic cases of AIHA. If administered only once every 1 to 2 weeks, it would be a very practical intravenous therapy to administer. Chronic use of liposomal clodronate remains speculative, however, because little is known (outside of the experiments detailed in this paper) about prolonged systemic depletion of macrophages with this agent.
Indeed, questions regarding the safety of liposomal clodronate, particularly when administered chronically, have not yet been thoroughly addressed. Other investigators have found that liposomal clodronate has no apparent direct effects on cells other than mononuclear phagocytes.21 No alterations in circulating lymphocytes or neutrophils have been reported. Furthermore, in our experiments using dye-labeled liposomal clodronate, nearly all dye-containing cells within the spleen or liver were CD68+. This result implies that no cells other than mononuclear phagocytes received a significant dose of the drug.
An important unanswered question, however, is how macrophage depletion with liposomal clodronate predisposes one to infection. This is a relevant question in light of the profound paralysis of macrophage function we observed in our model system. Other investigators have examined liposomal clodronate in a variety of short-term experimental models of infection. In some cases, it actually protects from mortality.38-42 In most, however, macrophage depletion worsens mortality or other infection-related end points.43-47 How this risk may compare with that of splenectomy has not yet been explored.
This work describes the novel use of liposomal clodronate as a therapeutic agent in a murine model of autoimmune hemolytic anemia. Because of its promising efficacy, we believe that it warrants further investigation as a potential experimental therapy for AIHA.
The authors would like to thank the staff of the National Jewish Medical and Research Center clinical laboratory, particularly Rhonda Emerick and Sharon Lyons, for assistance with hemoglobin determinations.
Prepublished online as Blood First Edition Paper, August 29, 2002; DOI 10.1182/blood-2001-11-0061.
Supported by US Public Health Service grants CA-82086, AI-07365, AI-17134, AI-18785, and AI-22295; National Cancer Institute grant CA-46934 (supporting a core facility); the Melvin Garb Endowed Fellowship in Basic Immunology; and a grant from the Swiss National Foundation for Scientific Research.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Michael B. Jordan, NJC/HHMI -k512, 1400 Jackson St, Denver, CO 80206; e-mail:jordanm@njc.org.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal